Abstract
Introduction
Dysphagia is a common, dose-limiting toxicity of combined chemoradiotherapy (CT/RT) in patients with locally advanced non-small cell lung cancer (NSCLC). This study assessed the efficacy and safety of palifermin in reducing dysphagia from CT/RT followed by consolidation chemotherapy (CT).
Methods
This randomized, double-blind, phase II trial enrolled adults with unresectable stage III NSCLC. Subjects received weekly paclitaxel (50 mg/m2) and carboplatin (AUC 2.0) with concurrent daily radiation (RT) of 6000 to 6600 cGy, followed by consolidation CT. Palifermin (n = 49) or placebo (n = 46) was administered before starting concurrent CT/RT and once weekly for 6 weeks. The primary end points were the incidence of grade ≥2 dysphagia and safety.
Results
The incidence of grade ≥2 and ≥3 dysphagia was numerically lower in palifermin subjects versus placebo subjects (61% versus 70%; p = 0.36; 22% versus 28%, p = 0.50, respectively). Mean duration of dysphagia (grade ≥2) was 25 days for palifermin subjects and 32 days for placebo subjects (p = 0.32). The incidence of adverse events was similar in the two treatment groups, and median overall survival and progression-free survival were not adversely affected by palifermin treatment (overall survival: 513 versus 319 days; progression-free survival: 262 versus 235 days for palifermin versus placebo arms, respectively). The palifermin arm received more doses of CT per study design and significantly more patients received RT doses ≥6000 cGy (84% versus 61%, p = 0.01).
Conclusions
The results of this exploratory trial suggest that additional larger studies may be warranted to further evaluate the effect of palifermin on dysphagia, exposure to CT/RT, and long-term survival.
Keywords: Non-small cell, Lung, Dysphagia
Concurrent chemoradiotherapy (CT/RT) is a standard treatment for locally advanced and unresectable nonsmall cell lung cancer (NSCLC), as studies reported that, compared with use of sequential therapy or radiotherapy (RT) only, use of concurrent CT/RT was associated with longer survival.1-3 Concurrent therapy, however, increases the risk and severity of esophagitis and pneumonitis as well as the rate of other treatment-induced toxicities.1,4-9
Esophagitis is a common adverse event (AE) in CT/RT treatment of stage III NSCLC, with dysphagia as the primary clinical symptom.10-12 Esophagitis may be severe and disabling and may result in pain, weight loss, hospitalization, and the need for a gastrostomy or jejunostomy tube for enteral feeding.13 In some patients, RT and chemotherapy (CT) interruptions that may have an adverse impact on tumor control and survival are necessary to allow for healing of the esophageal lining.13 Despite research into the potential of the cyto- and radioprotective agent amifostine,14 there is no effective treatment or preventive measure for RT-induced esophagitis/dysphagia.
Keratinocyte growth factor (KGF) is an endogenous protein in the fibroblast growth factor family that binds to the KGF receptor. Binding of KGF to its receptor has been reported to result in proliferation, differentiation, and migration of epithelial cells.15,16 Palifermin (Kepivance, Thousand Oaks, CA) is the recombinant human form of KGF and has been found to markedly reduce chemotherapy- and radiation-induced injury to the mucosal lining of the oral cavity and the lower gastrointestinal tract in a variety of animal models of CT, RT, and blood stem cell transplantation.17-19 Palifermin has an indication to decrease the incidence and duration of severe oral mucositis in patients with hematologic malignancies receiving myelotoxic therapy requiring hematopoietic stem cell support.20
This phase II, randomized, double-blind, placebo-controlled study evaluated the efficacy and safety of weekly palifermin 180 μg/kg in reducing the incidence and duration of dysphagia induced by CT/RT in patients with unresectable stage III NSCLC.
PATIENTS AND METHODS
Study Design
In this multicenter, double-blind study, subjects were screened for eligibility up to 6 weeks before the start of the study and randomly assigned using an interactive voice response system in a 1:1 ratio to palifermin or placebo. Subjects were stratified by disease stage (stage IIIa versus IIIb), Eastern Cooperative Oncology Group (ECOG) performance status (0–1 versus 2), and estimated weight loss in the 3 months before study randomization (<5% versus 5–10%). All subjects received weekly paclitaxel (50 mg/m2) and carboplatin (AUC 2.0), concurrent RT to a target of 6000 to 6600 cGy given as 200 cGy once daily for 30 to 33 fractions, followed by two cycles of consolidation CT of paclitaxel (225 mg/m2) and carboplatin (AUC 6.0). Lyophilized palifermin or placebo was supplied in 6.25-mg single-dose vials for reconstitution with 1.2 ml sterile water. Subjects received placebo or palifermin 180 μg/kg intravenously 3 days before initiation of concurrent CT/RT and then once weekly during weeks 1 through 6, for a total of seven doses (Figure 1).
FIGURE 1.
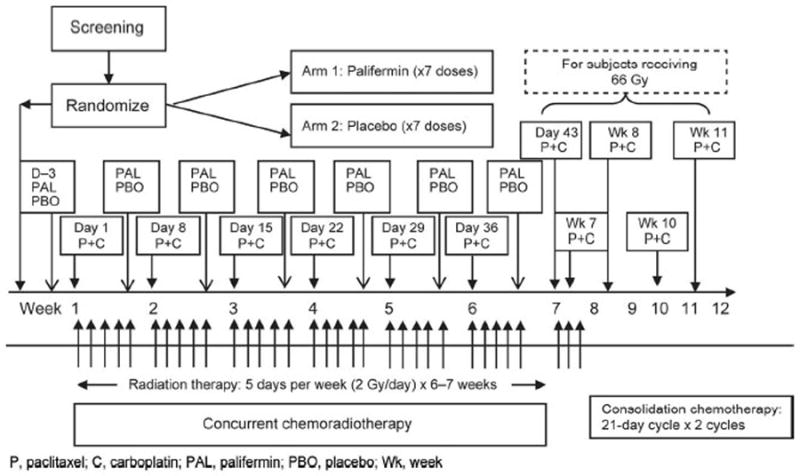
Study design and treatment schedule. P, paclitaxel; C, carboplatin; PAL, palifermin; PBO, placebo; Wk, week.
The primary end point was incidence of grade ≥2 dysphagia measured using the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0) dysphagia scale. Secondary end points included duration (days) of grade ≥2 dysphagia, incidence and duration of severe (grade ≥3) dysphagia, maximum severity of dysphagia, time to onset of grade ≥2 dysphagia, time to onset of severe dysphagia, change in ECOG performance status, and incidence of unplanned breaks or discontinuations of RT. Safety end points included incidence of chronic dysphagia (unresolved grade ≥2 dysphagia) at month 6, tumor response rate, progression-free survival (PFS) and overall survival (OS), laboratory abnormalities, and serum antipalifermin antibody formation.
Acute dysphagia was assessed twice weekly using CTCAE v3.0 during weeks 1 through 12 and once weekly after week 12 until dysphagia resolved to grade ≤1, up to week 16. Tumor response was evaluated by computed tomography scan or magnetic resonance imaging of the chest and abdomen at the end of week 12, as determined by the investigator. Positron emission tomography was permitted for assessment of disease status. All subjects were followed for disease progression, second primary tumors, other malignancies, and OS until death or loss to follow-up.
Subjects
Subjects were recruited from 25 centers across the United States and Europe. All subjects gave written informed consent, and the study was approved by Institutional Review Boards at participating centers. Eligible subjects were at least 18 years old, had unresectable stage IIIa or IIIb NSCLC with a life expectancy of at least 6 months, an ECOG status of 0 to 2, and an estimated weight loss of ≤10% in the previous 3 months. Patients were ineligible if they had stage IV disease; pleural or pericardial effusion estimated to be greater than 100 ml; prior CT, RT, or surgery for NSCLC; planned surgery to remove the tumor before completing the CT/RT course; or prior invasive malignancy in the past 3 years, except nonmelanomatous skin cancer.
Statistical Analysis
The primary objective of this study was to obtain preliminary data on whether palifermin had activity in this setting and indicate whether a larger phase 3 trial was warranted. As this was an exploratory analysis, the sample size was not powered to achieve statistical significance.
Efficacy analyses included all subjects who were randomized (intent-to-treat population), and data were analyzed based on the treatment group as per randomization. Missing data after discontinuation were imputed for the incidence and duration of dysphagia. Subjects with no dysphagia evaluations were assigned a grade ≥2 dysphagia. Duration of dysphagia was calculated in days from onset (first occurrence of grade ≥2) to resolution (grade ≤1). Safety analyses included all subjects who received at least one dose of study drug, and data were analyzed based on treatment actually received. Following advisement from one Institutional Review Board, four randomized subjects (two each in the placebo and palifermin group) from one site were excluded from the study, and all data were reanalyzed accordingly.
Summary statistics were provided by treatment group. For continuous variables, the mean, SD, median, and range were calculated. For categorical variables, the frequency and percentage were computed. The generalized Cochran-Mantel-Haenszel (CMH) method for general association, adjusted for the randomization stratification factors, was performed to compare the incidence- and duration-type end points between the palifermin and placebo groups. The time-to-onset end points were analyzed using the Kaplan-Meier method and log-rank test stratified by randomization factors to compare the treatment groups. The time to onset of dysphagia was calculated in days relative to the date of randomization. The OS and PFS time were calculated in days relative to the day when a subject received first dose of the study drug.
AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA). Long-term safety data of OS and PFS were analyzed using the Kaplan-Meier method.
Post hoc analyses were undertaken to gain insight into subjects who received the full RT dose (≥6000 cGy) in each treatment group. The CMH method for general association, adjusted for randomization stratification factors, was used to compare the proportion of subjects who received full dose of RT between the treatment groups.
RESULTS
Of 95 subjects enrolled, 49 were randomized to palifermin treatment and 46 to placebo treatment and were included in the intent-to-treat population (Figure 2). A total of 40 subjects (82%) in the palifermin group and 28 (61%) in the placebo group completed the study. The most common reasons for study discontinuation were withdrawal of consent (palifermin: three subjects, 6%; placebo: five subjects, 11%) and AEs (palifermin: two subjects, 4%; placebo: five subjects, 11%). The demographic and other baseline characteristics were similar in the palifermin and placebo groups (Table 1).
FIGURE 2.
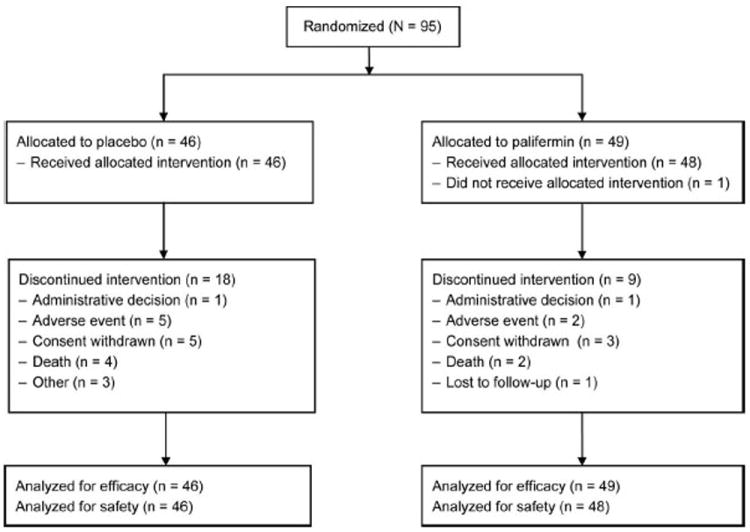
CONSORT diagram.
TABLE 1.
Subject Characteristics and Disease State
| Placebo (N = 46) | Palifermin (N = 49) | |
|---|---|---|
| Gender, male | 32 (70) | 34 (69) |
| Race/ethnicity | ||
| White | 43 (93) | 43 (88) |
| Black | 2 (4) | 2 (4) |
| Other | 1 (2) | 4 (8) |
| Age, yr, mean (SD) | 64.2 (7.7) | 61.6 (9.8) |
| AJCC stage | ||
| IIIa | 14 (30) | 19 (39) |
| IIIb | 32 (70) | 30 (61) |
| T stagea | ||
| T1 | 4 (9) | 3 (6) |
| T2 | 18 (39) | 18 (37) |
| T3 | 9 (20) | 10 (20) |
| T4 | 15 (33) | 17 (35) |
| N stage | ||
| N0/N1 | 9 (20) | 8 (16) |
| N2 | 19 (41) | 27 (55) |
| N3 | 18 (39) | 14 (29) |
| ECOG PS | ||
| 0–1 | 44 (96) | 47 (96) |
| 2 | 2 (4) | 2 (4) |
| Weight loss over last 3 mo | ||
| <5% | 35 (76) | 37 (76) |
| 5–10% | 11 (24) | 12 (24) |
| Current tobacco use | 16 (34) | 14 (29) |
| Current alcohol use | 14 (30) | 13 (27) |
Unless otherwise indicated, values are given as n (%).
One subject with nodal disease, unknown T stage.
AJCC, American Joint Committee on Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status.
The incidence of grade ≥2 dysphagia was numerically lower in the palifermin group (30 subjects, 61%) than in the placebo group (32 subjects, 70%, p = 0.36) (Figure 3). Lower values were also reported in the palifermin group than in the placebo group for the incidence of grade ≥3 dysphagia (11 subjects [22%] versus 13 subjects [28%]; p = 0.50) (Figure 3), the mean number of days of grade ≥2 dysphagia (25.3 versus 32.4 days; p = 0.32), and the incidence of unplanned RT breaks (9 subjects [18%] versus 15 subjects [33%]; p = 0.11). The overall distribution of fewer days of grade ≥2 dysphagia for subjects receiving palifermin is shown in Figure 4. Median time to onset of grade ≥2 dysphagia was 45 days in the palifermin group and 31 days in the placebo group (p = 0.21). Grade 4 dysphagia was reported for one subject in the palifermin group; this event did not result in drug or study discontinuation.
FIGURE 3.
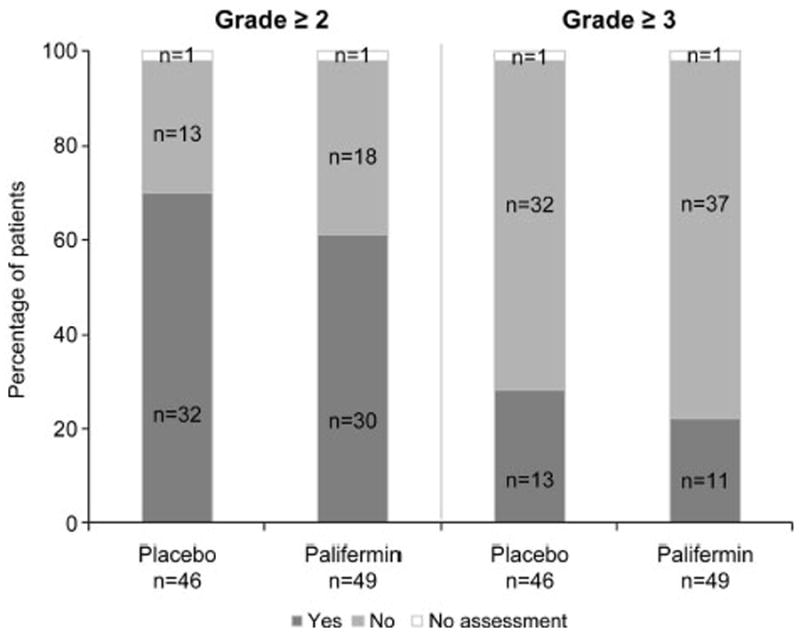
Incidence of grade ≥2 and ≥3 dysphagia.
FIGURE 4.
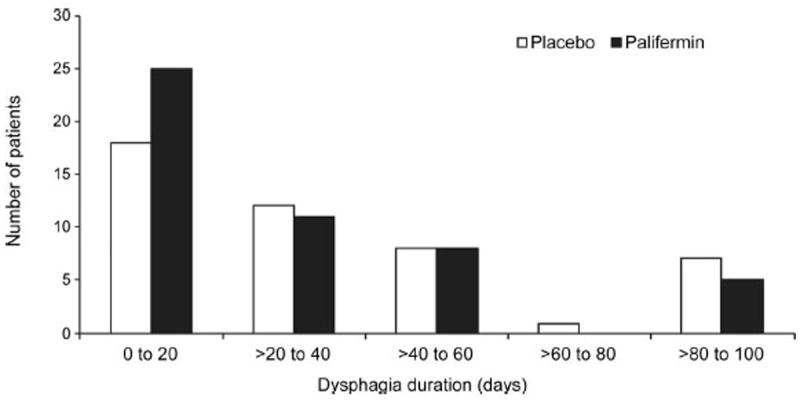
Distribution of mean duration of grade ≥2 dysphagia by treatment group.
Performance status of subjects deteriorated more in the placebo group than in the palifermin group, with a maximal mean (SD) ECOG score increase through week 12 of 0.9 (1.1) in the palifermin group and 1.5 (1.3) in the placebo group (p = 0.06).
Overall tumor response rate was numerically better for subjects in the palifermin group; 33 of 48 subjects (69%) in the palifermin group and 22 of 46 subjects (48%) in the placebo group had a complete or partial response.
Subjects in the palifermin group received more doses of study drug (palifermin) than subjects in the placebo group: 42 subjects (86%) in the palifermin group and 30 (65%) in the placebo group received seven or eight doses. This was also reflected in the mean (SD) number of doses received in the palifermin group, with 6.7 (1.2) doses received by the palifermin group and 5.8 (2.0) doses received in the placebo group. Mean (SD) relative dose intensity (RDI) showed similar exposure differences: 95% (17%) for the palifermin group and 83% (29%) for the placebo group.
Subjects in the palifermin group had greater exposure to RT than subjects in the placebo group. The mean (SD) total dose of RT for subjects who received palifermin was 5830 (836) cGy and 5220 (1611) cGy for the placebo group. The number of subjects receiving a cumulative RT dose ≥6000 cGy was 41 (84%) in the palifermin group and 28 (61%) in the placebo group (p = 0.01). These differences in dose were also reflected in the mean (SD) RDI for RT, which was 92% (14%) for the palifermin group and 82% (25%) for the placebo group. The distribution of cumulative RT dose received by treatment group is shown in Figure 5.
FIGURE 5.
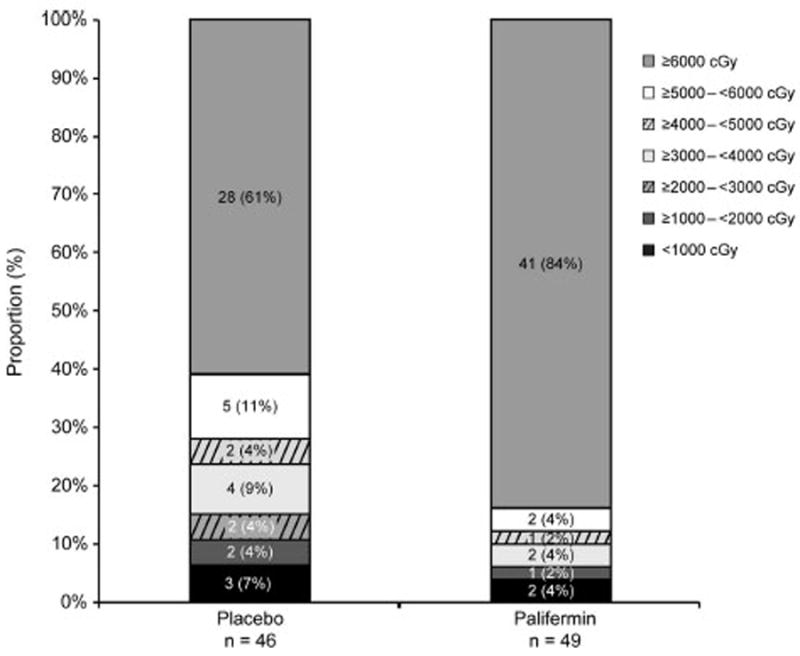
Distribution of cumulative radiation dose received.
When analyzed by RT dose received, the incidence of grade ≥2 dysphagia was numerically lower in the palifermin group (27/41 subjects, 66%) than in the placebo group (22/28 subjects, 79%) in subjects receiving a cumulative RT dose ≥6000 cGy (p = 0.29, Fisher’s exact test). Similar findings were observed in the incidence of grade ≥3 dysphagia for subjects receiving an RT dose ≥6000 cGy in the palifermin (8/41, 20%) and placebo (10/28, 36%) groups (p = 0.17, Fisher’s exact test).
Subjects in the palifermin group also received more doses of paclitaxel and carboplatin than subjects in the placebo group: 35 subjects (71%) in the palifermin group and 28 (61%) in the placebo group received six or more doses. The mean (SD) number of total doses was 5.8 (1.0) for the palifermin group and 5.1 (1.7) for the placebo group. The mean (SD) RDI for carboplatin was 93% (22%) for the palifermin group and 74% (27%) for the placebo group, and for paclitaxel it was 90% (15%) for the palifermin group and 81% (26%) for the placebo group. Consolidation CT with paclitaxel and carboplatin occurred during weeks 7 to 11. The exposure to this CT regimen was similar for the placebo and palifermin groups.
Adverse Events
One subject in the palifermin group did not receive study drug and was therefore not included in the safety analysis. Of the 94 subjects in the safety subset, AEs were experienced by all subjects except one in the placebo group. Serious AEs were reported for 51 subjects (54%) in the safety subset: 21 subjects (44%) in the palifermin group and 30 (65%) in the placebo group. Fatal AEs were reported for 7 subjects (7%), 2 of the 48 subjects in the palifermin group (4%) and 5 of the 46 subjects (11%) in the placebo group; none of these AEs was considered by the investigator to be related to palifermin treatment. Fatal AEs included pneumonia (two subjects, placebo), disease progression (one subject, palifermin), sudden death (one subject, placebo), hypoxia (one subject, palifermin), neoplasm progression (one subject, placebo), and circulatory collapse (one subject, placebo).
Treatment-related AEs were reported for 20 subjects: 13 of 48 subjects (27%) in the palifermin group and 6 of 46 (13%) in the placebo group (Table 2). Treatment-related serious AEs were reported for two subjects (4%) in the palifermin group and four subjects (9%) in the placebo group. All of these events occurred in one subject each; none of these were fatal.
TABLE 2.
Summary of Adverse Events
| Placebo (N = 46) | Palifermin (N = 48) | |
|---|---|---|
| Adverse events (AEs) | 45 (98) | 48 (100) |
| Serious AEs | 30 (65) | 21 (44) |
| Treatment-related AEs | 6 (13) | 13 (27) |
| Serious AEs | 4 (9) | 2 (4) |
| Most frequent (≥5%) treatment-related AEs | ||
| Rash | 3 (7) | 2 (4) |
| Diarrhea | 0 (0) | 3 (6) |
| Erythema | 0 (0) | 3 (6) |
| Flushing | 0 (0) | 3 (6) |
| Incidence of second primary tumors | 0 (0) | 0 (0) |
| Unresolved grade ≥2 dysphagia at month 6 | 2 (4) | 0 (0) |
| On-study death | 5 (11) | 2 (4) |
Values are given as n (%).
The incidence of clinical laboratory values that were considered AEs was similar between the treatment groups. No subject had a positive result for antipalifermin neutralizing antibodies during this study.
In the long-term safety evaluation at month 6, subjects in the placebo group had a numerically higher incidence of tumor progression or recurrence than subjects in the palifermin group: 9 of 48 subjects (19%) in the palifermin group and 12 of 46 subjects (26%) in the placebo group. OS and PFS were not adversely affected by palifermin treatment, with median time to death of 319 days for the placebo group and 513 days for the palifermin group (p = 0.42; hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.44 –1.50) (Figure 6A). Median time to disease progression or death was 262 days for the palifermin group and 235 days for the placebo group (p = 0.20; HR 0.74, CI 0.43–1.28) (Figure 6B). An ad-hoc analysis adjusted for tumor stages (N stage and AJCC stage) resulted similar hazard ratios (0.81 for OS and 0.74 for PFS).
FIGURE 6.
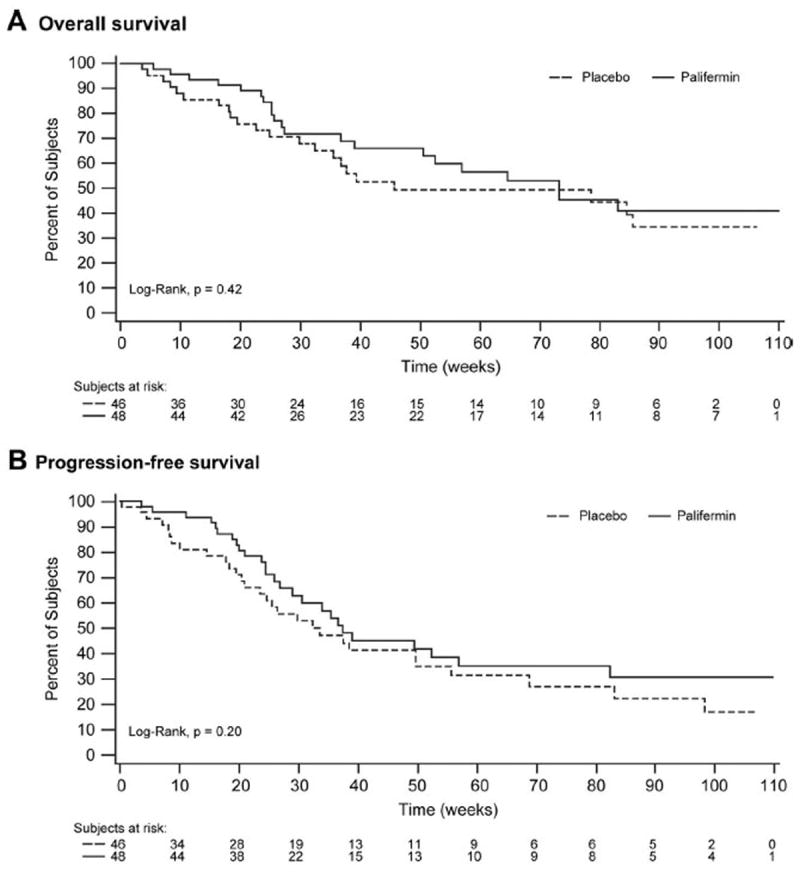
Kaplan-Meier curves for overall survival and progression-free survival: overall survival (A), progression-free survival (B).
DISCUSSION
This was the first study to assess the effect of palifermin on dysphagia in subjects with unresectable stage III NSCLC receiving concurrent CT/RT. There was a numerical decrease in the incidence and duration of dysphagia and a delay in time to onset of dysphagia in the palifermin arm, although this was not statistically significant in this exploratory trial. In addition, the palifermin group received more exposure than the placebo group to RT/CT, and post hoc analyses indicated that more subjects in the palifermin group than in the placebo group received the full RT dose (≥6000 cGy) (p = 0.01). Greater exposure to RT/CT in the palifermin group is not surprising as discontinuation was more common in the placebo group, with 39% of subjects discontinuing in the placebo group compared with 18% in the palifermin group, hence these subjects did not receive the scheduled RT/CT doses. Lower serious AEs, AEs leading to study discontinuation, and fewer on-study deaths were observed in the palifermin subjects compared with the placebo group, which may also have affected the number of doses received. Nevertheless, of those who received the full RT dose in either treatment group, a smaller percentage of palifermin subjects experienced grade ≥2 or ≥3 dysphagia. Increased exposure to CT and RT may have contributed to better ECOG performance status over time, higher response rates, and numerical difference in survival. No major acute or chronic safety concerns were identified.
Studies have suggested a relationship between higher doses of RT and CT and local tumor control.21-24 A minimum dose of 6000 cGY is considered a standard therapy following the results of the Radiation Therapy Oncology Group (RTOG) 73-01 study in which the intrathoracic failure rate was 33% at 3 years for NSCLC patients treated with 6000 cGy, 42% for those treated with 5000 cGy, and 52% for patients treated with 4000 cGy in a continuous course.24 While the standard RT dose (6000 cGy) has remained unchanged for the past 30 years, higher doses are being investigated for the effect on local control and survival, including the phase III RTOG 0617 study.25 Several groups have performed RT dose escalation trials in NSCLC patients and reported results supporting the safety of 7400 cGy26-29; however, studies also indicate that higher doses are associated with increased toxicity.30,31
Improved survival has also been reported in patients receiving higher CT dose intensity during concurrent CT/RT.23 In addition, clinical trials have shown that, compared with sequential therapy, concurrent CT/RT is associated with longer survival in patients with unresectable stage III NSCLC,1-4 with an expected 4-year survival rate of 21% for RT doses to 6000 cGy with concurrent CT in patients with good performance status and minimal weight loss.1 However, concurrent CT/RT is more toxic than the sequential approach, with an increased risk of RT-induced side effects, including esophagitis and dysphagia.1,4-9 Severe acute esophageal toxicity (grade 3– 4) increased from 3% with the sequential approach to 18% with concurrent RT/CT, with a relative risk of 5.7 (p < 0.0001).4 The current treatment options of CT/RT-induced esophagitis are symptomatic. Inadequate management can lead to pain, nutritional deficits, reduction in dose intensity, and unplanned treatment breaks.13,30 Amifostine has been investigated as a potential treatment option to reduce the rate of esophagitis in patients receiving concurrent RT/CT for locally advanced or inoperable NSCLC. Randomized clinical trials reported mixed results on the effectiveness of amifostine in reducing the incidence of esophagitis in this setting,10,32 and a large-scale trial (RTOG 98-01) failed to demonstrate a reduction in risk. Thus, there continues to be a lack of an effective therapy to reduce the risk of esophagitis despite the clinical need.
Palifermin is an epithelial growth factor, and some epithelial tumors may express the KGF receptor; therefore, it is theoretically possible for palifermin administration to interfere with disease outcomes, either through direct stimulation of tumor growth or through interference with the tumor response to cytotoxic treatment. The results of our study suggest that palifermin did not adversely affect disease outcomes in these patients. Unexpectedly, compared with the placebo group, the palifermin group appeared to have a better response rate, OS, and PFS, although the difference did not reach statistical significance. The significantly higher cumulative intensity of CT and higher RT doses may have contributed to these outcomes.
The activity of palifermin has been investigated previously in other solid tumors.33,34 A phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma reported that palifermin (60 μg/kg for 10 doses) was well tolerated and indicated activity with reductions observed in mucositis, dysphagia, and xerostomia during hyperfractionated radiotherapy but not standard radiotherapy.33 In a separate phase II study, a significant reduction in oral mucositis was reported with palifermin administration (40 μg/kg for 3 consecutive days) in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy.34
This exploratory study has several limitations. The sample size was not powered to achieve statistical significance, different RT techniques were used, and prespecified data evaluations were complemented by post hoc analyses. Fewer patients in the placebo group completed the study, which may have affected the results. While missing values for the incidence and duration of dysphagia were imputed for subjects who discontinued the study, doses of RT and CT were recorded until discontinuation. Hence, the higher cumulative doses of RT and CT observed in the study in the palifermin group are not unexpected, given that more subjects in the palifermin arm completed the study. Consequently, the results of this trial have to be interpreted with a hypothesis-generating approach.
In conclusion, the results of this exploratory trial suggest that additional larger studies may be warranted to further evaluate the effect of palifermin on dysphagia, exposure to RT/CT, and long-term survival.
Acknowledgments
Supported by Amgen Inc.
The authors thank Mandy Suggitt, on behalf of Amgen Inc., and Susanna Mac, MD, PhD, Amgen Inc., for medical writing assistance.
BM has received honorarium for Advisory Board participation from Amgen Inc. GO and YC have received research grants from Amgen Inc. RL, HW, DPB are/were employees of Amgen Inc., and own Amgen stock or stock options.
Footnotes
Disclosure: WS and MJK have no disclosures.
References
- 1.Curran W, Scott C, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22:621. [Google Scholar]
- 2.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 3.Pierre F, Maurice P, Gilles R. A randomized phase III trial of sequential chemoradiotherapy versus concurrent chemoradiotherapy in locally advanced non-small cell lung cancer (NSCLC) (GLOTGFPC NPC 95-01 study) Proc Am Soc Clin Oncol. 2001;20:312A. [Google Scholar]
- 4.Auperin A, Rolland E, Curran WJ, et al. Concomitant readio-chemotherapy (RT-CT) versus sequential RT-CT in locally advanced non-small cell lung cancer (CSCLC): a meta analysis using individual patient data (IPD) from randomised clinical trials. J Thorac Oncol. 2007;2:S310. [Google Scholar]
- 5.Belderbos J, Uitterhoeve L, van Zandwijk N, et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973) Eur J Cancer. 2007;43:114–121. doi: 10.1016/j.ejca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 7.Kepka L, Sprawka A, Casas F, et al. Combination of radiotherapy and chemotherapy in locally advanced NSCLC. Expert Rev Anticancer Ther. 2009;9:1389–403. doi: 10.1586/era.09.121. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 9.Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Antonadou D, Throuvalas N, Petridis A, et al. Effect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57:402–408. doi: 10.1016/s0360-3016(03)00590-x. [DOI] [PubMed] [Google Scholar]
- 11.Lau D, Leigh B, Gandara D, et al. Twice-weekly paclitaxel and weekly carboplatin with concurrent thoracic radiation followed by carboplatin/paclitaxel consolidation for stage III non-small-cell lung cancer: a California Cancer Consortium phase II trial. J Clin Oncol. 2001;19:442–447. doi: 10.1200/JCO.2001.19.2.442. [DOI] [PubMed] [Google Scholar]
- 12.Vokes EE, Herndon JEII, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Werner-Wasik M. Treatment-related esophagitis. Semin Oncol. 2005;32:S60–S66. doi: 10.1053/j.seminoncol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23:2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 15.Danilenko DM. Preclinical and early clinical development of keratinocyte growth factor, an epithelial-specific tissue growth factor. Toxicol Pathol. 1999;27:64–71. doi: 10.1177/019262339902700113. [DOI] [PubMed] [Google Scholar]
- 16.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 17.Farrell CL, Bready JV, Rex KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–939. [PubMed] [Google Scholar]
- 18.Farrell CL, Rex KL, Chen JN, et al. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif. 2002;35(Suppl 1):78–85. doi: 10.1046/j.1365-2184.35.s1.8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell CL, Rex KL, Kaufman SA, et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol. 1999;75:609–620. doi: 10.1080/095530099140258. [DOI] [PubMed] [Google Scholar]
- 20.Kepivance Prescribing Information. Stockholm, Sweden: Biovitrum AB; [Google Scholar]
- 21.Bradley J. A review of radiation dose escalation trials for non-small cell lung cancer within the Radiation Therapy Oncology Group. Semin Oncol. 2005;32:S111–S113. doi: 10.1053/j.seminoncol.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Hazuka MB, Turrisi AT, III, Lutz ST, et al. Results of high-dose thoracic irradiation incorporating beam’s eye view display in non-small cell lung cancer: a retrospective multivariate analysis. Int J Radiat Oncol Biol Phys. 1993;27:273–284. doi: 10.1016/0360-3016(93)90238-q. [DOI] [PubMed] [Google Scholar]
- 23.Machtay M, Swann RS, Komaki R, et al. O-265 Cisplatin dose delivery and survival in concurrent chemoradiation for stage III NSCLC: an RTOG secondary analysis. Lung Cancer. 2003;41:S77. [Google Scholar]
- 24.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–2753. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Radiation Therapy Oncology Group. High-dose or standard-dose radiation therapy and chemotherapy in treating patients with newly diagnosed stage III non-small cell lung cancer that cannot be removed by surgery. [February 23, 2010]; ClinicalTrials.gov Available at: http://www.clinicaltrials.gov/ct2/show/NCT00533949?term=rtog+0617&rank=1.
- 26.Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–2480. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayman JA, Martel MK, Ten Haken RK, et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol. 2001;19:127–136. doi: 10.1200/JCO.2001.19.1.127. [DOI] [PubMed] [Google Scholar]
- 28.Rosenman JG, Halle JS, Socinski MA, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002;54:348–356. doi: 10.1016/s0360-3016(02)02958-9. [DOI] [PubMed] [Google Scholar]
- 29.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 30.Pena-Greenberg E, Law E, Frankel J. Radiation-induced esophagitis: evidence-based nursing guidelines for lungs and esophageal cancer patients. Oncology Nursing Society 33rd Annual Congress Oncology Nursing Forum; 2008. p. 498. [Google Scholar]
- 31.Schild SE, McGinnis WL, Graham D, et al. Results of a Phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–1111. doi: 10.1016/j.ijrobp.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 32.Komaki R, Lee JS, Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369–1377. doi: 10.1016/j.ijrobp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Brizel DM, Murphy BA, Rosenthal DI, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489–2496. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- 34.Rosen LS, Abdi E, Davis ID, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol. 2006;24:5194–5200. doi: 10.1200/JCO.2005.04.1152. [DOI] [PubMed] [Google Scholar]


