Abstract
Background
The interval between neoadjuvant chemoradiation treatment and surgery has been described as an important predictor of pathologic response to therapy in non-esophageal cancer sites. We retrospectively reviewed our experience with patients who underwent neoadjuvant chemoradiation and esophagectomy to better understand the impact of the timing of surgery on pathologic complete response rates in esophageal cancer.
Methods
Two hundred thirty one sequentially treated patients from 2000 to 2011 were identified for this study, 88 of these patients completed neoadjuvant chemoradiation followed by esophagectomy at our institution. The interval between completion of chemoradiation and surgery was calculated for each patient. The patients were categorized into quartiles and also 3-week interval groups. Treatment factors and surgical morbidity data including the estimated blood loss and length of operative stay were also assessed.
Results
Quartiles for the neoadjuvant chemoradiation to surgery interval were <45 days, 46-50 days, 51-63 days, and 64+ days. Corresponding pathologic complete response rates were 12.5%, 20.0%, 22.7% and 40.9% (p=0.03). Results for 3-week intervals were similar (p=0.02). There was no association between increasing time interval between the ending of neoadjuvant chemoradiation to surgery and length of stay longer than 2 weeks.
Conclusions
A longer interval between completion of neoadjuvant chemoradiation and surgery was associated with higher pathologic complete response rates without an impact on surgical morbidity.
Keywords: esophagus, neoadjuvant, chemoradiation, esophagectomy, time interval
Introduction
Esophageal cancer is an aggressive malignancy that is associated with high mortality rates. Five-year survival for all stages is 17%, and even patients with localized disease have a survival rate of only 37%. Tri-modality treatment with pre-operative chemoradiotherapy has been shown to improve survival rates versus surgery alone and is accepted as a standard of care for esophageal cancer patients (1-5).Neoadjuvant chemoradiation treatment (NCRT) has been shown to decrease locoregional failure control (14.1% with NCRT vs. 33.5% with surgery alone at 45 months) as well as distant failure rates (31.5% vs. 47.8%, respectively), therefore improving outcomes (6). Studies have demonstrated that pathologic complete response (pCR) in patients undergoing tri-modality treatment for esophageal cancer predicts for decreased local and distant recurrence, as well as improved survival (7-9).
Due to the potential implications of pCR in patients with esophageal cancer, identifying variables effecting pCR is important. The interval between neoadjuvant treatment and surgery has been implicated as an important factor in other disease sites such as the rectal adenocarcinoma in predicting pathologic response to therapy. In squamous cell carcinoma of the anal canal, tumor regression after chemoradiation can be seen up to six months after completion of therapy. Increasing this interval following completion of pre-operative therapy may allow the tumor to continue to regress, thereby improving resectability, or allow a more accurate assessment of the maximal effect of NCRT. However, theoretically, waiting too long for regression may allow for tumor repopulation or increased radiation fibrosis, adding to the complexity and complications of surgery. We retrospectively reviewed our experience with patients who underwent esophagectomy to better understand the impact of the interval between the end of NCRT therapy and surgery on pCR rates in esophageal cancer.
Material and Methods
After institutional review board approval, all patients who underwent NCRT for esophageal cancer at Fox Chase Cancer Center between September 2000 and September 2011 were retrospectively reviewed. Clinical records identified 231 patients with esophageal cancer undergoing chemoradiation, of whom 91 underwent subsequent surgery. Two patients underwent radiation treatment at another facility and their records were not available at the time of analysis, and an additional patient did not complete radiation treatment. Thus, 88 patients were included in this study. For the evaluation of post-surgical outcomes, three patients were excluded because of surgery at an outside institution, and one patient was excluded because he underwent a total gastrectomy, not an esophagectomy.
All patients were staged pre- and postoperatively according to the tumor-node-metastasis classification of the American Joint Committee for Cancer Staging Version 7 (10). Pretreatment clinical staging routinely included CT scan, esophagogastroduodenoscopy and biopsy, bronchoscopy, endoscopic ultrasound and positron emission tomography scan. Patients with no viable tumor cells in the surgical specimen (ypT0N0M0) were classified as having a pCR; all other patients were considered to have either gross residual or microscopic disease.
Baseline data collected included general patient characteristics (age, sex, diagnosis date, histology), treatment characteristics (chemotherapy regimen, radiation dose, type of surgery), toxicity, and tumor recurrence. Follow-up data were obtained from patient medical records, referring physicians, and telephone interviews.
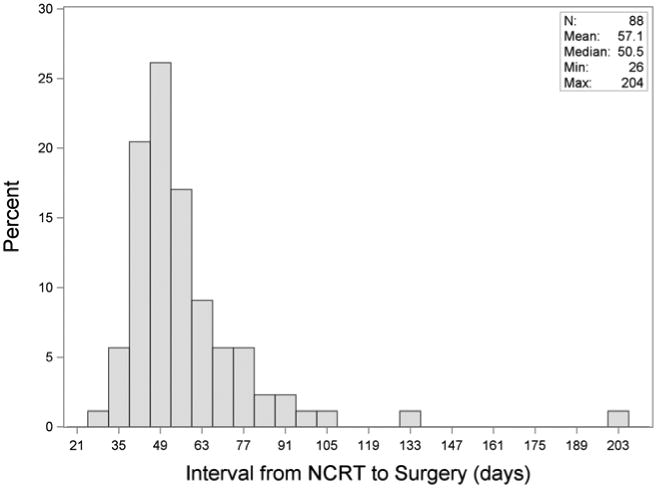
We determined the NCRT-to-surgery interval from the last day of radiation treatment to the day of surgery. This variable was examined as both a continuous and categorical predictor. Due to the skewed distribution (Figure 1), we also considered the log-transformed interval. As a categorical variable, we evenly classified patients according to interval quartiles and 3-week groups (3 to 6 weeks, 6 to ≤ 9 wks, 9 to ≤ 12 wks and > 12 wks). Both interval quartiles and 3-week groups were created in order to divide patients evenly by number (interval quartiles) and by regular time periods (3-week groups). The primary outcome was pCR (yes vs. no). We evaluated the association of quartiles and 3-week groups with the binary outcome of pCR using the Cochran-Armitage trend test. We used the odds ratio from univariate logistic regression to measure the association of the interval (as a continuous variable, log transformed variable, or categorical variable) with pCR. We also used logistic regression to evaluate potential confounders including age, gender, T-stage, N-stage, histology, type of chemotherapy and type of radiation. Statistically significant confounders would have been included as covariates in multivariable logistic regression to assess the impact on the association with the NCRT-surgery interval; however, none of the confounders were significantly related to the outcome. Overall survival (OS) was estimated using the Kaplan-Meier method. Cox proportional hazards model was used adjusting for age.
Figure 1. Elapsed Time from NCRT to Surgery.
Estimated blood loss (EBL) was considered as both a continuous and categorical variable (approximately 100 mL intervals). Spearman's rank correlation (non-parametric) was used to estimate the association between EBL and the interval as continuous variables. Linear regression was used to assess the trend between EBL as a categorical variable and the post NCRT-surgery interval. Length of hospital (LOS) stay of more than two weeks (yes/no) was evaluated similar to the pCR outcome, using the Cochran-Armitage trend test, and odds ratios from univariate logistic regression, and multivariable analysis to adjust for significant univariate confounders. Statistical significance was determined using a 5% Type I error. All analyses were conducted using SAS statistical software (Cary, NC), version 9.3.
Results
Of the patients completing tri-modality therapy, 75 were male and 13 were female, and the median age was 61 years (range 36-80 years). The cancer was adenocarcinoma in 74 patients (84.1%) and squamous cell carcinoma in 14 patients (15.9%). Eight patients (9.1%) had T1/T2 lesions and 80 patients (88.9%) had T3/T4 lesions. The median follow-up of the cohort was 87.7 months. Clinical characteristics of these patients can be found in Table 1.
Table 1. Patient Characteristics in each quartile, as number of patients (N) and percent unless otherwise specified.
| All | Q-I ≤45 Days | Q-II 46-50 Days | Q-III 51-63 Days | Q-IV 64+ Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % | N | % | p-value* |
| 88 | 24 | 20 | 22 | 22 | |||||||
| Age, years | 0.29 | ||||||||||
| Median | 61 | 57.5 | 66.5 | 58.5 | 65 | ||||||
| Range | 36-80 | 36-78 | 40-77 | 42-80 | 37-80 | ||||||
| Sex | 0.47 | ||||||||||
| Male | 75 | 85.2 | 21 | 87.5 | 17 | 85.0 | 20 | 90.9 | 17 | 77.3 | |
| Female | 13 | 14.8 | 3 | 12.5 | 3 | 15.0 | 2 | 9.1 | 5 | 22.7 | |
| T Stage | 0.22 | ||||||||||
| T1/T2 | 8 | 9.1 | 3 | 12.5 | 2 | 10.0 | 3 | 13.6 | 0 | 0.0 | |
| T3/T4 | 80 | 88.9 | 21 | 87.5 | 18 | 90.0 | 19 | 86.4 | 22 | 100.0 | |
| N Stage | 0.55 | ||||||||||
| Positive | 67 | 76.1 | 7 | 29.2 | 3 | 15.0 | 8 | 36.4 | 3 | 13.6 | |
| Negative | 21 | 23.9 | 17 | 70.8 | 17 | 85.0 | 14 | 63.6 | 19 | 86.4 | |
| M Stage | 0.49 | ||||||||||
| M0 | 71 | 80.7 | 20 | 83.3 | 16 | 80.0 | 19 | 86.4 | 16 | 72.7 | |
| M1 | 17 | 19.3 | 4 | 16.7 | 4 | 20.0 | 3 | 13.6 | 6 | 27.3 | |
| Histology | 0.04 | ||||||||||
| Adenocarcinoma | 74 | 84.1 | 23 | 95.8 | 16 | 80.0 | 20 | 90.9 | 15 | 68.2 | |
| SCC | 14 | 15.9 | 1 | 4.2 | 4 | 20.0 | 2 | 9.1 | 7 | 31.8 | |
| Chemotherapy | 0.49 | ||||||||||
| 5FU-based | 71 | 80.7 | 20 | 83.3 | 17 | 85.0 | 17 | 77.3 | 17 | 77.3 | |
| Taxol-based | 17 | 19.3 | 4 | 16.7 | 3 | 15.0 | 5 | 22.7 | 5 | 22.7 | |
| Type of RT | 0.65 | ||||||||||
| 3D-CRT | 72 | 81.8 | 21 | 87.5 | 17 | 85.0 | 15 | 68.2 | 19 | 86.4 | |
| IMRT | 16 | 18.2 | 3 | 12.5 | 3 | 15.0 | 7 | 31.8 | 3 | 13.6 | |
Cochrane-Armitage trend test for categorical variables, linear regression model for age
SCC: Squamous Cell Carcinoma
3D-CRT: 3D-conformal radiotherapy
IMRT: intensity modulated radiotherapy
The induction regimen varied with the median radiation dose being 50.4 Gy (45-60). Sixteen patients (18.1%) received intensity modulated radiation therapy (IMRT), and 72 (81.8%) underwent three-dimensional conformal radiotherapy (3D-CRT). Seventy-one patients (80.7%) received 5FU-based therapy and 17 patients (19.3%) received Taxol-based therapy.
Surgical treatment was guided by the location of the tumor as well as surgeon preference. Eighteen patients underwent three-hole minimally invasive esophagectomy, 68 patients underwent Ivor-Lewis esophagectomy, one patient underwent a transhiatal esophagectomy, and one patient underwent total gastrectomy. Twenty one patients (23.9%) had a pCR following NCRT and 67 patients (76.1%) had residual or microscopic disease on pathology of the primary tumor or lymph nodes. The majority of patients with residual or microscopic disease had it in the lymph nodes and esophagus (47.8%) or the esophagus alone (44.8%).
The median number of days between completion of NCRT and surgery was 50.5 (26 to 204 days). The median time from completion of NCRT to surgery was 57 days for patients achieving a pCR and 50 days for patients with residual or microscopic disease on pathology. When divided evenly by number of patients, quartiles for the NCRT to surgery interval were <45 days, 46-50 days, 51-63 days, and 64+ days (Figure 2A). There were no significant differences in terms of patient characteristics between the groups aside from histology (p=0.04) (Table 1). PCR was 12.5%, 20.0%, 22.7% and 40.9% in each one of these quartiles, respectively (p=0.03, Table 2). For patients with an interval >64 days, 8 patients (36.4%) had a longer interval due to a post-NCRT hospitalization and 8 patients (36.4%) had a poor performance status following NCRT. Other reasons for a delayed interval included patient decision (3 patients), scheduling difficulties (2 patients), and peri-operative clearance problems (1 patients). NCRT to surgery intervals were also categorized into 3-week interval groups, including 3-6 weeks, 6-9 weeks, 9-12 weeks, and 12+ weeks (Figure 2B). PCR was 11.8% (2/17) in the shortest interval and 50.0% (3/6) in the longest interval group, with a significant across the four groups (p=0.02). Univariable logistic regression also showed a difference in pCR rates when the interval between NCRT and surgery was examined as a continuous variable (Odds ratio for pCR for a difference of 1 week = 1.20, 95% CI = 1.01-1.42, p=0.04) and a log transformed continuous variable (p=0.02) (Table 3).
Figure 2. Pathologic Response by (A) Interval Quartile and (B) 3-week Interval Group.
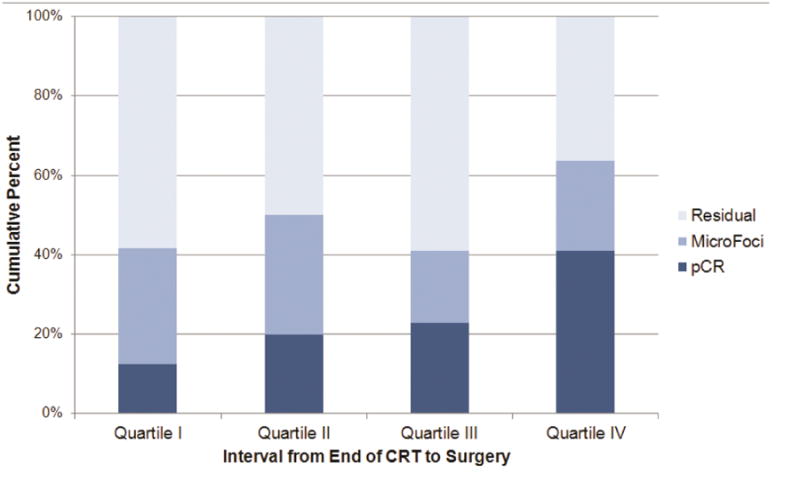
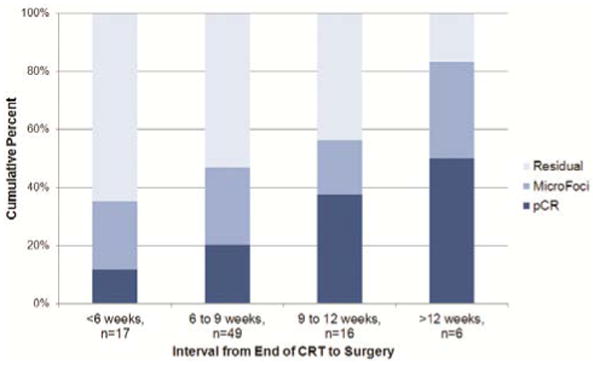
Table 2. Interval from NCRT to surgery and pCR.
| ALL | Residual Cancer† | pCR | |||
|---|---|---|---|---|---|
| N | n | % with resid ca | n | % with pCR | |
| Quartile§ | |||||
| I: 26-45 Days | 24 | 21 | 87.5 | 3 | 12.5 |
| II: 46-50 Days | 20 | 16 | 80.0 | 4 | 20.0 |
| III: 51-63 Days | 22 | 17 | 77.3 | 5 | 22.7 |
| IV: 64+ Days | 22 | 13 | 59.1 | 9 | 40.9 |
| 3-week interval group¥ | |||||
| 3-6 weeks (26-42 days) | 17 | 15 | 88.2 | 2 | 11.8 |
| 6-9 weeks (43-63 days) | 49 | 39 | 79.6 | 10 | 20.4 |
| 9-12 weeks (64-84 days) | 16 | 10 | 62.5 | 6 | 37.5 |
| 12+ weeks (85+ days) | 6 | 3 | 50.0 | 3 | 50.0 |
NCRT: Neoadjuvant chemoradiation
pCR: Pathologic complete response
Cochran Armitage Trend Test, p=0.0265
Cochran Armitage Trend test, p=0.0203
Residual cancer includes microscopic foci
Table 3. Logistic Regression for Outcome Complete Pathologic Response (Yes v. No).
| pCR | Univariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| N | N | % pCR | OR | 95% CI | p-value | |
| Interval from end of NCRT to Surgery | ||||||
| Interval in weeks (continuous) | 1.20 | 1.01-1.42 | 0.038 | |||
| Log transformation of Interval in weeks(continuous) | 6.77 | 1.32-34.67 | 0.022 | |||
| By interval quartiles | ||||||
| ≤45 Days | 24 | 3 | 12.5 | 1.00 | Ref | |
| 46-50 Days | 20 | 4 | 20.0 | 1.75 | 0.34-8.95 | 0.50 |
| 51-63 Days | 22 | 5 | 22.7 | 2.06 | 0.43-9.87 | 0.37 |
| 64+ Days | 22 | 9 | 40.9 | 4.85 | 1.11-21.26 | 0.036 |
| By 3 week intervals | ||||||
| 3-6 weeks (26-42 days) | 17 | 2 | 11.8 | 1.00 | Ref | |
| 6.1-9 weeks (43-63 days) | 49 | 10 | 20.4 | 1.92 | 0.38-9.82 | 0.43 |
| 9.1-12 weeks (64-84 days) | 16 | 6 | 37.5 | 4.50 | 0.75-26.93 | 0.099 |
| >12 weeks (85+ days) | 6 | 3 | 50.0 | 7.50 | 0.85-66.12 | 0.070 |
| Other Characteristics | ||||||
| Age (continuous) | 1.04 | 0.99-1.09 | 0.16 | |||
| Gender | ||||||
| Female | 13 | 2 | 15.4 | 1.00 | Ref | |
| Male | 75 | 19 | 25.3 | 1.87 | 0.38-9.19 | 0.44 |
| T Stage | ||||||
| T1/T2 | 8 | 2 | 25.0 | 1.00 | Ref | |
| T3/T4 | 80 | 19 | 23.8 | 0.93 | 0.17-5.02 | 0.94 |
| N Stage | ||||||
| Positive | 67 | 16 | 23.9 | 1.00 | 0.99 | |
| Negative | 21 | 5 | 23.8 | 1.00 | Ref | |
| Mstage | ||||||
| M0 | 71 | 19 | 26.8 | 1.00 | Ref | |
| Any M1 | 17 | 2 | 11.8 | 0.37 | 0.08-1.75 | 0.21 |
| Histology | ||||||
| Adenocarcinoma | 74 | 16 | 21.6 | 1.00 | Ref | |
| Squamous Cell Carcinoma | 14 | 5 | 35.7 | 2.01 | 0.26 | |
| Type of Chemotherapy | ||||||
| 5FU-based | 71 | 16 | 22.5 | 1.00 | Ref | |
| Taxol-based | 17 | 5 | 29.4 | 1.43 | 0.44-4.67 | 0.55 |
| Type of RT | ||||||
| 3D-CRT | 72 | 18 | 25.0 | 1.00 | Ref | |
| IMRT | 16 | 3 | 18.8 | 0.69 | 0.18-2.71 | 0.60 |
NCRT: Neoadjuvant chemoradiation
3D-CRT: 3D-conformal radiotherapy
IMRT: intensity modulated radiotherapy
Analysis of tumor characteristics including tumor stage, node positivity, and histology revealed no significant difference in tumor response (Table 3). Furthermore, when stratifying patients according to chemotherapy regimen or radiation dose, there was no difference in tumor response to treatment. Patient characteristics such as sex or age also did not affect the response of the tumor following tri-modality treatment. Since none of these characteristics were significantly related to pCR, they were not considered potential confounders of the interval-pCR association and thus not added as covariates to the model for interval and pCR.
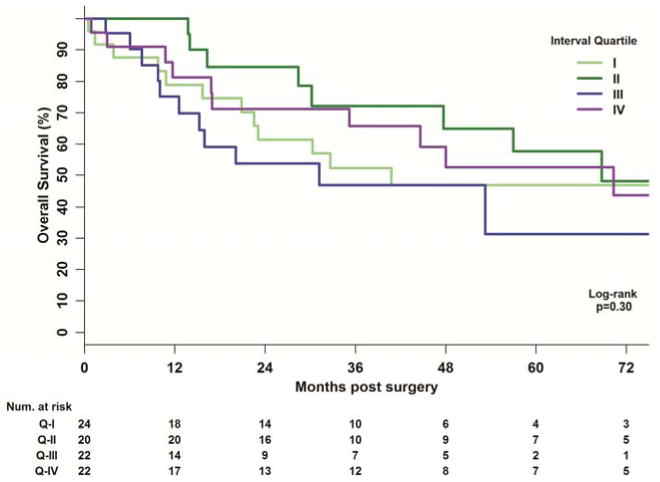
Patients achieving a pCR had an improved OS versus patients with residual or microscopic disease (p=0.05). When analyzing by interval between NCRT to surgery, there was no difference in OS by interval quartile (p=0.30) or by 3-week interval groups (p=0.13) (Figure 3). In a Cox-model adjusting for age, the interval quartile (p=0.24) and 3-week quartile (p=0.17) still did not reach statistical significance.
Figure 3. Kaplan Meier Plots for Overall Survival by Interval Quartile.
Of the 85 patients for whom surgical data were available, the median LOS was 12 days (8-35 days). Of these patients, 23 (27.1%) had LOS > 14 days. There was no significant trend between LOS > 14 days and either interval quartile (p=0.17) or 3-week interval group (p=0.60). The median amount of EBL was 200 cc (range 50 to 800 cc). No significant relationship between time interval and EBL was found (p=0.54).
Comment
Despite improvements in technology and treatment modalities, the prognosis for esophageal cancer patients remains poor with 5-year survival rates ranging from 17% to 37%. The cornerstone of managing these patients remains esophagectomy, although the addition of NCRT has become the standard of care in many institutions throughout the country.
The ideal interval between NCRT and surgery is not well defined. The typical goal time period of four to six weeks is somewhat arbitrary, with the intent of allowing resolution of acute inflammation as well as allowing for tumor regression while minimizing the chronic fibrotic changes in the surgical field. In theory, this may improve both resectability and decrease morbidity following esophagectomy.
A study by Berger et al retrospectively reviewed 171 patients who received an esophagectomy either with or without NCRT (11). Patients who achieved a pCR following tri-modality treatment showed a median overall survival of 50 months and a 5-year survival rate of 48% versus a median survival of 25 months and a 5-year survival of 15% of patients without neoadjuvant therapy. The value of a pCR has also been shown in a prospectively collected data set by Stahl, who demonstrated that each of the 8 patients who achieved a pCR with neoadjuvant therapy was alive and disease-free at last follow-up (9).
Multiple studies have demonstrated that patients with a pCR following NCRT therapy have improved survival outcomes. Kleinberg et al reviewed 92 patients with esophageal cancer who underwent NCRT followed by esophagectomy. At a median follow-up of 63.5 months, a total of 30 patients (33%) achieved a pCR. Patients achieving a pCR had a survival rate of 67% versus 27% for those without a pCR. Other studies have demonstrated similar response rates following neoadjuvant treatment (2-5, 12, 13). Our findings were consistent with the literature as patients achieving a pCR had an improved OS versus those with residual or microscopic disease. Although patients with a longer interval between NCRT and surgery had a higher rate of pCR, this did not translate into an improvement in OS. Reasons for this disconnect may be small sample sizes in each cohort.
For rectal cancer, the Lyon R90-01 trial examined the outcomes of patients following preoperative radiation treatment (14). Interestingly, patients with a long interval (6-8 weeks) between radiation and surgery versus a short interval (within 2 weeks) were found to have a better clinical response and pathologic downstaging, although both groups had similar local control and overall survival. Other studies in rectal cancer have demonstrated similar findings (15, 16). This brings into question whether time elapsed between neoadjuvant treatment and surgery alters patient outcomes at other disease sites.
Our study examined tumor regression rates by evaluating the number of patients who had pCR or residual disease following NCRT. When stratifying patients according to time elapsed between completion of NCRT and surgery, there was an increased pCR rate in patients with a greater time elapsed between NCRT and surgery. Unlike the Lyon R0-01 study, our cohort of patients also received neoadjuvant chemotherapy yet the outcome was similar. Patients with a longer interval to surgery appeared to have better down staging and response rates.
Tessier et al recently examined the optimal timing of surgery in patients undergoing neoadjuvant chemoradiation for esophageal cancer. In their cohort of 257 patients, they found no association between delay in surgical intervention and outcomes (17). A study by Kim et al examined 266 patients who underwent tri-modality therapy in order to determine whether increased interval between NCRT and surgery was associated with perioperative complications, pathologic response, and overall survival. In their data set, there was no correlation between time and the above outcomes (18). A study by Rizk et al examined 276 patients undergoing tri-modality treatment for esophageal cancer (19). With a median interval of time from completion of radiation to surgery of 49 days, they reported no difference in patient outcomes based on the interval from radiation completion and surgery. Similarly, Koshy et al, found that with a median time lapse of 7 weeks between treatments, there was no significant effect on tumor response (20).
Although other studies have demonstrated no significant impact of the time between NCRT and surgery, our data suggests a possible advantage of delayed resection. This disparity may be due to various reasons. Prior studies have had a much shorter median interval between NCRT and surgery, thus possibly preventing any significant difference in response to be assessed. Also, prior retrospective studies have examined different chemotherapy regimens and a mix of histologies weighted more toward squamous cell carcinomas (5). These factors may impact the velocity of treatment response. Inherent biases may also impact the different outcomes in these studies. Although our study groups do not appear to have any differences in terms of treatment related factors, other disparities between the groups may be unaccounted for. Finally, statistical methods between these studies vary. In our analysis, we examine differences in these cohorts using the end-point as both a continuous and categorical predictor. Other analysis, have used timing as a dichotomous variable which may not account for the varied distribution as evident in analysis.
In our patient analysis, longer time intervals were associated with increased length of stay. This is not surprising, as gaps between completion of NCRT and surgery are often because of the toxicity of initial therapy. Although patients completing NCRT are typically presumed to have worse perioperative outcomes, a recent study demonstrated that patients who completed NCRT followed by esophagectomy were more likely to have a reduced length of stay compared with patients who only underwent surgery (21).
As with any retrospective analysis, the main limitation of this study is the non-randomization of patients allocated to each group. Due to the nature of NCRT, patients may have significant morbidity which makes surgeons more apt to suggest additional recovery time prior to surgery. Surgery may also be delayed due to suspicion of distant metastasis during NCRT; therefore, patients with delays who do not have evidence of distant disease may have been biologically selected as responders to therapy. However, prospective randomized trials have strict timeframes for protocol treatment, which by their nature would limit an analysis of the type we have performed. In addition, not all patients undergoing chemoradiation undergo surgical resection. In our cohort, of 231 patients undergoing chemoradiation, only 91 patients underwent surgical resection and 88 were evaluable.
In summary, this study suggests that similar to findings at other disease sites, patients with esophageal cancer may have better response rates with longer time interval between chemoradiotherapy and surgery. To our knowledge, this is the first study reporting such a finding in esophageal cancer patients receiving NCRT. When examining pCR as a surrogate endpoint, it may be important to keep the interval from NCRT to surgery in mind, as early surgery may underestimate the efficacy of chemoradiation. Importantly, although longer time to surgery improved response rates, it did not significantly affect overall surgical morbidity as measured by LOS and EBL. This study emphasizes the importance of future studies to gain a better understanding of the ideal interval between NCRT and surgery in esophageal cancer patients.
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh TN, Grennell M, Mansoor S, Kelly A. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2002;15(2):121–124. doi: 10.1046/j.1442-2050.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 2.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(2):305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 3.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepato-gastroenterology. 1994;41(4):391–393. [PubMed] [Google Scholar]
- 4.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase iii trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: Calgb 9781. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(7):1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the cross trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5):385–391. doi: 10.1200/JCO.2013.51.2186. [DOI] [PubMed] [Google Scholar]
- 7.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 8.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(19):4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Stahl M, Walz MK, Stuschke M, et al. Phase iii comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(6):851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB American Joint Committee on Cancer., American Cancer Society. Ajcc cancer staging handbook : From the ajcc cancer staging manual. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Watson DI, Jamieson GG, Lally C, Bessell JR, Devitt PG. Outcome of oesophagectomy for adenocarcinoma of the oesophagus and oesophagogastric junction. ANZ journal of surgery. 2005;75(7):513–519. doi: 10.1111/j.1445-2197.2005.03433.x. [DOI] [PubMed] [Google Scholar]
- 12.Aad G, Abbott B, Abdallah J, et al. Search for the higgs boson in the h→ww(*)→l(+)nul(-)nu decay channel in pp collisions at radicals=7 tev with the atlas detector. Physical review letters. 2012;108(11):111802. doi: 10.1103/PhysRevLett.108.111802. [DOI] [PubMed] [Google Scholar]
- 13.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. The New England journal of medicine. 1996;335(7):462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 14.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The lyon r90-01 randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(8):2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 15.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Annals of surgery. 2009;250(4):582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 16.Chiu CH, Chao YK, Chang HK, et al. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: Does delayed surgery impact outcome? Annals of surgical oncology. 2013;20(13):4245–4251. doi: 10.1245/s10434-013-3139-7. [DOI] [PubMed] [Google Scholar]
- 17.Tessier W, Gronnier C, Messager M, et al. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? The Annals of thoracic surgery. 2014;97(4):1181–1189. doi: 10.1016/j.athoracsur.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? The Annals of thoracic surgery. 2012;93(1):207–212. doi: 10.1016/j.athoracsur.2011.05.021. discussion 212-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizk NP, Venkatraman E, Bains MS, et al. American joint committee on cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(5):507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- 20.Koshy M, Greenwald BD, Hausner P, et al. Outcomes after trimodality therapy for esophageal cancer: The impact of histology on failure patterns. Am J Clin Oncol. 2011;34(3):259–264. doi: 10.1097/COC.0b013e3181e841ce. [DOI] [PubMed] [Google Scholar]
- 21.Markar SR, Bodnar A, Rosales J, Song G, Low DE. The impact of neoadjuvant chemoradiotherapy on perioperative outcomes, tumor pathology, and survival in clinical stage ii and iii esophageal cancer. Annals of surgical oncology. 2013 doi: 10.1245/s10434-013-3137-9. [DOI] [PubMed] [Google Scholar]


