Abstract
Background
Second-line chemotherapy for advanced non-small cell lung cancer (NSCLC) improves survival modestly but new strategies are needed. This trial was designed to evaluate an antivascular endothelial growth factor strategy with or without standard chemotherapy in previously treated NSCLC.
Methods
Patients with stage IIIB/IV NSCLC with performance status 0 to 1 progressive after first-line chemotherapy were eligible for randomization to pemetrexed, sunitinib, or the combination. Patients were stratified by performance status, stage, and sex. Primary objective was 18-week progression-free survival (PFS) rate; secondary objectives included response, overall survival (OS), and toxicity. Target accrual was 225. The study was terminated early because of decreasing accrual rates.
Results
Between April 2008 and September 2011, 130 patients were registered and randomized; of this, 125 patients were treated. Baseline characteristics in the three arms were well balanced. Toxicity was higher in the sunitinib-containing arms. The 18-week PFS rate in the pemetrexed, sunitinib, and combination arms was 54% (95% confidence interval [CI], 40–71), 37% (95% CI, 25–54), and 48% (95% CI, 35–66), respectively (p= 0.25). Median PFS in the pemetrexed, sunitinib, and combination arms in months was 4.9 (2.1–8.8), 3.3 (2.3–4.2), and 3.7 (2.5–5.8), respectively (p= 0.18). There was an overall statistically significant difference in OS between the three arms: median OS in months was 10.5 (8.3–20.2) for pemetrexed, 8.0 (6.8–13.5) for sunitinib, and 6.7 (4.1–10.1) for the combination (p= 0.03).
Conclusion
Pemetrexed had a superior toxicity profile to either sunitinib or the combination of pemetrexed and sunitinib. The 18-week PFS rate was not significantly different between the arms. OS was significantly better with pemetrexed alone compared with the two sunitinib-containing arms, with the doublet performing worst for OS.
Keywords: CALGB 30704, Lung cancer
Antiangiogenic therapy as a therapeutic target in non–small-cell lung cancer (NSCLC) was demonstrated as beneficial by Eastern Cooperative Oncology Group 4599, which showed that the addition of bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), to standard chemotherapy improved overall survival (OS) among advanced nonsquamous NSCLC patients treated in the first-line setting.1 Multiple other antiangiogenic drugs, including many small-molecule tyrosine kinase inhibitors of VEGF receptor (VEGFR), have been developed and are in clinical testing across a broad range of tumor types.2-4
We hypothesized that the addition of antiangiogenic therapy to standard chemotherapy in the second-line setting could lead to improved outcomes. We therefore designed this randomized phase 2 trial to treat patients on one of three arms: Arm I, pemetrexed alone; Arm II, sunitinib alone; or Arm III, the combination of pemetrexed and sunitinib, with a primary end point of improvement in 18-week progression-free survival (PFS).
Sunitinib is a small-molecule inhibitor of multiple receptor tyrosine kinases, including VEGFR, platelet-derived growth factor receptor (PDGFR), FGFR1, cKIT, FLT3, and RET kinases.5 Sunitinib as monotherapy in patients with advanced NSCLC has been tested in multiple studies.6-8 Among previously treated NSCLC patients, at 50 mg/day on a 4-week on and 2-week off schedule, overall response rate (RR) was 11.1% with median PFS of 12 weeks and median OS of 23.4 weeks.8 Subsequent studies in NSCLC showed that continuous daily dosing at 37.5 mg/day resulted in similar median PFS of 11.9 weeks and median OS of 37.1 weeks.7 On the basis of these phase 2 data, sunitinib was a rational choice to test in the second-line setting, either as monotherapy or combined with second-line chemotherapy. Combination of sunitinib, at either 37.5 mg/day continuous dosing or 50 mg/day on a 2-week on and 1-week off schedule, with pemetrexed at 500 mg/m2 on day 1 of every 21 days, has been found to be well tolerated in the phase 1 setting.9
PATIENTS AND METHODS
Patients
Patients aged 18 years or older with histologically or cytologically proven advanced NSCLC (stage IIIB or IV) with evidence of progression after first-line therapy were eligible. Prior bevacizumab was allowed. There were no restrictions regarding histologic subtype of NSCLC, and central review was not required. Patients were required to have Eastern Cooperative Oncology Group performance status (PS) of 0 or 1 and adequate hematologic, liver, and kidney function defined by laboratory testing. Treated, asymptomatic brain metastases were allowed. Exclusion criteria included: symptomatic congestive heart failure, active coronary artery disease defined as myocardial infarction or unstable angina in the past year, cerebrovascular accident or transient ischemic accident in the past year, uncontrolled hypertension, hemoptysis, cavitary pulmonary lesions, history of thromboembolism, or requirement for full-dose therapeutic anticoagulation.
Study Design and Treatment
This was a randomized, open-label phase 2 study with patients randomized in a 1:1:1 allocation to one of three arms: Arm I, pemetrexed alone at 500 mg/m2 on day 1; Arm II, sunitinib alone at 37.5 mg/day; and Arm III, pemetrexed 500 mg/m2 on day 1 with sunitinib 37.5 mg daily. One cycle was considered 21 days. Randomization was stratified by PS (0/1), stage (IIIB/IV), and sex (male/female) using a stratified permuted block-randomization scheme.
The primary objective of the study was to estimate the 18-week PFS survival rate in each of the three arms of the study. Secondary objectives included: RRs, PFS, OS, and toxicity in the three arms.
The study was planned to randomize a total 225 eligible patients. With 75 patients in each arm, the study has at least 80% power to detect a 18-week PFS rate of 56.3% for either experimental arm (Arm II or III) versus the expected 18-week PFS rate of 37.1% for the control arm (Arm I) with each comparison performed at the one-sided 0.10 significance level.
The study was activated on April 15, 2008, and closed on September 15, 2011, after 130 patients had been registered. Each participant signed an Institutional Review Board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines. The study was terminated early because of slow accrual and changing paradigms of therapy in metastatic NSCLC, specifically, the increased use of first-line or maintenance pemetrexed and the restriction of pemetrexed use to patients with nonsquamous histologies. The 130 registered patients were considered eligible and were randomized. Of them, five patients withdrew after randomization and did not receive protocol treatment. All 130 patients who were registered and randomized were included in the final intention-to-treat analysis conducted in June 2013. Of note, analyses were also done excluding the five patients who did not receive protocol treatment, and the results were similar to those reported here. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Assessments
Computed tomography scans of the chest and upper abdomen were obtained at baseline and at 6-week intervals. Radiographic review was done locally and Response Evaluation Criteria in Solid Tumors version 1.0 criteria were used to define response and progression. Specifically, progression was defined as at least a 20% increase in the sum of the longest diameter (LD) of target lesions, taking as references the smallest sum LD recorded since the treatment started, or the appearance of one or more new lesions. PFS was defined as the time from registration to disease progression or death from any cause, whichever came first. OS was defined as the time from registration to death of any cause. Adverse events were assessed and graded using National Cancer Institute Common Toxicity Criteria version 4.0. For additional safety monitoring, the initial cohort of 12 patients in the two experimental arms (Arms II and III) were followed for adverse events during the first cycle of therapy, with a stopping rule that if more than one-third of the initial patient cohort experienced a dose-limiting toxicity, then patient accrual would be stopped for the study team to review the cumulative safety data and make recommendations regarding changing the dose level or terminating the trial.
Statistical Analysis
The balance of baseline covariates between the treatment arms, including age, sex, race, histology, stage, PS, prior radiotherapy, prior surgery, and number of prior chemotherapy regimens (1, 2+), was tested using χ2 exact tests. The Kaplan–Meier product limit estimator10 was used to graphically describe OS and PFS. Median survival and 18-week survival rates by treatment arm, with 95% confidence intervals (CIs), were derived from the product limit estimates. Comparisons of PFS and OS were conducted using a log-rank test. The Cox proportional hazards model was used to estimate the hazard ratios and their 95% CIs of the experimental regimens relative to the control arm, with and without adjusting for baseline prognostic factors and their interactions with treatments. Backward selection was used for selecting significant baseline covariates and the interactions. The proportion of patients who responded (completely or partially) to each treatment regimen was estimated as well as their associated 95% CIs. Differences in RR (complete and partial response) were evaluated using the Cochran–Mantel–Haenszel test and using a multivariate logistic regression model. The type and grade of toxicity associated with each treatment regimen were summarized. The planned one-sided test on 18-week PFS between the arms was conducted and is reported in this article. However, because the outcome is contrary to the hypothesized direction, we also report the two-sided p value for this test, as two-sided p values are not dependent on the direction of hypothesized effects and are more appropriate for descriptive purposes. For all other statistical tests, two-sided p values were reported, and because of the small size of the final accruals, these p values are considered purely descriptive.
RESULTS
Patients and Treatment Exposure
Between April 2008 and September 2011, 130 patients were registered: 42 in Arm I (pemetrexed alone), 47 in Arm II (sunitinib alone), and 41 in Arm III (pemetrexed + sunitinib). Median follow-up time was 36 months. Five patients did not receive protocol treatment after randomization (Fig. 1). Table 1 shows the clinical and demographic characteristics of the 130 patients. Baseline characteristics in the three arms were well balanced (all p > 0.10). The median age was 63 years, with a range from 38 to 84. Fifty-three percent were male; 12% had stage IIIB disease and 88% stage IV. There were no significant differences between the treatment arms in age, sex, stage, PS, or number of prior chemotherapy regimens. Histologic diagnosis was predominantly adenocarcinoma (64% overall), with 13% squamous histology and no significant differences in histology distribution between the three arms.
FIGURE 1.
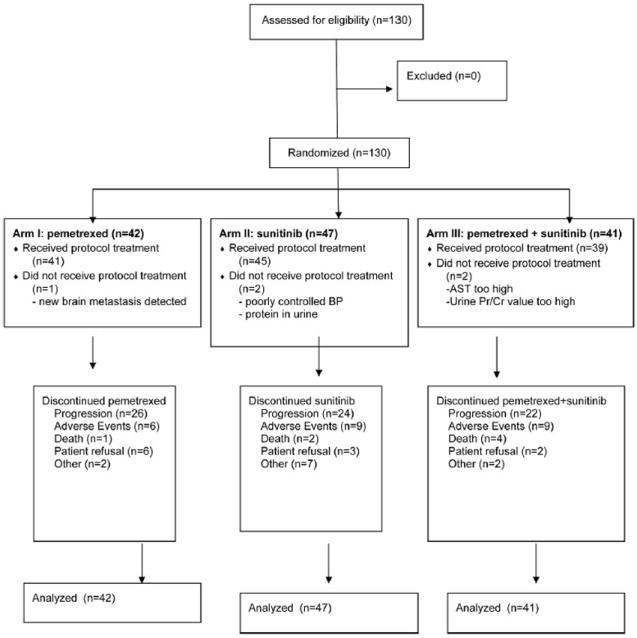
CONSORT diagram. AST, aspartate transaminase, BP, blood pressure.
TABLE 1.
Patient Characteristics
| Characteristic | Arm I n = 42 |
Arm II n = 47 |
Arm III n = 41 |
Total N = 130 |
|---|---|---|---|---|
| Age, median: 63; range (38–84) | ||||
| <60 | 16 (38%) | 15 (32%) | 15 (37%) | 46 (35%) |
| 60–70 | 15 (36%) | 24 (51%) | 13 (32%) | 52 (40%) |
| ≥70 | 11 (26%) | 8 (17%) | 13 (32%) | 32 (25%) |
| Sex | ||||
| Male | 22 (52%) | 25 (53%) | 22 (54%) | 69 (53%) |
| Female | 20 (48%) | 22 (47%) | 19 (46%) | 61 (47%) |
| Race | ||||
| White | 36 (86%) | 43 (91%) | 32 (78%) | 111 (85%) |
| Black | 5 (12%) | 3 (6%) | 8 (20%) | 16 (12%) |
| Asian | 0 (0%) | 1 (2%) | 1 (2%) | 2 (1%) |
| More than one race | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Ethnicity | ||||
| Non-Hispanic | 39 (93%) | 47 (100%) | 36 (88%) | 122 (94%) |
| Hispanic | 2 (5%) | 0 (0%) | 1 (2%) | 3 (2%) |
| Unknown | 1 (2%) | 0 (0%) | 4 (10%) | 5 (4%) |
| Histology | ||||
| Adenocarcinoma | 28 (67%) | 28 (60%) | 27 (66%) | 83 (64%) |
| Squamous cell | 4 (10%) | 7 (15%) | 6 (15%) | 17 (13%) |
| Large cell | 2 (5%) | 4 (9%) | 0 (0%) | 6 (5%) |
| Undifferentiated NSC | 3 (7%) | 6 (13%) | 1 (2%) | 10 (8%) |
| Other | 3 (7%) | 2 (4%) | 7 (17%) | 12 (9%) |
| Missing | 2 (5%) | 0 (0%) | 0 (0%) | 2 (2%) |
| Stage | ||||
| IIIB | 5 (12%) | 8 (17%) | 3 (7%) | 16 (12%) |
| IV | 37 (88%) | 39 (83%) | 38 (93%) | 114 (88%) |
| Prior surgery | ||||
| No | 24 (57%) | 34 (72%) | 30 (73%) | 88 (68%) |
| Yes | 16 (38%) | 12 (26%) | 11 (27%) | 39 (30%) |
| Missing | 2 (5%) | 1 (2%) | 0 (0%) | 3 (2%) |
| Prior XRT | ||||
| No | 22 (52%) | 26 (55%) | 21 (51%) | 69 (53%) |
| Yes | 17 (40%) | 21 (45%) | 20 (49%) | 58 (45%) |
| Missing | 3 (7%) | 0 (0%) | 0 (0%) | 3 (2%) |
| No. of prior chemo regimens | ||||
| 1 | 38 (90%) | 43 (91%) | 39 (95%) | 120 (92%) |
| 2+ | 1 (2%) | 4 (9%) | 0 (0%) | 5 (4%) |
| Missing | 3 (7%) | 0 (0%) | 2 (5%) | 5 (4%) |
| Performance status | ||||
| 0 | 13 (31%) | 17 (36%) | 14 (34%) | 44 (34%) |
| 1 | 29 (69%) | 30 (64%) | 27 (66%) | 86 (66%) |
NSC, non-small cell lung cancer; XRT, radiation.
Efficacy
Table 2 summarizes the best overall response. There were no complete responses. The rates of partial response and stable disease in the three arms were: pemetrexed (14% partial response [PR]/50% stable disease [SD]), sunitinib (17% PR/38% SD), pemetrexed + sunitinib (22% PR/51% SD). Despite a numerically higher PR rate in the combination arm, these differences were not statistically significant (p = 0.34).
TABLE 2.
Best Overall Response
| Best Response | Arm I n = 42 |
Arm II n = 47 |
Arm III n = 41 |
Total N = 130 |
|---|---|---|---|---|
| Partial response | 6 (14%) | 8 (17%) | 9 (22%) | 23 (18%) |
| Stable | 21 (50%) | 18 (38%) | 21 (51%) | 60 (46%) |
| Progression | 13 (31%) | 13 (28%) | 6 (15%) | 32 (25%) |
| Missing or unevaluable | 2 (5%) | 8 (17%) | 5 (12%) | 15 (12%) |
| Response rate (95% CI)a | 14 (5%–29%) | 17 (8%–31%) | 22 (11%–38%) | 18 (12%–25%) |
The 95% CI was exact confidence intervals.
The Cochran–Mantel–Haenszel test p value is 0.363 for testing response (CR + PR) homogeneity across three treatment arms.
CR, complete response; PR, partial response.
More importantly, these PR rates did not translate to advantages in either PFS or OS for the combination arm (Table 3). The 18-week PFS rate in the three arms was not significantly different (2-df Wald test, one-sided p = 0.88 and two-sided p = 0.25), with an 18-week PFS rate in the pemetrexed arm of 54% (95% CI, 40–71), sunitinib 37% (95% CI, 25–54), and pemetrexed + sunitinib 48% (95% CI, 35–66). Median PFS was 4.9 months (95% CI, 2.1–8.8) for pemetrexed alone, 3.3 months (95% CI, 2.3–4.2) for sunitinib alone, and 3.7 months (95% CI, 2.5–5.8) for pemetrexed + sunitinib (p = 0.18; Fig. 2A). The hazard ratio for sunitinib alone over pemetrexed alone is 1.4 (95% CI, 0.9–2.2) and that for pemetrexed + sunitinib over pemetrexed is 1.3 (95% CI, 0.9–2.1), estimated from a multivariate Cox model with adjustment for significant baseline covariates.
TABLE 3.
Eighteen-Week PFS and Median PFS and OS (95% CI) by Treatment Arm
| Arm I n = 42 |
Arm II n = 47 |
Arm III n = 41 |
Two-df Wald or Log-Rank Test | Total N = 130 |
|
|---|---|---|---|---|---|
| Eighteen-week PFS rate | 53.7 (40.4–71.3) | 37.0 (25.4–53.9) | 48.1 (34.9–66.3) | 0.252 | 45.9 (38.0–55.4) |
| Median PFS (mo) | 4.9 (2.1–8.8) | 3.3 (2.3–4.2) | 3.7 (2.5–5.8) | 0.191 | 3.6 (2.8–4.4) |
| Median OS (mo) | 10.5 (8.3–20.2) | 8.0 (6.8–13.5) | 6.7 (4.1–10.1) | 0.019 | 8.3 (7.3–10.4) |
PFS, progression-free survival; OS, overall survival; CI, confidence interval.
FIGURE 2.
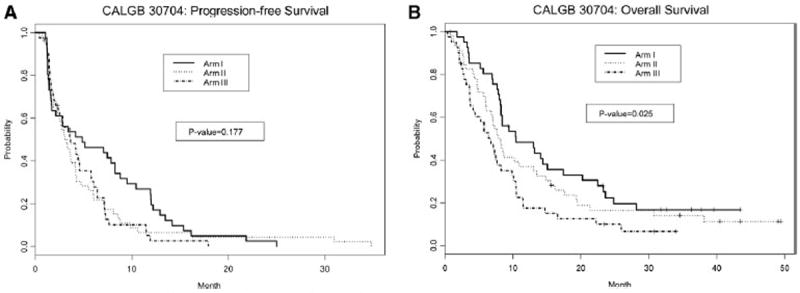
A, Progression-free survival. B, Overall survival.
OS was significantly better with pemetrexed alone. Median OS was 10.5 months (95% CI, 8.3–20.2) for pemetrexed alone, 8.0 months (95% CI, 6.8–13.5) for sunitinib alone, and 6.7 months (95% CI, 4.1–10.1) for pemetrexed + sunitinib (p = 0.03; Fig. 2B). The hazard ratio for sunitinib alone over pemetrexed alone is 1.4 (95% CI, 0.9–2.3) and that for pemetrexed + sunitinib over pemetrexed is 2.0 (95% CI, 1.2–3.2), estimated from a multivariate Cox model with adjustment for significant baseline covariates.
Analysis of the squamous subset is exploratory only as there were only 17 squamous patients on study (Table 4). There was no benefit in PFS or OS to the sunitinib or pemetrexed + sunitinib arms in either the squamous or nonsquamous subsets. The OS benefit seen with pemetrexed alone was seen only in the nonsquamous arm.
TABLE 4.
PFS and OS by Squamous and Nonsquamous Histology
| Median Survival (95% CI) (mo)
|
Two-df Log-Rank Test
|
|||
|---|---|---|---|---|
| Arm I (Pem) | Arm II (Sun) | Arm III (Pem + Sun) | p | |
| Squamous | N = 4 | N = 7 | N = 6 | |
| PFS | 2.02 (1.22–7.85) | 4.10 (1.35–5.52) | 1.77 (0.39–7.20) | 0.650 |
| OS | 9.28 (7.85–.) | 10.18 (6.80–15.54) | 4.70 (0.39–14.72) | 0.259 |
| Nonsquamous | N = 38 | N = 40 | N = 35 | |
| PFS | 5.13 (1.71–9.49) | 2.96 (1.68–4.17) | 4.17 (2.53–5.78) | 0.210 |
| OS | 13.04 (8.25–17.45) | 7.29 (4.83–13.47) | 7.13 (3.68–9.89) | 0.089 |
PFS, progression-free survival; OS, overall survival; CI, confidence interval.
Safety
Table 5 shows the rates of grade 3 to 5 toxicities in the three arms. Overall, toxicity was higher in the sunitinib-containing arms compared with pemetrexed alone. There was significantly more fatigue in the sunitinib-containing arms, as well as more gastrointestinal side effects. Cardiovascular events and thrombotic/hemorrhagic events also clustered more in the sunitinib-containing arms although the absolute numbers of these were small. Of the five grade 5 events, two (hemoptysis in the sunitinib arm and pneumonia in setting of neutropenia in pemetrexed + sunitinib arm) were thought to be treatment related, and one (pulmonary embolus in pemetrexed + sunitinib arm) was thought probably treatment related. The grade 5 hemoptysis death occurred in a patient with adenocarcinoma.
TABLE 5.
Treatment-Related ≥Grade 3 Adverse Events
| Arm I
|
Arm II
|
Arm III
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| Overall AEs | 9 (22%) | 3 (7%) | 0 | 24 (55%) | 3 (7%) | 2 (5%) | 18 (46%) | 10 (26%) | 3 (8%) |
| Hematologic AEs | 6 (15%) | 1 (2%) | 0 | 8 (18%) | 2 (5%) | 0 | 8 (21%) | 10 (26%) | 0 |
| Low hemoglobin | 1 (2%) | 0 | 0 | 3 (7%) | 0 | 0 | 3 (8%) | 0 | 0 |
| Low ANC | 2 (5%) | 0 | 0 | 2 (5%) | 1 (2%) | 0 | 3 (7%) | 8 (21%) | 0 |
| Low platelets | 1 (2%) | 1 (2%) | 0 | 2 (5%) | 1 (2%) | 0 | 6 (15%) | 2 (5%) | 0 |
| Febrile neutropenia | 0 (0%) | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 0 |
| Nonhematologic AEs | 5 (12%) | 3 (7%) | 0 | 23 (52%) | 1 (2%) | 2 (5%) | 20 (51%) | 1 (3%) | 3 (8%) |
| Constitutional | |||||||||
| Fatigue | 3 (7%) | 1 (2%) | 0 | 12 (27%) | 0 | 0 | 9 (23%) | 0 | 0 |
| Infection | 1 (2%) | 0 | 0 | 2 (4%) | 0 | 0 | 0 | 0 | 2 (6%) |
| Gastrointestinal | |||||||||
| Nausea | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 3 (8%) | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5%) | 0 | 0 |
| Mucositis | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 3 (8%) | 0 | 0 |
| Diarrhea | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elevated ALT | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 1 (3%) | 0 | 0 |
| Elevated AST | 1 (2%) | 0 | 0 | 2 (5%) | 0 | 0 | 0 | 0 | 0 |
| Dermatologic | |||||||||
| Rash (hand-foot) | 0 | 0 | 0 | 3 (7%) | 0 | 0 | 0 | 0 | 0 |
| Cardiovascular | |||||||||
| Cardiac ischemia | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 |
| Hypertension | 1 (2%) | 0 | 0 | 2 (5%) | 0 | 0 | 2 (5%) | 0 | 0 |
| Pulmonary hemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 |
| Other hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 0 |
| Thrombosis/embolism | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 1 (3%) |
ANC, absolute neutrophil count; ALT, alanine aminotransferase; AST, aspartate transaminase; AEs, adverse events.
Because there were only 17 squamous patients on study, a comparison of toxicity between squamous and nonsquamous is limited by small sample size. However there was no obvious signal for increased toxicity in the squamous patients.
DISCUSSION
In this randomized phase 2 study, we investigated the role of sunitinib in the second-line setting in NSCLC by treating patients with either pemetrexed alone, sunitinib alone, or the combination of pemetrexed and sunitinib. The primary end point, 18-week PFS rate, was not significantly different between the three arms. However, there was a trend toward improved PFS with pemetrexed alone, and OS was significantly better with pemetrexed alone. In addition, we found that pemetrexed had a superior safety profile to either sunitinib or the combination of pemetrexed and sunitinib.
This study was closed prematurely because of slowing accrual rates. The slow accrual was thought to be a result of global changes in management in NSCLC, specifically the increased adoption of maintenance chemotherapy as well as the increasing limitation of pemetrexed to nonsquamous histology only. Therefore, the study as completed is not adequately powered to address the primary end point of difference in 18-week PFS rate. Despite this, the study suggests that pemetrexed remains the best treatment option in this setting and the single-agent activity of sunitinib or the addition of sunitinib to pemetrexed yielded no benefit. This validates our approach of carefully exploring treatment options in a phase 2 setting before committing to phase 3 trials and reconfirms the very limited role of antiangiogenic drugs thus far in lung cancer.
It is not entirely clear why the OS was superior in the pemetrexed alone arm. Of note, there were significantly fewer patients in the pemetrexed plus sunitinib arm who subsequently went on to receive further lines of therapy (54% in pemetrexed alone arm received subsequent chemotherapies, 63% in sunitinib arm, and 22% in pemetrexed plus sunitinib arm). Within each treatment arm, patients who received subsequent therapies had significantly longer survival than those without. Whether the relative lack of subsequent therapies in the combination arm is simply a reflection of the worse outcomes seen in that arm, or possibly contributed to that worse survival, is unknown. Although similar percentages of patients in the sunitinib only and pemetrexed only arms went on to receive further therapies, the median PFS in the sunitinib arm is the shortest of the three treatment regimens and it is possible that the limited activity of sunitinib in the second-line setting contributed to the shorter OS in that arm relative to the pemetrexed only arm.
Other trials combining VEGFR tyrosine kinase inhibitors with chemotherapy have yet to show a benefit to the combination. Many of these studies demonstrate higher rates of adverse events with combination therapy. A phase 3 study in the first-line setting of carboplatin and paclitaxel with or without sorafenib was terminated early when interim analysis concluded that the study was highly unlikely to meet its primary end point of OS benefit. Of note, patients with squamous histology had greater mortality in the combination arm.11 BR24, a large randomized phase 2/3 study in first-line NSCLC of carboplatin and paclitaxel with cediranib or placebo, was halted after the phase 2 portion as rates of serious adverse events were significantly higher in the cediranib arm.12 However, because of signals of potential clinical benefit, with a statistically significant higher RR and trend toward better PFS in the cediranib arm, plans to proceed at a 20 mg dose of cediranib in the phase 3 setting are underway. In the previously treated setting, the combination of sunitinib with erlotinib did not improve OS over erlotinib alone, despite statistically significant improvements in RR and PFS.13 Again, treatment-related adverse events were higher with the combination.
Similar to these studies, we found a higher rate of adverse events in the sunitinib-containing arms. Although we did not have a large proportion of squamous patients on the current study, there was no signal that the squamous patients in particular did any worse in terms of toxicity than the non-squamous patients. Whether the relatively less toxicity seen with pemetrexed alone was another factor in the improved OS is also a possibility. Overall grade 3 to 4 toxicity was higher in this study in the sunitinib-containing arms as compared with the pemetrexed alone arm. Differences in these side effects can be quite clinically significant for patients, particularly as significant fatigue or gastrointestinal side effects may impact patients’ PS, ability to tolerate current therapies, and fitness and tolerance for future therapies.
Whether a subset of patients exists who would derive benefit from sunitinib remains an open question. As a multitargeted agent, sunitinib inhibits multiple kinases other than the VEGFR and is not solely an antiangiogenic agent. In particular, the inhibitory activity against RET and PDGFR are of interest. Recently, RET kinase fusions have been identified as a novel potential oncogenic driver in lung cancer.14-17 The most common fusion partner in lung cancer is KIF5B although CCDC6-RET fusions have been reported as well. The fusion gene causes overexpression of the RET receptor tyrosine kinase and occurs in approximately 1% to 2% of lung adenocarcinomas. Cell lines engineered to harbor the RET fusion have been shown to be transforming, and sensitive to several multitargeted kinase inhibitors that inhibit RET, including sunitinib, sorafenib, and vandetanib. Another potential biomarker for sunitinib response may be PDGFRA amplification. High throughput cell line screening revealed that two of 637 cell lines had significant sensitivity to single-agent sunitinib.18 Both of these cell lines (one NSCLC and one rhabdomyosarcoma) showed PDGFRA activation with high levels of expression of phosphorylated PDGFRA, with focal PDGFRA gene amplification seen in the NSCLC cell line.
Although these data from cell lines for RET fusion and PDGFRA amplification are suggestive, there are as yet no clinical data that such molecular changes in patients confer sensitivity to sunitinib. This trial did not mandate tumor collection from patients and the small sample size would make the likelihood of finding any specific genetic alteration quite low. However, future studies investigating this question would be of interest to determine whether specific molecularly defined subsets of patients may benefit from sunitinib.
This study has several limitations that should temper any interpretation of these results. We were able to accrue only a little over half the goal accrual for this study. The limited sample size severely limits our ability to draw definitive conclusions from this data. Variation between the arms may be a function of small numbers. Ultimately, however, this study does not support the use of sunitinib in the second-line setting in NSCLC.
Acknowledgments
The research for CALGB 30704 (Alliance) was supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, MD, Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, PhD, CA33601). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Baptist Cancer Institute CCOP, TN, Lee S. Schwartzberg, MD, supported by CA71323; Christiana Care Health Services, Inc., CCOP, Wilmington, DE, Stephen Grubbs, MD, supported by CA45418; Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD, supported by CA32291; Greenville CCOP, Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, MD, supported by CA29165; Heartland Cancer Research CCOP, St. Louis, MO, Alan P. Lyss, MD, supported by CA114558 (Missouri Baptist); Massachusetts General Hospital, Boston, MA, Jeffrey W. Clark, MD, supported by CA32291; Georgetown University Medical Center, Washington, DC, Bruce Cheson, MD, supported by CA77597; Missouri Baptist Medical Center, St. Louis, MO, Alan P. Lyss, MD, supported by CA114558-02; Missouri Valley Consortium CCOP, Omaha, NE, Gamini S. Soori, MD; Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, MD, supported by CA45564; University of California at San Diego, San Diego, CA, Barbara A. Parker, MD, supported by CA11789; New Hampshire Oncology-Hematology PA, Concord, NH, Douglas J. Weckstein, MD; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN, Rafat Ansari, MD, supported by CA86726; Southeast Cancer Control Consortium Inc., CCOP, Goldsboro, NC, James N. Atkins, MD, supported by CA45808; The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD, supported by CA77658; State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD, supported by CA21060; University of Chicago, Chicago, IL, Hedy L. Kindler, MD, supported by CA41287; University of Iowa, Iowa City, IA, Daniel A. Vaena, MD, supported by CA47642; University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Karl E. Freter, MD, supported by CA12046; University of Nebraska Medical Center, Omaha, NE, Apar Ganti, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD, supported by CA47559; University of Vermont, Burlington, VT, Steven M. Grunberg, MD, supported by CA77406; Kinston Medical Specialists, P.A., Kinston, North Carolina; supported by CA047559; Dana-Farber Cancer Institute, Boston, Massachusetts; supported by CA032291.
Footnotes
Presented in part at the 48th Annual Meeting of the Annual Society of Clinical Oncology, June 1–5, 2012, Chicago, IL.
Disclosure: The authors declare no conflict of interest.
References
- 1.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti G, Govindan R. Targeting angiogenesis with multitargeted tyrosine kinase inhibitors in the treatment of non-small cell lung cancer. Oncologist. 2010;15:436–446. doi: 10.1634/theoncologist.2009-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulahannan SV, Brahmer JR. Antiangiogenic agents in combination with chemotherapy in patients with advanced non-small cell lung cancer. Cancer Invest. 2011;29:325–337. doi: 10.3109/07357907.2011.554476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vokes EE, Salgia R, Karrison TG. Evidence-based role of bevacizumab in non-small cell lung cancer. Ann Oncol. 2013;24:6–9. doi: 10.1093/annonc/mds608. [DOI] [PubMed] [Google Scholar]
- 5.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 6.Novello S, Scagliotti GV, Rosell R, et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer. 2009;101:1543–1548. doi: 10.1038/sj.bjc.6605346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novello S, Camps C, Grossi F, et al. Phase II study of sunitinib in patients with non-small cell lung cancer and irradiated brain metastases. J Thorac Oncol. 2011;6:1260–1266. doi: 10.1097/JTO.0b013e318219a973. [DOI] [PubMed] [Google Scholar]
- 8.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa K, Okamoto I, Shimizu T, et al. Phase I study of sunitinib in combination with pemetrexed in patients with advanced solid tumors. J Clin Oncol. 2009;27:e14630. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 12.Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 13.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol. 2012;30:2070–2078. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 14.Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 17.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott U, Ames RY, Iafrate AJ, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69:3937–3946. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]


