Abstract
In this multicenter, open-label, randomized controlled trial, we determined whether 2-month prednisolone therapy for steroid-sensitive nephrotic syndrome was inferior or not to 6-month therapy despite significantly less steroid exposure. The primary end point was time from start of initial treatment to start of frequently relapsing nephrotic syndrome. The pre-specified non-inferiority margin was a hazard ratio of 1.3 with one-sided significance of 5%. We randomly assigned 255 children with an initial episode of steroid-sensitive nephrotic syndrome to either 2 - or 6-month treatment of which 246 were eligible for final analysis. The total prednisolone exposure counted both initial and relapse prednisolone treatment administered over 24 months. Median follow-up in months was 36.7 in the 2-month and 38.2 in the 6-month treatment group. Time to frequent relaps was similar in both groups; however, the median was reached only in the 6-month group (799 days). The hazard ratio was 0.86 (90% confidence interval, 0.64–1.16) and met the non-inferior margin. Time to first relapse was also similar in both groups: median day 242 (2-month) and 243 (6-month). Frequency and severity of adverse events were similar in both groups. Most adverse events were transient and occurred during initial or relapse therapy. Thus, 2 months of initial prednisolone therapy for steroid-sensitive nephrotic syndrome, despite less prednisolone exposure, is not inferior to 6 months of initial therapy in terms of time to onset of frequently relapsing nephrotic syndrome.
Keywords: initial treatment, nephrotic syndrome, pediatric nephrology, randomized controlled trial, steroid
Idiopathic nephrotic syndrome (NS) is a disorder affecting the kidneys that is mainly characterized by high excretion of protein in the urine. Pediatric idiopathic NS is understood to be the most common cause of primary glomerular diseases, and it frequently occurs in infants aged 2–6 years. Most patients are presumed to have minor glomerular abnormality. Cellular immunologic abnormalities are believed to contribute to the condition, although its pathology remains unknown. In Europe and the United States, two in 100,000 children will develop idiopathic NS in a single year.1 An 8-week corticosteroid regimen is the standard initial treatment for children with idiopathic NS, as outlined by the International Study of Kidney Disease in Children (ISKDC).2, 3 Although corticosteroids induce the remission of proteinuria in more than 80% of children with idiopathic NS, ∼60% undergo proteinuria relapse. Previous research has shown that a high number of children undergo frequent relapse, and corticosteroid toxicities occur after repeated therapy.2, 3 Although some controlled studies4, 5, 6, 7 and a meta-analysis8 show that long-term corticosteroid treatment up to 7 months maximum leads to a longer sustained remission of NS than ISKDC-recommended administration, the optimum dose and duration of initial therapy are still unknown. A Cochrane review concluded that a well-designed and adequately powered randomized controlled trial is required to establish the optimum dose and duration of treatment.8 The purpose of this study is to investigate whether 2 months of initial prednisolone therapy (ISKDC regimen) is not inferior to 6 months of initial therapy with an increasing cumulative dose, and to compare adverse events between treatment regimens.
RESULTS
Patient population
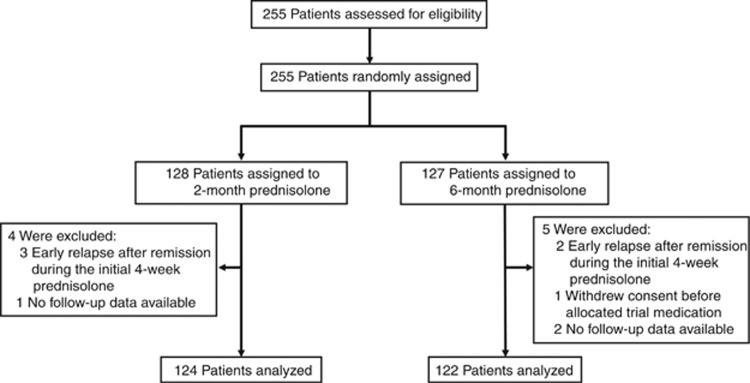
The study was conducted from September 6, 2007 until February 8, 2013. Figure 1 shows the trial profile. We assessed 255 patients from 90 hospitals (61 general, 7 children's, and 22 university hospitals) for eligibility. We randomly assigned 128 patients to the 2-month prednisolone group and 127 patients to the 6-month prednisolone group. We excluded nine patients from the analysis: six did not receive trial medication because of either early relapse after remission during the initial 4-week prednisolone treatment, or withdrawn consent, and three were excluded owing to a lack of participant data. Thus, we analyzed data for 246 patients. Median follow-up was 36.7 months in the 2-month group (interquartile range 27.8–46.4 months) and 38.2 months in the 6-month group (interquartile range 28.6–48.5 months). There was no difference in characteristics between the two groups (Table 1).
Figure 1.
Trial profile.
Table 1. Baseline characteristics.
| 2-Month prednisolone (n=124) | 6-Month prednisolone (n=122) | P-value | |
|---|---|---|---|
| Male, n (%) | 89 (71.8) | 87 (71.3) | 0.94 |
| Age, mean (s.d.), years | 6.7 (4.1) | 6.3 (4.1) | 0.42 |
| Age group, years | |||
| 1–5, n (%) | 67 (54.0) | 66 (54.1) | 0.99 |
| 6–10, n (%) | 33 (26.6) | 33 (27.1) | |
| 11–15, n (%) | 24 (19.4) | 23 (18.9) | |
| Blood pressure, mean (s.d.), mm Hg | |||
| Systolic | 104.4 (10.7) | 106.4 (12.0) | 0.16 |
| Diastolic | 62.4 (10.0) | 62.5 (11.3) | 0.98 |
| Serum albumin, mean (s.d.), g/l | 1.4 (0.5) | 1.4 (0.5) | 0.90 |
| Hospital, n (%) | |||
| General | 47 (65.3) | 46 (64.8) | 1.00 |
| Children's | 7 (9.7) | 7 (9.9) | |
| University | 18 (25.0) | 18 (25.4) | |
| Quarterly distribution of disease onset, n (%) | |||
| January–March | 23 (18.7) | 24 (19.7) | 0.99 |
| April–June | 36 (29.3) | 34 (27.9) | |
| July–September | 30 (24.4) | 31 (25.4) | |
| October–December | 34 (27.6) | 33 (27.0) | |
| Duration from the first episode to remission, mean (s.d.), days | 9.7 (3.1) | 10.0 (3.1) | 0.45 |
Abbreviation: s.d., standard deviation.
Primary end point
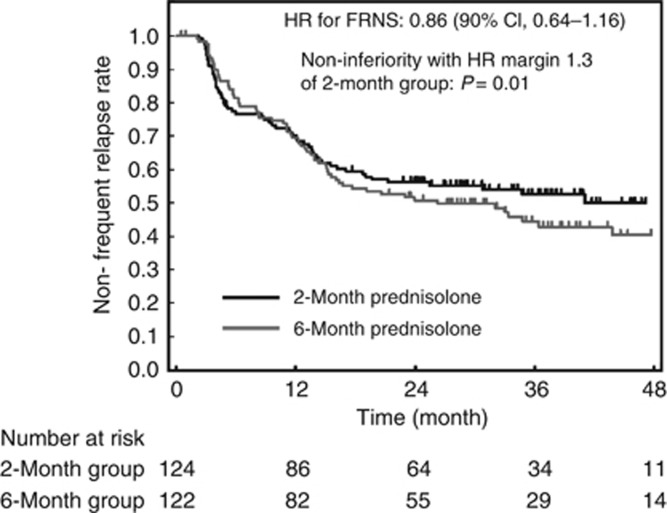
The primary end point was defined as the duration from start of initial treatment to diagnosis of frequently relapsing nephrotic syndrome (FRNS), or ‘time to FRNS'. By the end of the 24-month intervention period, we observed 54 events in the 2-month group (comprising 46 FRNS [definition 1, 28; definition 2, 18], and 8 requiring immunosuppressant administration) and 58 events in the 6-month group (comprising 45 FRNS [definition 1, 23; definition 2, 22], and 13 requiring immunosuppressant administration). Twenty-one patients required immunosuppressants owing to steroid-dependent or steroid-resistant relapse. Times to FRNS were similar in both groups: however, the median duration of time to FRNS was reached only in the 6-month group (at 799 days). The hazard ratio (HR) was 0.86 (90% confidence interval (CI), 0.64–1.16; Figure 2), and noninferiority of the 2-month group was confirmed significantly, with an HR margin of 1.3 (P=0.01). Post-hoc analyses showed that age groups did not affect the median duration of time to FRNS. The HRs (95% CI) were 0.92 (0.59–1.45), 0.86 (0.41–1.84), and 0.74 (0.31–1.77) for the age groups 1–5 years, 6–10 years, and 11–15 years, respectively.
Figure 2.
Kaplan–Meier estimates of time to frequently relapsing nephrotic syndrome (FRNS). HR, hazard ratio.
Secondary end points
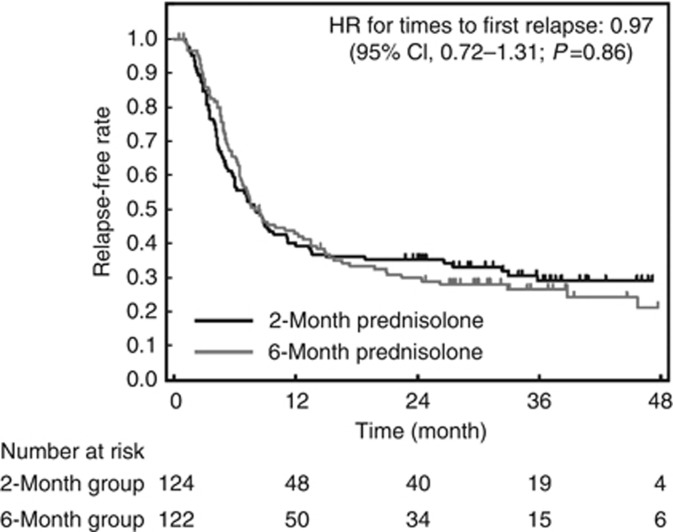
Times to first relapse were similar in both groups: the median was 242 days and 243 days in the 2-month and 6-month treatment groups, respectively (HR=0.97; 95% CI, 0.72–1.31; P=0.86; Figure 3). The number of relapses per person-year during the trial intervention period was 1.25 times in the 2-month group and 1.33 times in the 6-month group, and the ratio was 0.94 (95% CI, 0.71–1.22; P=0.65, Table 2). The median cumulative dose of prednisolone during the 2-year trial period in the 2-month group was also significantly lower than in the 6-month group (4621.9 [interquartile range=2191.3–7472.5] vs. 6484.8 [interquartile range=3701.0–9577.9], P<0.001).
Figure 3.
Kaplan–Meier estimates of time to first relapse. HR, hazard ratio.
Table 2. Number of relapses.
| Total number of relapses | Duration of observation (person-year) | The number of relapses (per person-year) | Ratio of the number of relapses (CI) | P-value | |
|---|---|---|---|---|---|
| 2-Month prednisolone | 301 | 240.93 | 1.25 | 0.94 | 0.65 |
| 6-Month prednisolone | 309 | 232.62 | 1.33 | (0.71–1.22) |
Abbreviation: CI, confidence interval.
Adverse events
Frequency and severity of adverse events were similar in both groups (Table 3). Most adverse events were transient and occurred during initial therapy or relapse therapy. In our study, steroid dependency did not greatly affect the occurrence of adverse events. Two patients in the 2-month group had severe adverse events requiring hospitalization. One patient discontinued because of acute kidney failure during relapse (month 10) and recovered in 22 days. Another patient had pneumonia with influenza infection on the last date of the 2-month prednisolone treatment and recovered in 10 days. Height standard deviation scores show a significant decrease in growth at 2 months of follow-up compared with baseline (P<0.003). In both groups, this was restored within 9 months after initial treatment commenced. Notably, one patient in the 6-month group was diagnosed with possible adrenal insufficiency owing to steroid withdrawal according to clinical symptoms (mild headache and mild nausea) when the patient switched to trial medication after the initial dose of prednisolone. Symptoms disappeared on the same day of onset without further treatment.
Table 3. Adverse events during the 24-month trial intervention perioda.
| Event | 2-Month prednisolone n=124 | 6-Month prednisolone n=122 | P-value |
|---|---|---|---|
| Hypertensionb | 15 | 9 | 0.24 |
| Cushingoid appearance | |||
| Cushing (moon face)b | 54 | 61 | 0.46 |
| Central obesityb | 20 | 34 | 0.052 |
| Striae | 1 | 0 | 1.00 |
| Adrenal insufficiency | 0 | 1 | 1.00 |
| Ophthalmological abnormalities | |||
| Glaucomab | 19 | 13 | 0.31 |
| Cataract | 0 | 0 | |
| Severe infections | |||
| Pneumoniac | 1 | 0 | 1.00 |
| Peptic ulcer | 1 | 0 | 1.00 |
| Acute kidney failurec | 1 | 0 | 1.00 |
| Hyperglycemia | 2 | 3 | 0.64 |
| Increased laboratory data | |||
| ASTb | 14 | 11 | 0.58 |
| ALTb | 26 | 16 | 0.14 |
| Amylase | 3 | 0 | 1.00 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Data are expressed as the number of events.
Multiple reports were recorded for these adverse events.
Severe adverse events requiring hospitalization.
DISCUSSION
Extension of initial steroid treatment for more than 3 months to decrease the risk of relapse in children with steroid-sensitive (SS) NS has been widely described in previous studies.4, 5, 6, 7, 8, 9, 10 However, 80–90% of children diagnosed with SSNS who are given new corticosteroid treatments continue to relapse, and ∼50% relapse frequently.11 Therefore, initial approaches to SSNS therapy are likely to be substantially different.12 Our results demonstrate that extending initial steroid treatment, and even increasing the total dose of prednisolone (2240 vs. 3885 mg/m2), does not improve clinical outcomes (time to FRNS, time to first relapse, the number of relapses, total prednisolone dose, and adverse events) for pediatric NS. Our results add to the Cochrane review by Hodgson et al.8 by comparing treatment regimens of 2 vs. 6 months.
Our findings build on a 2013 study from the Netherlands. This well-designed, randomized, double-blind, placebo-controlled trial of children with NS clearly showed no improvement in clinical outcomes when the initial prednisolone treatment was extended from 3 to 6 months without an increasing cumulative dose.13 However, despite the results of this trial, the most effective duration and dosage of prednisolone treatment for an initial episode of SSNS is still under debate.14 Although our study has demonstrated that increasing the total dose in 2-month or 6-month regimens does not improve clinical outcomes, further investigation is still required to determine the most effective duration and dosage regimen for initial SSNS.
A key strength of our trial is its unique design. This is a multicenter, randomized, controlled trial for noninferiority that compares the efficacy of the 2-month ISKDC regimen with a 6-month, long-term prednisolone regimen. The most important clinical objective of initial treatment of SSNS is to prevent frequent relapses. Consequently, the primary end point was set to time to FRNS rather than initial relapses.
Our data from several analyses consistently support noninferiority of the ISKDC regimen. In the current trial, a HR threshold of 1.3 for noninferiority of the primary end point is slightly high given the feasible study size. However, the posterior probability that a HR would be smaller than 1.1 and 1.2 (HRs that are commonly used as an upper equivalence margin) was 91 and 97%, respectively.
In our trial, noninferiority was found in FRNS (primary end point) and first relapse (secondary end point) between the ISKDC and 6-month regimens. This means that many patients relapse even while taking tapering-dose prednisolone (Figure 3). Previous studies vary in their observation of (frequent) relapses from either the start or end of initial therapy.8, 13 However, if analyses are performed from the end of initial therapy, relapses during tapering-dose prednisolone cannot be counted, possibly resulting in an inadequate interpretation. Therefore, we selected observations primarily from the start of initial therapy. In our study design, steroid-dependent NS was predicted to occur more frequently because of its definition in the 6-month regimen. This is one reason why we selected FRNS as a primary end point, and not steroid-dependent NS. In fact, time to steroid-dependent NS was significantly higher in the ISKDC regimen group (data not shown).
No significant difference in adverse events was observed between the two regimens in our large-scale trial, which is a similar finding to previous small-scale trials.4, 5, 6, 7 Most adverse events were transient and occurred during initial or relapse therapy. However, because the ISKDC regimen is generally less likely to cause adverse events owing to the lower dosage and the shorter duration, its use can be recommended.
A limitation of our trial is the open-label design, which may have introduced preconception bias. However, as our trial design is a noninferiority trial with regular visits, and relapses are measured objectively, we cannot assume positive placebo effects. Therefore, the open-label design of this study may have limited impact on preconception bias. Moreover, the results of this trial may partially be due to the relatively high rate of relapse compared with other studies.5 The high rate of relapse may be owing to our definition of relapse (proteinuria 2+ or higher). It still remains unknown whether long prednisolone therapy consisting of a dose of 60 mg/m2 per day for 6 weeks, followed by alternate-day doses of 40 mg/m2 per 2 days for 6 weeks, is more effective against time to FRNS compared with the ISKDC regimen for treating idiopathic NS. In our study, only one patient withdrew consent. A low rate of consent withdrawal is common in Japan.15, 16 Cultural differences between countries may account for variations in rates of consent withdrawal.
In our study, steroid sensitivity was confirmed by day 21 in order to ensure time for eligibility screening. Generally, remission after 3 weeks is uncommon.17 Therefore, as the effect of early confirmation of steroid sensitivity was slight, we decided to confirm steroid sensitivity by day 21.
We conducted a meta-analysis to address the differences between corticosteroid regimens in children with an initial episode of SSNS. We searched randomized controlled trials that compared durations of steroid therapy in children and reported the number of FRNS cases within 2 years (see the Supplementary Information online for a detailed search strategy). Meta-analysis of our study and five studies6, 13, 18, 19 showed a risk ratio (long vs short) of 0.99 (95% CI, 0.68–1.44, see Supplementary Figure 2A online), whereas meta-analysis of our study and the published studies only showed an risk ratio of 1.15 (95% CI: 0.95–1.40, Supplementary Figure 2B online). This result might indicate that long-term treatment is not superior but almost equivalent to ISKDC-standard therapy.
In conclusion, our study shows that extending initial prednisolone treatment from 2 to 6 months with an increasing dose does not improve clinical outcomes for pediatric NS. The original ISKDC regimen is not inferior to 6 months of initial therapy with an increasing cumulative dose. We assert that the ISKDC regimen is recommended as an initial treatment for pediatric idiopathic NS.
MATERIALS AND METHODS
Study design and patients
We conducted a multicenter, randomized, noninferiority, open-label trial at 90 hospitals in Japan and compared prednisolone treatment of 2 months (ISKDC regimen) with 6 months for children with a first episode of idiopathic NS. We diagnosed idiopathic NS and remission according to the ISKDC.1 NS was defined as a urinary protein–creatinine ratio ⩾1.8 and albumin levels ⩽25 g/l in serum. Remission was defined as a negative dipstick analysis for 3 consecutive days. Patients aged 1–15 years with a first episode of idiopathic NS were eligible if they had remission within 3 weeks of prednisolone administration. Patients were ineligible if they had secondary NS, renal insufficiency defined as creatinine clearance of ⩽60 ml/min per 1.73 m2, active infections, poorly controlled hypertension, severe liver dysfunction, pregnancy, or a history of immunosuppressant administration.
Before enrollment, patients' guardians provided written informed consent, and informed assent was obtained from older children. This study was approved by the institutional review boards of participating hospitals, complied with the Declaration of Helsinki and the Declaration of Istanbul, and adhered to the International Conference on Harmonisation Guidelines on Good Clinical Practice.
Randomization
Patients were randomly assigned to either the 2-month or 6-month group in a 1:1 ratio at the Japan Clinical Research Support Unit. We applied a minimization method using a computer-generated sequence (SAS PROC PLAN) with age (1–10 years or 11–15 years), sex, and institution as adjustment (stratification) factors. Patients, patients' guardians, treating physicians, and individuals assessing outcomes and analyzing data were not blinded to the patients' treatment assignments. Apart from the trial statistician and the data-monitoring committee, all treating physicians and other investigators remained blinded to the trial results until follow-up was completed.
Procedures
The first patient was randomized in September 2007, and the last patient in January 2011. Follow-up started at diagnosis and was truncated when the last enrolled patients finished the 24-month intervention.
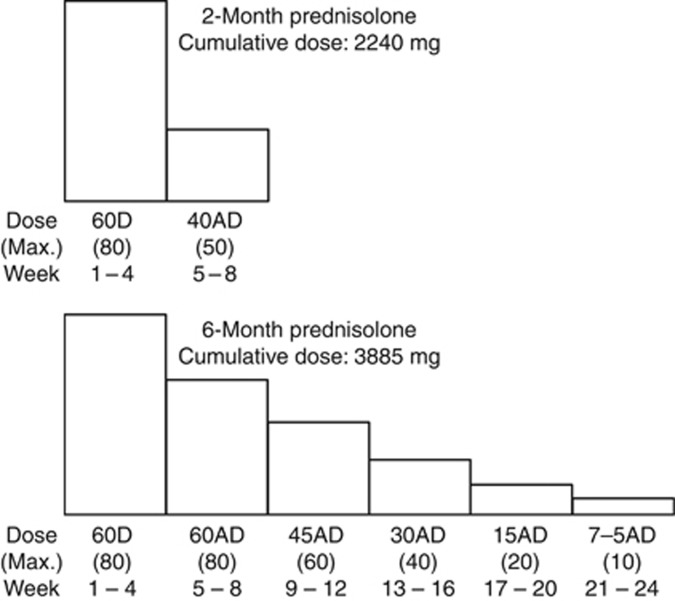
All patients diagnosed with a first episode of idiopathic NS started initial therapy of 60 mg/m2 oral prednisolone in three divided doses (maximum of 80 mg/day) daily for 4 weeks. Patients underwent a screening examination and were registered after their eligibility, including remission, was verified. Participants switched to trial medication after initial doses of prednisolone were given (Figure 4). If participants relapsed after remission during the initial 4-week prednisolone treatment, they were excluded.
Figure 4.
Initial treatment regimens. Upper doses are in mg/m2 per day. Maximum doses are in mg/day. D, daily; AD, alternate days.
Trial medication consisted of initial treatment regimens and relapse treatment, and was completed within a total of 24 months in both groups (Figures 4 and 5). The duration of long-term prednisolone treatment was set to 6 months, which is consistent with recommendations from a non-Japanese randomized controlled trial.8 The cumulative dose of initial treatment was 2240 mg/m2 (2-month group) and 3885 mg/m2 (6-month group). Participants who relapsed during the 24-month trial medication period received relapse treatment regimens (Figure 5). Relapse treatment was the same in both groups. However, relapse treatment given during the 6-month initial treatment was adjusted according to the initial treatment regimen in the 6-month group.
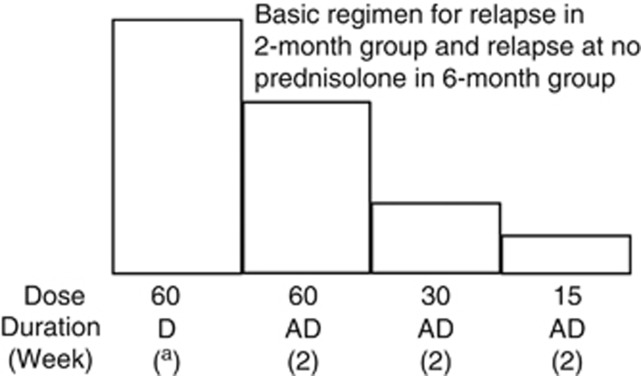
Figure 5.
Treatment regimens for relapse. aUntil urinary protein is negative on 3 consecutive days using a urine dipstick test. Upper doses are in mg/m2/day. A maximum dose of each is the same as initial treatments in Figure 4. AD, alternate days; D, daily.
Participant characteristics (age, sex, physical characteristics, blood, and urine test results) were recorded at baseline. Clinical assessment and urine tests (urinalysis, qualitative and quantitative proteinuria, quantitative creatinine) were performed at 1, 2, 4, and 6 months after enrollment and every 3 months thereafter, and also on diagnosis of relapse. Blood analysis (complete blood cell count, blood chemistry) was performed at 1, 2, 6, and 12 months after enrollment, at the end of treatment, and at diagnosis of relapse only.
Adverse events were recorded throughout the trial period and assessed using Common Terminology Criteria for Adverse Events v3.0. Within 4 weeks of starting initial treatment, participants were screened for glaucoma by an ophthalmologist. Details about monitoring adverse events are described in the Supplementary Information online. Briefly, name of diagnosis, severity, seriousness, date of onset and disappearance, outcome, and assessment of causal relationship to the study drug were investigated at the start of study treatment and at 1, 2, 4, 6, 9, 12, 15, 18, 21, and 24 months after the start of study treatment (each within ±2 weeks).
Outcomes
The primary end point was defined as the duration from start of initial treatment to diagnosis of FRNS, or ‘time to FRNS'. Diagnosis of FRNS was based on the relapse dates according to the ISKDC. In our study, FRNS was defined as two relapses within 6 months of initial remission (definition 1), or four relapses within any 12-month period (definition 2), which included relapses during initial tapering treatment but excluded relapses with spontaneous remissions. Patients were observed for at least 2 years, on the basis of the Cochrane review, which states that the risk of relapse after 1–2 years was lower after long-term prednisolone treatment relative to the ISKDC regimen.8 Relapse was defined as proteinuria 2+ or higher on dipstick analysis for 3 or more consecutive days or proteinuria 2+ or higher on dipstick analysis and serum albumin ⩽25 g/l. Immunosuppressant administration was prohibited in the protocol. However, if administration was undertaken for clinical necessity, e.g., steroid dependency, it was treated as an event in the primary analysis. Data for patients who did not experience these events were considered censored at the last examination. Important secondary end points were time to first relapse, the number of relapses per person-year, total prednisolone dose, and adverse events.
Statistical analyses
The primary objective of this trial was to confirm noninferiority of initial therapy with 2-month treatment compared with 6-month treatment, with respect to time to FRNS. The noninferiority margin of HR for the 2-month to 6-month group was predefined to 1.3, and the significance level was set to 5% (one-sided). The former was determined based on surveys conducted among practicing pediatric nephrologists and other specialists before the protocol was developed.
On the basis of results from a previous study,20 we assumed an event rate of 15 and 19% at 1 year in the 2- and 6-month groups, respectively. With a sample size of 125 patients in each treatment group, an HR test with a one-sided 5% significance level would have 70% power to confirm noninferiority. Accrual and follow-up times were specified to be 3 and 2 years, respectively.
As the previous study20 was conducted more than 10 years earlier, it is possible that the current event rate of our study is lower than the previous study. We scheduled an interim analysis to take place just before the accrual completion date, which was performed in October 2010. A statistical test regarding the primary end point was not performed. The number of events observed matched that of the assumption, and the study plan was not changed.
Statistical analyses followed the protocol and the intention-to-treat principle. The Cox proportional hazard model was used to test noninferiority and estimate the HR with a 90% CI of the primary end point. The Kaplan–Meier method was used to summarize time to FRNS. These methods and the log-rank test were used to analyze time to first relapse. The number of relapses per person-year was calculated as the total number of relapses divided by the total observed person-years in each treatment group (Table 2). A permutation test was used to compare the number of relapses per person-year between groups. We compared the prednisolone total dose using the Wilcoxon test. The number of adverse events was compared using Poisson regression. For baseline characteristics, we compared distributions of continuous variables between groups using the t-test or Wilcoxon test, depending on the shape of the distribution. We analyzed categorical variables using the chi-squared test or Fisher's exact test. Posterior probability was calculated with the improper flat prior and the normal distribution to which log-HR was approximated. Except for noninferiority testing of the primary end point, we regarded a two-sided P-value<0.05 to indicate statistical significance. We analyzed data using SAS software (version 9.3) and calculated the sample size using the SAS POWER procedure.
Acknowledgments
We thank all our patients, their families, and the site investigators. We thank Emma Barber for editing the article. We thank Drs Jonathan Craig, Patrick Niaudet, and Tohru Kobayashi for their helpful advice. The trial was supported by a grant from the Ministry of Health, Labour and Welfare, Japan (H19-shouni-002). The results of this trial were presented in abstract form at the annual meeting of the American Society of Nephrology, November 7–10, 2013, Atlanta, USA. This trial is registered at the University Hospital Medical Information Network clinical trial registry (UMIN-CTR) (http://www.umin.ac.jp/ctr/), registration number UMIN000000747. See the Supplementary Information online for complete Methods (Clinical Study Protocol).
Appendix
Japanese Study Group of Kidney Disease in Children (JSKDC). JSKDC has been supported by grants from the Ministry of Health, Labour and Welfare, Japan. Members of the JSKDC are as follows.
Steering Committee: Norishige Yoshikawa (chair), Kazumoto Iijima, Hidefumi Nakamura, Masataka Honda, and Mayumi Sako.
JSKDC study office: Koichi Nakanishi (chair) and Yuko Shima.
Data Coordinating Center: Yasuo Ohashi (chair).
Statistical Center: Mari S Oba (chair).
Data and Safety Monitoring Board: Takashi Igarashi, Yasuo Ohashi, Tetsuya Kawamura, and Michio Nagata.
Clinical Investigators (Institution): Satoshi Sasaki (Hokkaido University Hospital), Yutaka Yamada (Hakodate Central General Hospital), Tetsuro Nagashima (Kushiro Red Cross Hospital), Yoshinori Saita (Japanese Red Cross Kitami Hospital), Tetsuji Morimoto (Tohoku University Hospital), Yohei Ikezumi (Niigata University Medical & Dental Hospital), Toshio Yanagihara (Niigata Prefecture Yoshida Hospital), Noriko Oonishi (Fujita General Hospital), Tomoko Sato (Jusendo General Hospital), Kenji Nemoto (Shirakawa Kosei General Hospital), Katsutoshi Nagasawa (Takeda General Hospital), Masahiko Katayose (Public Soma General Hospital), Yoshiyuki Namai (Ohta General Nishinouchi Hospital), Shigeo Suzuki (Ohara General Hospital), Yasuaki Kobayashi (Japanese Red Cross Ashikaga Hospital), Yoko Owada (Dokkyo Medical University Hospital), Hideaki Kurayama (National Hospital Organization Chiba-East-Hospital), Shuichiro Fujinaga (Saitama Children's Medical Center), Midori Awazu (Keio University Hospital), Hirotaka Takahashi (Tokyo Metropolitan Ohtsuka Hospital), Shori Takahashi (Surugadai Nihon University Hospital), Motoshi Hattutori (Tokyo Women's Medical University Hospital), Shuichi Ito and Koichi Kamei (National Center for Child Health and Development), Masayasu Ohta (Toride Kyodo General Hospital), Tae Omori (Tokyo Metropolitan Bokutoh Hospital), Nobuyuki Kurosawa (Tsuchiura Kyodo General Hospital), Masuhiro Shimoda (Japanese Red Cross Musashino Hospital), Shiro Tsuchiya (Soka Municipal Hospital), Kenichiro Miura (The University of Tokyo Hospital), Atsushi Inatomi (Yaizu City Hospital), Yoshiyuki Otomo (Juntendo University Nerima Hospital), Tomonosuke Someya (Juntendo University Hospital), Shoichi Oyama (Saiseikai Kawaguchi General Hospital), Hiroshi Hataya and Kenji Ishikura (Tokyo Metropolitan Children's Medical Center), Takeshi Matsuyama (Fussa Hospital), Masahiro Banba (Yokosuka Kyosai Hospital), Kiyoshi Araki (Saitama Social Insurance Hospital), Hitoshi Wakaki (Yokohama City Hospital), Cho Hideo (Kawasaki Municipal Hospital), Tomonori Harada (Yokohama City University Medical Center), Tomoko Nakamura (Odawara Municipal Hospital), Shoko Goto (Saiseikai Yokohama City Nanbu Hospital), Fumio Niimura (Tokai University Hospital), Naohiro Wada (Shizuoka Children's Hospital), Masami Shirai (Iwata City Hospital), Kozo Muto (Shimada Municipal Hospital), Osamu Uemura (Aichi Children's Health and Medical Center), Yoshimitsu Goto (Japanese Red Cross Nagoya Daini Hospital), Naoya Fujita (Seirei Hamamatsu General Hospital), Kazuhide Ohta (National Hospital Organization Kanazawa Medical Center), Masaki Shimizu (Kanazawa University Hospital), Koichi Tsukahara (Fukui University Hospital), Yukiko Mori (Fukui Red Cross Hospital), Hiroshi Akutagawa (Hyogo Prefectural Tsukaguchi Hospital), Toshihiro Sawai (Shiga University of Medical Science Hospital), Kashiro Nishizawa (Omihachiman City Hospital), Akira Ashida (Osaka Medical College Hospital), Naohisa Kawamura (Osaka Rosai Hospital), Takuya Tanabe (Hirakata City Hospital), Koji Taira (Nara Prefectural Nara Hospital), Seiji Kinoshita (Higashi-Osaka City General Hospital), Shinya Tanaka (Hyogo Prefectural Nishinomiya Hospital), Nobuhiko Shimizu (Sakai City Hospital), Katsuhisa Yamamoto (Minoh City Hospital), Shinichi Sumimoto (Osaka Red Cross Hospital), Koichi Nakanishi (Wakayama Medical University Hospital), Noriyuki Aoyagi (Wakayama Rosai Hospital), Seiji Iwahashi (Hidaka General Hospital), Masakazu Miyawaki (Social Insurance Kinan Hospital), Ritsuko Miyashita (Izumiotsu Municipal Hospital), Masamitsu Nishino (Takatsuki General Hospital), Daisuke Hata (Kitano Hospital), Mikio Goto (Kishiwada City Hospital), Ryojiro Tanaka (Hyogo Children's Hospital), Kandai Nozu and Hiroshi Kaito (Kobe University Hospital), Sakiko Konohana (Ono Municipal Hospital), Ichiro Kamioka (Kakogawa City Hospital), Masayuki Yamane (Saiseikai Hyogoken Hospital), Katsuji Kuwakado (Kurashiki Central Hospital), Shoji Kagami (Tokushima University Hospital), Yuhei Ito (Kurume University Medical Center), Yoshihiko Murakami (Omuta City Hospital), Jiro Iwamoto (Iizuka Hospital), Yoshitsugu Kaku (Fukuoka Children's Hospital & Medical Center for Infectious Diseases), Kentaro Kamesaki (Kokuritsukokura Hospital), Ken Hatae (Japanese Red Cross Fukuoka Hospital), Hitoshi Nakazato (Kumamoto University Hospital), Yasushi Otsuka (Saga University Hospital), Tomohiro Ichimaru (Saga-ken Medical Centre Koseikan), and Tadashi Sato (National Hospital Organization Ureshino Medical Center).
This study was supported by a grant from the Ministry of Health, Labour and Welfare, Japan. All expenses were covered by the grant. NY has received grants from Novartis Pharma K.K. and Asahi Kasei Pharma Corporation and has also received lecture fees from Novartis Pharma K.K. and Asahi Kasei Pharma Corporation. KN has received lecture fees from Novartis Pharma K.K. and Asahi Kasei Pharma Corporation. KIs has received lecture fees from Novartis Pharma K.K. HH has received lecture fees from Asahi Kasei Pharma Corporation. MH has received lecture fees from Novartis Pharma K.K. and Asahi Kasei Pharma Corporation. SI has received lecture fees from Novartis Pharma and Asahi Kasei Pharma Corporation. YS has received lecture fees from Novartis Pharma K.K. HN owns stocks in Asahi Kasei Pharma Corporation. TI has received lecture fees from Takeda Pharmaceutical, K.K. KIi has received grants from Takeda Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corporation, and Novartis Pharma K.K., and lecture fees from Novartis Pharma K.K. and Asahi Kasei Pharma Corporation. No other disclosures were reported.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S2. (A) Meta-analysis of studies comparing 2–3 months of prednisolone to 5–6 months of prednisolone for children with their first episode of nephrotic syndrome, with an outcome showing the number of children with frequent relapses after 1–2 years. (B) Sensitivity analysis excluding Sharma 2002 (unpublished conference proceeding) from Fig. A.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Schlesinger ER, Sultz HA, Mosher WE, et al. The nephrotic syndrome: its incidence and implications for the community. Am J Dis Child. 1968;116:623–632. [PubMed] [Google Scholar]
- van Husen M, Kemper MJ. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol. 2011;26:881–892. doi: 10.1007/s00467-010-1717-5. [DOI] [PubMed] [Google Scholar]
- Tarshish P, Tobin JN, Bernstein J, et al. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8:769–776. doi: 10.1681/ASN.V85769. [DOI] [PubMed] [Google Scholar]
- Ueda N, Chihara M, Kawaguchi S, et al. Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. J Pediatr. 1988;112:122–126. doi: 10.1016/s0022-3476(88)80136-7. [DOI] [PubMed] [Google Scholar]
- Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr. 1993;152:357–361. doi: 10.1007/BF01956754. [DOI] [PubMed] [Google Scholar]
- Ksiazek J, Wyszyńska T. Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr. 1995;84:889–893. doi: 10.1111/j.1651-2227.1995.tb13787.x. [DOI] [PubMed] [Google Scholar]
- Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol. 1999;13:824–827. doi: 10.1007/s004670050708. [DOI] [PubMed] [Google Scholar]
- Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2010;4:CD001533. doi: 10.1002/14651858.CD001533.pub4. [DOI] [PubMed] [Google Scholar]
- Hodson EM, Alexander SI. Evaluation and management of steroid-sensitive nephrotic syndrome. Curr Opin Pediatr. 2008;20:145–150. doi: 10.1097/MOP.0b013e3282f4307a. [DOI] [PubMed] [Google Scholar]
- Hodson EM, Craig JC, Willis NS. Evidence-based management of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2005;20:1523–1530. doi: 10.1007/s00467-005-1968-8. [DOI] [PubMed] [Google Scholar]
- Hodson EM, Willis NS, Craig JC. Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev. 2008;23:CD002290. doi: 10.1002/14651858.CD002290.pub3. [DOI] [PubMed] [Google Scholar]
- MacHardy N, Miles PV, Massengill SF, et al. Management patterns of childhood-onset nephrotic syndrome. Pediatr Nephrol. 2009;24:2193–2201. doi: 10.1007/s00467-009-1282-y. [DOI] [PubMed] [Google Scholar]
- Teeninga N, Kist-van Holthe J, van Rijskwijk N, et al. Extending prednisolone therapy does not reduce relapse in childhood nephrotic syndrome. J Am Soc Nephrol. 2013;24:149–159. doi: 10.1681/ASN.2012070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson EM, Craig JC. Corticosteroid therapy for steroid-sensitive nephrotic syndrome in children: dose or duration. J Am Soc Nephrol. 2013;24:7–9. doi: 10.1681/ASN.2012111093. [DOI] [PubMed] [Google Scholar]
- Iijima K, Sako M, Oba MS, et al. Japanese Study Group of Kidney Disease in Children. Cyclosporine C2 monitoring for the treatment of frequently relapsing nephrotic syndrome in children: a multicenter randomized phase II trial. Clin J Am Soc Nephrol. 2014;9:271–278. doi: 10.2215/CJN.13071212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Saji T, Otani T, et al. RAISE study group investigators. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Iijima K, Ishikura K, et al. Two-year outcome of the ISKDC regimen and frequent-relapsing risk in children with idiopathic nephrotic syndrome. Clin J Am Soc Nephrol. 2013;8:756–762. doi: 10.2215/CJN.09010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra OP, Thakur N, Mishra RN, et al. Prolonged versus standard prednisolone therapy for initial episode of idiopathic nephrotic syndrome. J Nephrol. 2012;25:394–400. doi: 10.5301/jn.5000016. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Tsukahara H, Matsubara K, et al. A randomized study of two long-course prednisolone regimens for nephrotic syndrome in children. Am J Kidney Dis. 2003;4:1155–1162. doi: 10.1016/s0272-6386(03)00346-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H, Takehoshi Y, et al. Standard versus long-term prednisolone with Sairei-to in childhood steroid-responsive nephrotic syndrome: a prospective controlled study. Jpn J Nephrol. 1998;40:587–590. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.