Abstract
BACKGROUND
Hypertension develops early in patients with autosomal dominant polycystic kidney disease (ADPKD) and is associated with disease progression. The renin–angiotensin–aldosterone system (RAAS) is implicated in the pathogenesis of hypertension in patients with ADPKD. Dual blockade of the RAAS may circumvent compensatory mechanisms that limit the efficacy of monotherapy with an angiotensin-converting–enzyme (ACE) inhibitor or angiotensin II–receptor blocker (ARB).
METHODS
In this double-blind, placebo-controlled trial, we randomly assigned 486 patients, 18 to 64 years of age, with ADPKD (estimated glomerular filtration rate [GFR], 25 to 60 ml per minute per 1.73 m2 of body-surface area) to receive an ACE inhibitor (lisinopril) and placebo or lisinopril and an ARB (telmisartan), with the doses adjusted to achieve a blood pressure of 110/70 to 130/80 mm Hg. The composite primary outcome was the time to death, end-stage renal disease, or a 50% reduction from the baseline estimated GFR. Secondary outcomes included the rates of change in urinary aldosterone and albumin excretion, frequency of hospitalizations for any cause and for cardiovascular causes, incidence of pain, frequency of ADPKD-related symptoms, quality of life, and adverse study-medication effects. Patients were followed for 5 to 8 years.
RESULTS
There was no significant difference between the study groups in the incidence of the composite primary outcome (hazard ratio with lisinopril–telmisartan, 1.08; 95% confidence interval, 0.82 to 1.42). The two treatments controlled blood pressure and lowered urinary aldosterone excretion similarly. The rates of decline in the estimated GFR, urinary albumin excretion, and other secondary outcomes and adverse events, including hyperkalemia and acute kidney injury, were also similar in the two groups.
CONCLUSIONS
Monotherapy with an ACE inhibitor was associated with blood-pressure control in most patients with ADPKD and stage 3 chronic kidney disease. The addition of an ARB did not alter the decline in the estimated GFR. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; HALT-PKD [Study B] ClinicalTrials.gov number, NCT01885559.)
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by the progressive development of kidney cysts.1 Hypertension develops early in patients with ADPKD and is associated with the progression of disease.2 The renin–angiotensin–aldosterone system (RAAS) is implicated in the pathogenesis of hypertension in patients with ADPKD.2–7 Angiotensin-converting–enzyme (ACE) inhibitors slow the progression of renal dysfunction in non-diabetic kidney diseases.8,9 On the basis of these data, the use of ACE inhibitors as first-line agents to treat hypertension in patients with ADPKD has become standard clinical practice, although no randomized, clinical trials of sufficient size and quality have shown their superiority over other antihypertensive agents.10–14
The Halt Progression of Polycystic Kidney Disease (HALT-PKD) trials were designed to have sufficient power to ascertain the effect of intensive blockade of the RAAS and blood-pressure control on the progression of kidney disease in persons with an early15 or moderately advanced stage of ADPKD. Here we present the results of a study that involved patients with an estimated glomerular filtration rate (GFR) of 25 to 60 ml per minute per 1.73 m2 of body-surface area.
METHODS
TRIAL DESIGN AND STUDY OVERSIGHT
This randomized, double-blind, placebo-controlled clinical trial was designed to assess the efficacy of a combination of an ACE inhibitor (lisinopril) and an ARB (telmisartan), as compared with lisinopril and placebo, in reducing disease progression in patients with moderately advanced ADPKD. A steering committee of investigators designed the trial. An external advisory committee selected by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health reviewed the protocol and served as the data and safety monitoring board. The institutional review board at each site approved the study protocol, which is available with the full text of this article at NEJM.org and has been published previously.16,17
Data collected by site investigative teams were managed by the data coordinating center and analyzed by study statisticians. The first author wrote the first draft of the manuscript, with substantial contributions from the coauthors, and all the authors jointly decided to submit the manuscript for publication. All the authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of this report to the protocol. The study drugs were donated by Boehringer Ingelheim Pharmaceuticals (telmisartan and matched placebo) and Merck (lisinopril). Neither company had any role in the design of the study, accrual or analysis of data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
PATIENTS AND INTERVENTIONS
Eligible patients were enrolled from February 2006 through June 2009. Written informed consent was obtained from all the patients. Inclusion criteria were a diagnosis of ADPKD and hypertension or high-normal blood pressure,16,18 an age of 18 to 64 years, and an estimated GFR of 25 to 60 ml per minute per 1.73 m2.19 Patients were randomly assigned in a 1:1 ratio to lisinopril plus telmisartan or to lisinopril plus placebo with the use of a central system, with stratification according to age, sex, race, baseline estimated GFR, and clinical site.16 The last study visit was in June 2014.
TRIAL ASSESSMENTS
Assessments at screening, washout of antihypertensive medications, and baseline visits were performed as described in the related study by Schrier et al.15 except for magnetic resonance imaging, which was not performed in this study. After randomization, study medications were adjusted to achieve blood-pressure targets (systolic blood pressure, 110 to 130 mm Hg; diastolic blood pressure, 70 to 80 mm Hg), as measured at home, and plasma levels of creatinine and potassium were monitored 1 week after any dose increase. Second-, third-, and fourth-line antihypertensive agents were added if needed (Table S1 in the Supplementary Appendix, available at NEJM.org). Follow-up clinical-site visits took place, adherence to treatment was monitored, and central and local measurements of laboratory values were performed as described by Schrier et al.15
OUTCOME MEASURES
The composite primary outcome was the time to death, end-stage renal disease (ESRD; defined as the initiation of dialysis or preemptive transplantation), or a 50% reduction from the baseline estimated GFR (confirmed by assessment of a second sample and adjudicated by the end-points committee, whose members were unaware of the study-medication assignments). Secondary outcomes included the rates of change in urinary albumin and aldosterone excretion, the frequency of hospitalizations for any cause and for cardiovascular causes, quality of life, incidence of pain, the frequency of symptoms related to ADPKD, and adverse study-medication effects. Hospitalizations were adjudicated by the end-points committee and evaluated with respect to the principal diagnosis, whether hospitalization was related to ADPKD, and whether the criteria for acute kidney injury, as described by Schrier et al.,15 were met.
STATISTICAL ANALYSIS
The primary analysis examined the effect of an ACE inhibitor plus an ARB versus an ACE inhibitor plus placebo on the time to the composite outcome of death, ESRD, or a 50% reduction from the baseline estimated GFR. A Cox proportional-hazards model was fitted to model the hazard ratio as a function of month, month by treatment group, age, sex, race, baseline estimated GFR, and clinical site. Data were censored if the participants completed study follow-up without having an event or if they discontinued study participation.
The estimated GFR, determined according to the Chronic Kidney Disease Epidemiology Collaboration equation,19 was calculated with the use of central measurements of the serum creatinine level at baseline, at 4 and 12 months, and at every subsequent 6-month visit until the participant met an end point or data were censored. As a result of informative censoring of the data on the estimated GFR, shared parameter models20 were used to jointly model the estimated GFR and the event times. The same applied to other secondary outcomes with identity or logit-link functions for trajectories. The effect of the study groups on hospitalizations for any cause and hospitalizations related to cardiovascular disease was tested with the use of a Cox regression analysis for recurrent events.21 Safety outcomes were compared with the use of logistic regression.22
We tested for a priori hypothesized differential treatment effects on the time to disease progression according to age at screening, sex, baseline estimated GFR, and baseline urinary albumin excretion, using tests for interactions. Interim analyses for the primary outcome were to occur when 5%, 20%, and 55% of the study data had been accrued. A Lan–DeMets spending function was used to define O’Brien–Fleming upper boundaries.23 In 2012, the study was extended until July 1, 2014, owing to a lower-than-expected number of end points.
Power was based on the trajectory of the estimated GFR from the Modification of Diet in Renal Disease study.24 We simulated data with an average slope of −4.1 ml per minute per 1.73 m2 per year in the control group as well as standard deviations of the intercept, slope, and noise of 8.57, 0.1956, and 2.1836, respectively. We simulated end points for each participant and compared the 8-year event rates resulting from between-group differences that ranged from 25% to 40%. We estimated that 435 participants would need to be enrolled for the study to detect a difference in outcomes resulting from a 25% reduction in the underlying rate of change in the estimated GFR, with at least 90% power and a significance level of 5%.
RESULTS
PATIENTS
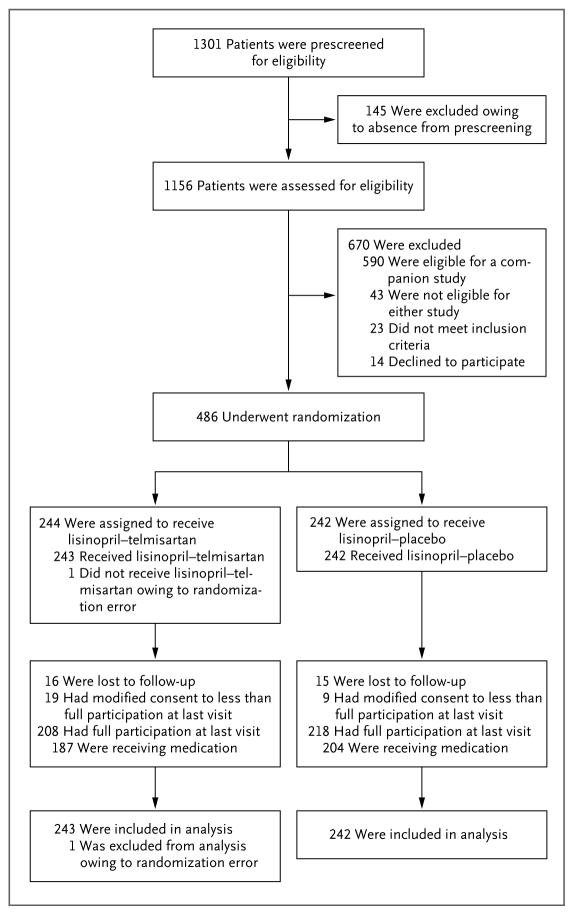
Of 1156 patients assessed for eligibility, 486 underwent randomization: 244 patients were assigned to lisinopril–telmisartan, and 242 to lisinopril–placebo (Fig. 1). Owing to a randomization error, 1 patient in the lisinopril–telmisartan group did not receive the assigned study drug. Demographic and clinical characteristics at baseline were balanced between the two study groups (Table 1, and Tables S2 and S3 in the Supplementary Appendix). Overall, 208 patients (85.2%) assigned to lisinopril–telmisartan and 218 (90.1%) assigned to lisinopril–placebo completed the trial according to the protocol (full participation); 5.8% of the patients discontinued the study medication, reduced the number of study visits or assessments, or both (i.e., modified their consent to less than full study participation), and 6.4% were lost to follow-up (Fig. 1); the average follow-up was 5.2 years. Across the two groups, visit completion rates (the proportion of participants completing a particular visit) ranged from 80 to 100%. For some visits, the rate was as low as 80%, whereas it was as high as 100% for other visits. Treatment adherence was approximately 78% in each treatment group.
Figure 1. Enrollment, Study-Group Assignments, and Follow-up.
We screened 1156 patients, 486 of whom were randomly assigned to receive lisinopril–telmisartan or lisinopril–placebo. Overall, 426 participants completed the trial according to the protocol (i.e., full participation), and 5.8% of the patients discontinued the study medication, reduced the number of study visits or assessments, or both (i.e., modified consent to less than full participation). All but 1 participant who was ineligible for randomization (485 participants) were included in the analysis of the primary outcome.
Table 1.
Demographic, Clinical, and Laboratory Characteristics of the Participants at Baseline.*
| Characteristic | Lisinopril–Telmisartan (N = 244) | Lisinopril–Placebo (N = 242) |
|---|---|---|
| Age — yr | 48.6±8.5 | 48.9±8.1 |
| Male sex — no. (%) | 115 (47.1) | 120 (49.6) |
| Race — no. (%)† | ||
| White | 230 (94.3) | 224 (92.6) |
| Black | 5 (2.0) | 7 (2.9) |
| Other | 9 (3.7) | 11 (4.5) |
| PKD genotype — no./total no. (%)‡ | ||
| PKD1 | 179/223 (80.3) | 183/224 (81.7) |
| PKD2 | 30/223 (13.5) | 30/224 (13.4) |
| No mutation detected | 14/223 (6.3) | 11/224 (4.9) |
| Body-mass index§ | 28.0±4.9 | 28.0±5.5 |
| Serum creatinine — mg/dl¶ | 1.5±0.4 | 1.6±0.4 |
| Estimated GFR — ml/min/1.73 m2|| | 48.5±11.5 | 47.9±12.2 |
| Urinary sodium — mmol/24 hr | 177.4±78.2 | 178.2±84.0 |
| Urinary aldosterone — μg/24 hr | 10.2±8.4 | 9.1±5.8 |
| Urinary albumin — mg/24 hr | ||
| Median | 29.7 | 28.1 |
| Interquartile range | 16.6–71.8 | 17.3–78.0 |
Plus–minus values are means ±SD. There were no significant between-group differences in the baseline characteristics. PKD denotes polycystic kidney disease.
Race was self-reported.
The mutated genes PKD1 and PKD2 encode polycystin-1 and polycystin-2, respectively.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
To convert the values for creatinine to micromoles per liter, multiply by 88.4.
The estimated glomerular filtration rate (GFR) was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration equation.19
BLOOD PRESSURE, HYPERTENSION CONTROL, AND URINARY ALDOSTERONE EXCRETION
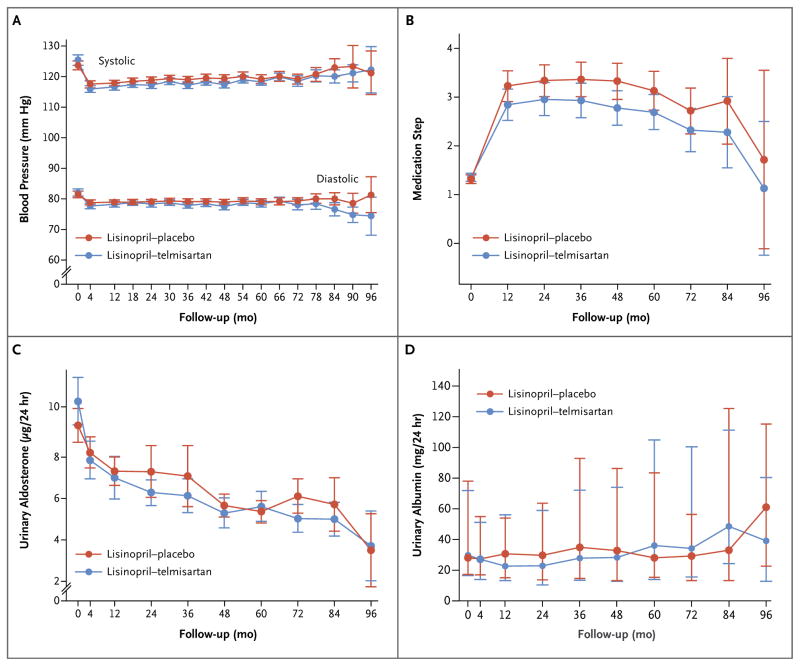
The systolic blood pressures and mean arterial pressures, as assessed at home, were within the target range throughout the trial in 73 to 86% and 70 to 83% of the participants, but fewer participants had diastolic pressures in the target range (56 to 65% of the participants during 72 months of follow-up) (Fig. S1 in the Supplementary Appendix). In most patients, adequate blood-pressure control was achieved with lisinopril–placebo or lisinopril–telmisartan (Tables S3 and S4 in the Supplementary Appendix). As compared with patients treated with lisinopril–telmisartan, those in the lisinopril–placebo group had a higher systolic blood pressure (difference, 1.23 mm Hg; 95% confidence interval [CI], 0.24 to 2.21; P = 0.02) and mean arterial pressure (difference, 0.89 mm Hg; 95% CI, 0.15 to 1.63; P = 0.02), as assessed at home (Fig. 2A). Although trends toward higher steps in the medication-dose adjustments (Fig. 2B, and Table S1 in the Supplementary Appendix) and higher doses of lisinopril (Tables S3, S4, and S5 in the Supplementary Appendix) were not significant, the patients in the lisinopril–placebo group received diuretics and beta-adrenergic blockers or alpha–beta–adrenergic blockers more frequently than did those in the lisinopril–telmisartan group (Table S6 in the Supplementary Appendix).
Figure 2. Blood-Pressure Levels, Medication Steps, and Urinary Aldosterone and Albumin Excretion.
The graphs show the mean systolic and diastolic blood pressures (Panel A), steps in the medication-dose adjustments (Panel B, and Table S1 in the Supplementary Appendix), urinary aldosterone excretion (Panel C), and median urinary albumin excretion (Panel D) in participants treated with lisinopril–telmisartan and those who received lisinopril–placebo. I bars indicate 95% confidence intervals (Panels A, B, and C) or interquartile ranges (Panel D).
In the two study groups, urinary excretion of aldosterone decreased after the baseline visit and remained low throughout the study (P<0.001 for both comparisons) (Fig. 2C). Urinary aldosterone excretion did not differ significantly between the two groups (P = 0.08), and there were no significant between-group differences in the rates of change in urinary albumin excretion (Fig. 2D).
PRIMARY AND SECONDARY OUTCOMES
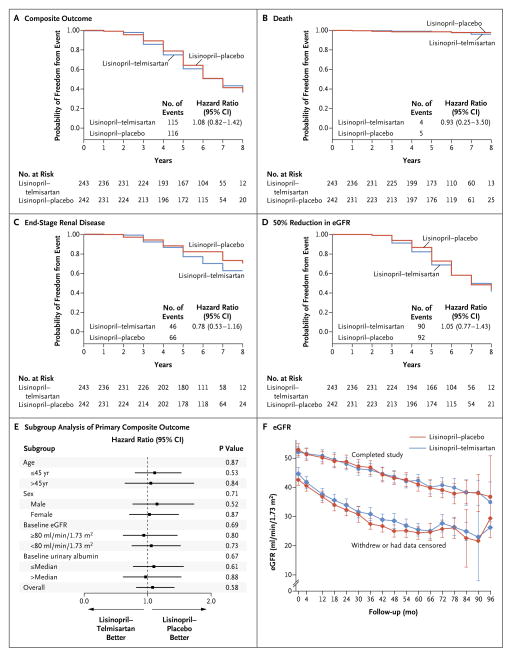
No significant difference in the composite primary outcome of time to death, ESRD, or 50% reduction from the baseline estimated GFR was detected between the two treatment groups (Fig. 3A through 3E). After accounting for informative censoring due to an end point being met or withdrawal from the study, there was no difference in the overall rate of change in the estimated GFR between the lisinopril–placebo group and the lisinopril–telmisartan group (−3.91 ml per minute per 1.73 m2 per year [95% CI, −3.65 to −4.17] and −3.87 ml per minute per 1.73 m2 per year [95% CI, −3.61 to −4.14], respectively) (Fig. 3F, and Tables S7A and S7B in the Supplementary Appendix).
Figure 3. Effect of Lisinopril–Telmisartan, as Compared with Lisinopril–Placebo, on the Time to Primary-Outcome Events and on the Estimated Glomerular Filtration Rate (eGFR).
The graphs show the probability of freedom from the composite outcome (Panel A) and its components: death (Panel B), end-stage renal disease (Panel C), and a 50% reduction from the baseline eGFR (Panel D). Panel E shows the hazard ratios for the primary composite outcome for the lisinopril–telmisartan group, as compared with the lisinopril–placebo group, according to prespecified subgroups. The P values are for the interaction of subgroup by treatment. Horizontal bars indicate 95% confidence intervals. Panel F shows the mean eGFR values at baseline, after 4 months of treatment, and yearly thereafter from 12 to 96 months after the start of treatment for all participants in the two treatment groups who either had an end point or withdrew from the study (diamonds) and for those who completed the study follow-up (circles). I bars represent 95% confidence intervals. The change in the eGFR over time for participants who completed the study follow-up is linear. The appearance of a deceleration in the rate of decline of the eGFR for participants who met an end point or withdrew is probably due to the early attrition of participants with rapid disease progression.
There were no significant differences between the lisinopril–placebo group and the lisinopril–telmisartan group in the rate of hospitalization for any reason (13.75 events per 100 person-years and 10.90 events per 100 person-years, respectively) or hospitalization for cardiovascular disorders (2.30 events per 100 person-years and 1.28 events per 100 person-years, respectively). No significant differences between the treatment groups were detected in the remaining secondary outcomes, including frequency of symptoms related to ADPKD, quality of life, and incidence of pain (Tables S7A and S7B in the Supplementary Appendix).
ADVERSE EVENTS
The frequencies of serious adverse events (Table 2) and of common symptoms (Table S8 in the Supplementary Appendix) were similar in the two treatment groups. Five participants (2.1%) in the lisinopril–placebo group and four (1.6%) in the lisinopril–telmisartan group died during the trial. None of the deaths were thought by the investigators (who were unaware of the study assignments) to be related to the study medications or participation in the trial (with the exception of one death for which relatedness to the study was unknown). Episodes of hyperkalemia or acute kidney injury occurred with similar frequency in the two groups, and most were mild (Table S9 in the Supplementary Appendix). Cancer was diagnosed with similar frequency in the two groups.
Table 2.
Serious Adverse Events.*
| Event | Lisinopril–Telmisartan (N = 244) | Lisinopril–Placebo (N = 242) |
|---|---|---|
| Mean duration of follow-up — yr | 5.2 | 5.2 |
| Death — no. of participants (%)† | 4 (1.6) | 5 (2.1) |
| Cardiac disorder | ||
| No. of events | 12 | 18 |
| No. of participants (%) | 11 (4.5) | 13 (5.4) |
| Coronary artery disease | ||
| No. of events | 3 | 9 |
| No. of participants (%) | 3 (1.2) | 9 (3.7) |
| Arrhythmia | ||
| No. of events | 5 | 6 |
| No. of participants (%) | 4 (1.6) | 3 (1.2) |
| Other | ||
| No. of events | 4 | 3 |
| No. of participants (%) | 4 (1.6) | 3 (1.2) |
| Gastrointestinal disorder | ||
| No. of events | 18 | 33 |
| No. of participants (%) | 15 (6.1) | 25 (10.3) |
| Nervous system disorder | ||
| No. of events | 9 | 10 |
| No. of participants (%) | 8 (3.3) | 9 (3.7) |
| Cerebrovascular event | ||
| No. of events | 4 | 3 |
| No. of participants (%) | 4 (1.6) | 3 (1.2) |
| Headache | ||
| No. of events | 2 | 2 |
| No. of participants (%) | 2 (0.8) | 2 (0.8) |
| Other | ||
| No. of events | 3 | 5 |
| No. of participants (%) | 3 (1.2) | 4 (1.7) |
| Renal or urinary system disorder | ||
| No. of events | 14 | 34 |
| No. of participants (%) | 14 (5.7) | 19 (7.9) |
| Renal hemorrhage or hematuria | ||
| No. of events | 5 | 2 |
| No. of participants (%) | 5 (2.0) | 2 (0.8) |
| Nephrolithiasis or renal colic | ||
| No. of events | 1 | 12 |
| No. of participants (%) | 1 (0.4) | 4 (1.7) |
| Acute kidney injury | ||
| No. of events | 3 | 5 |
| No. of participants (%) | 3 (1.2) | 5 (2.1) |
| Other | ||
| No. of events | 5 | 15 |
| No. of participants (%) | 5 (2.0) | 12 (5.0) |
All serious adverse events were classified with the use of the Common Terminology Criteria for Adverse Events, version 4.0. Patients may have had more than one event in an overall category but were counted only once in the overall-category total.
Causes of death in the lisinopril–telmisartan group were glioblastoma multiforme, sudden death, respiratory failure, and renal failure (in one patient each). Causes of death in the lisinopril–placebo group were metastatic melanoma, cerebral hemorrhage, ruptured aneurysm, pulmonary embolism, and renal failure (in one patient each).
DISCUSSION
The results of this study show that an ACE inhibitor alone or in combination with an ARB was associated with reduced urinary excretion of aldosterone and with adequate blood-pressure control in the majority of patients with ADPKD and stage 3 chronic kidney disease (mean [±SD] estimated GFR, 48.2±11.8 ml per minute per 1.73 m2). The overall rates of change in the estimated GFR in the lisinopril–placebo group and the lisinopril–telmisartan group (−3.91 and −3.87 ml per minute per 1.73 m2 per year, respectively) are similar to the rates of −5.4 and −3.5 ml per minute per 1.73 m2 per year that have been observed in two other studies24,25 involving patients with similar stages of ADPKD.
Although ACE inhibitors have become the first-line therapy for hypertension in patients with chronic kidney disease, including ADPKD, their renoprotective effect may be limited by compensatory-feedback increases in renin release and the generation of angiotensin. Dual RAAS blockade with the combination of an ACE inhibitor and an ARB, a direct renin inhibitor, or an aldosterone antagonist has been proposed as a strategy to circumvent this compensatory feedback.26 This study showed that, as compared with other anti-hypertensive agents that were used more frequently in the lisinopril–placebo group, telmisartan added to lisinopril resulted in slightly lower blood pressures but did not reduce the incidence of primary-outcome events, the rate of decline in the estimated GFR, or the incidence of other secondary outcomes.
Short-term studies of dual RAAS blockade in patients with chronic kidney disease have shown greater reductions in blood pressure and albuminuria but more frequent episodes of hyperkalemia and acute kidney injury, as compared with ACE-inhibitor or ARB monotherapy.27–30 The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), which involved patients with coronary artery, peripheral artery, or cerebrovascular disease or diabetes with end-organ damage and a mean baseline age of 66.4 years, showed that the use of a fixed combination of ramipril (at a dose of 10 mg per day) and telmisartan (at a dose of 80 mg per day) was associated with a higher rate of syncope, a doubling of the serum creatinine level, or greater use of dialysis, as compared with telmisartan monotherapy, without a benefit with respect to the composite end point of fatal and nonfatal cardiovascular events.31,32 Two additional studies of dual RAAS blockade involving patients with mean ages of 64.5 years and 64.6 years who had type 2 diabetes mellitus, cardiovascular disease, or both were terminated early because of a lack of effect on primary cardiovascular or renal outcomes as well as safety concerns (increased risk of hyperkalemia and acute kidney injury).33,34
These results have led to cautionary warnings against the use of dual RAAS blockade in patients with chronic kidney disease. In contrast to the safety findings in these studies, episodes of hyperkalemia and acute kidney injury in our study were infrequent, generally mild, and not more common in the dual-therapy group than in the monotherapy group, probably owing to the younger age of the participants, the exclusion of patients with a high risk of diabetic or cardiovascular complications, and the use of a dose-adjustment protocol for antihypertensive agents that was aimed at achieving a specific blood-pressure target with the use of home blood-pressure values and avoiding symptoms of hypotension.
Both this study and the study by Schrier et al.,15 the results of which are now published in the Journal, and the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 trial,35 are relatively large clinical trials of treatment for ADPKD. The strengths of the present study include a high rate of achieved blood-pressure targets with the use of home monitoring and a long duration of follow-up with a high rate of study completion. A relative weakness is the lack of a treatment group that did not receive a RAAS-blocking agent — a decision made during the design of the trial because the inclusion of such a group was deemed ethically questionable.
In conclusion, this study showed that monotherapy with an ACE inhibitor was sufficient to achieve blood-pressure control in the majority of patients with ADPKD and stage 3 chronic kidney disease. The addition of an ARB did not appear to confer an additional benefit.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK62410 to Dr. Torres, DK62408 to Dr. Chapman, DK62402 to Dr. Schrier, DK082230 to Dr. Moore, DK62411 to Dr. Perrone, and DK62401 to Washington University at St. Louis) and the National Center for Research Resources General Clinical Research Centers (RR000039 to Emory University, RR000585 to the Mayo Clinic, RR000054 to Tufts Medical Center, RR000051 to the University of Colorado, RR023940 to the University of Kansas Medical Center, and RR001032 to Beth Israel Deaconess Medical Center), National Center for Advancing Translational Sciences Clinical and Translational Science Awards (RR025008 and TR000454 to Emory University, RR024150 and TR00135 to the Mayo Clinic, RR025752 and TR001064 to Tufts University, RR025780 and TR001082 to the University of Colorado, RR025758 and TR001102 to Beth Israel Deaconess Medical Center, RR033179 and TR000001 to the University of Kansas Medical Center, and RR024989 and TR000439 to Cleveland Clinic), by funding from the Zell Family Foundation (to the University of Colorado), and by a grant from the PKD Foundation.
We thank the participants for taking part in the study, the research program coordinators and program managers at Washington University (Gigi Flynn and Robin Woltman) and the University of Pittsburgh (Susan Spillane and Patty Smith), the study coordinators at the clinical centers (Sabira Bacchus, Rita Brienza, Sheri Copeland, Elizabeth Courtney, Julie Driggs, Maria Fishman, Michelle Garcia, Diana George, Amy Haun, Carol Horner, Cathy Jackman, Andee Jolley, Pamela Lanza, Barbara Maxwell, Pamela Morgan, Sarah Nicholls, Troy Ofstie, Kristine Otto, Heather Ondler, Sue Saunders, Gertrude Simon, Rita Spirko, Lydia Sweeney, Veronika Testa, and Diane Watkins), and all the other members of the Halt Progression of Polycystic Kidney Disease teams at the study sites, including those who left before the end of the study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Grantham JJ. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–85. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20:1888–93. doi: 10.1681/ASN.2008080882. [DOI] [PubMed] [Google Scholar]
- 3.Graham PC, Lindop GB. The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 1988;33:1084–90. doi: 10.1038/ki.1988.115. [DOI] [PubMed] [Google Scholar]
- 4.Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin–angiotensin–aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–6. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 5.Torres VE, Wilson DM, Burnett JCJ, Jr, Johnson CM, Offord KP. Effect of inhibition of converting enzyme on renal hemodynamics and sodium management in polycystic kidney disease. Mayo Clin Proc. 1991;66:1010–7. doi: 10.1016/s0025-6196(12)61724-8. [DOI] [PubMed] [Google Scholar]
- 6.Torres VE, Donovan KA, Scicli G, et al. Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;42:364–73. doi: 10.1038/ki.1992.297. [DOI] [PubMed] [Google Scholar]
- 7.Loghman-Adham M, Soto CE, Inagami T, Cassis L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2004;287:F775–F788. doi: 10.1152/ajprenal.00370.2003. [DOI] [PubMed] [Google Scholar]
- 8.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414. [Google Scholar]
- 10.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting–enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334:939–45. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 11.Ecder T, Chapman AB, Brosnahan GM, Edelstein CL, Johnson AM, Schrier RW. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:427–32. doi: 10.1016/s0272-6386(00)70195-8. [DOI] [PubMed] [Google Scholar]
- 12.van Dijk MA, Breuning MH, Duiser R, van Es LA, Westendorp RG. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2003;18:2314–20. doi: 10.1093/ndt/gfg417. [DOI] [PubMed] [Google Scholar]
- 13.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–52. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 14.Zeltner R, Poliak R, Stiasny B, Schmieder RE, Schulze BD. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2008;23:573–9. doi: 10.1093/ndt/gfm731. [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW, Abebe KZ, Perrone RD, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. doi: 10.1056/NEJMoa1402685. [DOI] [PubMed] [Google Scholar]
- 16.Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5:102–9. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres VE, Chapman AB, Perrone RD, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–85. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–7. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [Erratum, Ann Intern Med 2008;149:519.] [DOI] [PubMed] [Google Scholar]
- 20.Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Stat Med. 2006;25:143–63. doi: 10.1002/sim.2249. [DOI] [PubMed] [Google Scholar]
- 21.Prentice RL, Williams J, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373–9. [Google Scholar]
- 22.Seber GAF, Wild CJ. Nonlinear regression. New York: John Wiley; 1989. [Google Scholar]
- 23.Demets DL. Group sequential procedures: calendar versus information time. Stat Med. 1989;8:1191–8. doi: 10.1002/sim.4780081003. [DOI] [PubMed] [Google Scholar]
- 24.Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. J Am Soc Nephrol. 1995;5:2037–47. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 25.Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–40. doi: 10.1056/NEJMoa1003491. [Errata, N Engl J Med 2010;363:1190, 1977.] [DOI] [PubMed] [Google Scholar]
- 26.Susantitaphong P, Sewaralthahab K, Balk EM, Eiamong S, Madias NE, Jaber BL. Efficacy and safety of combined vs. single renin-angiotensin-aldosterone system blockade in chronic kidney disease: a meta-analysis. Am J Hypertens. 2013;26:424–41. doi: 10.1093/ajh/hps038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 28.Jennings DL, Kalus JS, Coleman CI, Manierski C, Yee J. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486–93. doi: 10.1111/j.1464-5491.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- 29.MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis. 2006;48:8–20. doi: 10.1053/j.ajkd.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 30.Laverman GD, Navis G, Henning RH, de Jong PE, de Zeeuw D. Dual renin-angiotensin system blockade at optimal doses for proteinuria. Kidney Int. 2002;62:1020–5. doi: 10.1046/j.1523-1755.2002.00536.x. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 32.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multi-centre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 33.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 34.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 35.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.