Abstract
Significant impairment in endothelial-derived nitric oxide is present in the diabetic corpus cavernosum. RhoA/Rho-kinase may suppress endothelial nitric oxide synthase (eNOS). Here, we tested the hypothesis that RhoA/Rho-kinase contributes to diabetes-related erectile dysfunction and down-regulation of eNOS in the streptozotocin (STZ)-diabetic rat penis. Colocalization of Rho-kinase and eNOS protein was present in the endothelium of the corpus cavernosum. RhoA/Rho-kinase protein abundance and MYPT-1 phosphorylation at Thr-696 were elevated in the STZ-diabetic rat penis. In addition, eNOS protein expression, cavernosal constitutive NOS activity, and cGMP levels were reduced in the STZ-diabetic penis. To assess the functional role of RhoA/Rho-kinase in the penis, we evaluated the effects of an adeno-associated virus encoding the dominant-negative RhoA mutant (AAVTCMV19NRhoA) on RhoA/Rho-kinase and eNOS and erectile function in vivo in the STZ-diabetic rat. STZ-diabetic rats transfected with AAVCMVT19NRhoA had a reduction in RhoA/Rho-kinase and MYPT-1 phosphorylation at a time when cavernosal eNOS protein, constitutive NOS activity, and cGMP levels were restored to levels found in the control rats. There was a significant decrease in erectile response to cavernosal nerve stimulation in the STZ-diabetic rat. AAVT19NRhoA gene transfer improved erectile responses in the STZ-diabetic rat to values similar to control. These data demonstrate a previously undescribed mechanism for the down-regulation of penile eNOS in diabetes mediated by activation of the RhoA/Rho-kinase pathway. Importantly, these data imply that inhibition of RhoA/Rho-kinase improves eNOS protein content and activity thus restoring erectile function in diabetes.
Keywords: gene therapy, neuronal NO synthase, endothelium
Relaxation of corporal smooth muscle is essential for normal erectile function, and evidence exists to implicate neuronal- and endothelial-derived nitric oxide (NO) as the principal mediator of corporal smooth muscle relaxation (1-3). Impairments in neurogenic and endothelium-dependent corporal smooth muscle relaxation is observed in diabetes mellitus and is responsible for erectile impairment in diabetic patients (4, 5).
Contraction of smooth muscle is primarily mediated by phosphorylation of the regulatory myosin light chain (MLC) by the Ca2+/calmodulin-dependent activation of MLC kinase and actin/myosin cross-bridge formation (6). Relaxation is mediated by the dephosphorylation of MLC by smooth muscle myosin phosphatase. Recent evidence has established the importance of Ca2+-sensitization through the Ca2+-independent stimulation of MLC kinase or the attenuation of MLC phosphatase activity (7). A principle regulator of MLC phosphatase is the serine/threonine kinase, Rho-kinase. Data from peripheral arteries suggest that RhoA, a GTP-binding protein, mediates agonist-induced activation of Rho-kinase (8). The exchange of GDP for GTP on RhoA and translocation of RhoA from the cytosol to the membrane are markers of activation, and enable the downstream stimulation of Rho-kinase. Recent evidence has established the importance of Rho-kinase activity in the maintenance of corporal vasoconstriction and penile detumescence (9-11).
Endothelial-derived NO is an important mediator of erectile function through its ability to induce vasodilation and to inhibit vasoconstriction (3, 12). The interaction of endothelial-derived NO with RhoA has recently been investigated. The potential role of RhoA in endothelial dysfunction arose from studies using HMG-CoA reductase inhibitors, which lower cholesterol and improve endothelial function by decreasing posttranslational acylation of RhoA GTPase and restore diminished endothelial nitric oxide synthase (eNOS) expression (13). The RhoA/Rho-kinase pathway plays an important role in the suppression of eNOS in endothelial cells, which contributes to decreased NO synthesis, a hallmark feature in diabetes-related erectile dysfunction (ED) (14).
The role of RhoA/Rho-kinase in diabetic penile vascular dysfunction is not completely understood. Rho-kinase protein expression is increased in diabetic corporal tissue of experimental animals, suggesting that the RhoA/Rho-kinase signaling pathway may contribute to diabetes-related ED (15). The aims of our study were to investigate the levels of expression of RhoA/Rho-kinase and eNOS in the penises of diabetic rats, and to examine the effects of adeno-associated viral gene transfer of the dominant-negative RhoA mutant (an inhibitor of RhoA) to the penis to determine the consequence of inhibition of RhoA on corporal Rho-kinase, eNOS expression/activity, cGMP levels, and erectile function in diabetes.
Materials and Methods
Development of Diabetes. Adult male CD rats (300-330 g; Harlan Sprague-Dawley, San Diego) were divided into three groups: (i) age-matched control receiving i.p. injection of citrate buffer (0.1 M citric acid/0.2 M disodium phosphate), (ii) rats receiving i.p. injection of streptozotocin (60 mg/kg; STZ; Sigma) and subsequently transfected with AAVCMVβgal, and (iii) rats receiving i.p. injection of STZ and subsequently transfected with AAVCMVT19NRhoA, an inhibitor of RhoA (11, 16). STZ-diabetic rats received one intracavernosal injection of adeno-associated viruses ≈2 months after i.p. injection of STZ. Total body weight and blood glucose levels were determined before and after i.p. injection of STZ and when the in vivo experiments were conducted (Table 1).
Table 1. Weight (g) and blood glucose levels (mg/dl).
| Control | Diabetic + AAVCMVβgal | Diabetic + AAVCMVT19NRhoA | |
|---|---|---|---|
| Initial weight | 306 ± 9 | 308 ± 4 | 310 ± 8 |
| Final weight | 421 ± 23* | 287 ± 11* | 274 ± 8* |
| Initial blood glucose | 104 ± 3 | 99 ± 6 | 101 ± 8 |
| Final blood glucose | 104 ± 4 | 424 ± 22* | 421 ± 23* |
P < 0.05 value is significantly different when compared to initial values.
In Vivo Gene Delivery to the Corpus Cavernosum. Adeno-associated viruses encoding β-galactosidase (AAVCMVβgal) and T19NRhoA (AAVCMVT19NRhoA; dominant-negative RhoA) were prepared according to standard procedures at Johns Hopkins University (16). Packaging, propagation, and purification of AAV viral particles were carried out by standard procedures (17). Rats were anesthetized with sodium pentobarbital (30 mg/kg i.p.), the penis was exposed, and 20 μl of AAVCMVβgal (1 × 109 parts per ml) or AAVCMVT19NRhoA (1 × 109 parts per ml) was injected into the corpora with a 30-gauge needle (11, 18, 19).
Confocal Microscopy. After fixation of the control rat's penile shaft, the samples were frozen in OCT (BDH), and serial cryosections (20 μm) were prepared. The sections were incubated with PBS (0.1% Triton X-100/5% horse and donkey serum) and subsequently incubated with antibodies against Rho-kinase (ROCK-α or ROCK-2; raised in goat; Santa Cruz Biotechnology, 1:100) and eNOS (NOS3; monoclonal; Becton Dickinson, 1:100) overnight at 4°C followed by detection with FITC-conjugated anti-goat and rhodamine-conjugated anti-mouse secondary antibodies (20). The images were obtained by using a laser-scanning confocal microscope (Leica TCS-DMRE, Germany). No significant fluorescence was observed when the primary antibodies were omitted.
Western Blot Analysis. Corporal tissue from control and AAVCMVβgal and AAVCMVT19NRhoA-transfected STZ-diabetic rats were homogenized (Polytron, Brinkmann Instruments), and cytosolic and membrane fraction were isolated for RhoA, Rho-kinase, MYPT-1 phosphorylation, eNOS, and neuronal NOS (nNOS) Western blot analysis. The membrane fraction was used for RhoA and eNOS protein expression because it contains ≈80% eNOS and activated RhoA (8, 21). Samples were quantified for protein content (Lowry), and equal amounts of protein were run on SDS/PAGE gels, transferred to nitrocellulose membranes, and incubated with monoclonal anti-RhoA, rabbit-anti-Rho-kinase (ROCK-α or ROCK-2), rabbit-anti-phospho-Thr-696-MYPT-1, polyclonal rabbit anti-nNOS, and monoclonal rabbit anti-eNOS primary antibody (Santa Cruz Biotechnology, BD Transduction Laboratories, and Upstate Biotechnology, respectively). Membranes were then incubated with appropriate horseradish peroxidase-linked secondary antibody, and bands were visualized by using enhanced chemiluminescence (Amersham Pharmacia).
Measurement of Cavernosal NOS Activity and cGMP. Cavernosal NOS activity and cGMP levels were measured in control corpora and AAVCMVβgal and AAVCMVT19NRhoA-transfected corporal tissue of STZ-diabetic rats. For the determination of constitutive NOS activity (Calbiochem), l-arginine to L-citrulline conversion, in the presence of Ca2+, was assayed in cavernosal extracts (22). For cGMP levels, cavernosal samples were assayed for cGMP by using an enzyme immunoassay kit (Cayman Chemical; ref. 18).
Measurement of Erectile Responses. Control and STZ-diabetic rats 7 days after adeno-associated virus administration were anesthetized with pentobarbital (30 mg/kg i.p.), and an in vivo experimental erectile protocol was conducted (9, 23). The ratio between the maximal intracavernosal pressure (ICP) and mean arterial pressure (MAP) obtained at the peak of erectile response after cavernous nerve stimulation (CNS) and direct intracavernous injection of Y-27632 (Calbiochem), a selective Rho-kinase inhibitor, was determined to control for variations in MAP. Y-27632 was diluted in 50 μl for infusion into the sinuses over a period of ≈1 min (doses used in this study may inhibit other protein kinases). Tulane Animal Care and Use Committee has approved all procedures.
Statistical Analysis. The data are expressed as mean ± SEM and were analyzed by using a one-way ANOVA with repeated-measures and Neumann-Kuels post hoc test for multiple group comparisons. A P value of <0.05 was used as the criterion for statistical significance.
Results
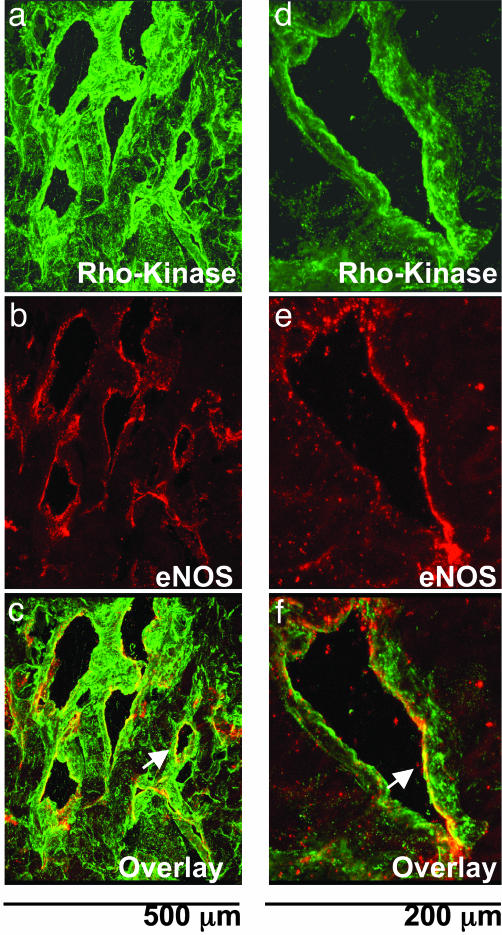
Confocal Microscopic Examination of Rho-Kinase and eNOS. Immunofluorescence for Rho-kinase was found in both corporal smooth muscle fibers and in the endothelium lining the cavernosal sinusoids of control rats (Fig. 1 a and d). eNOS immunofluorescence was predominately found in the endothelium of the corpus cavernosum with little to no immunostaining in the smooth muscle layer (Fig. 1 b and e). Colocalization of Rho-kinase and eNOS as observed by the yellow color of the overlay of Rho-kinase and eNOS images and the coexpression was found in the endothelial lining of the corpus cavernosum, suggesting the presence of these two proteins in the endothelium (Fig. 1 c and f).
Fig. 1.
Colocalization of Rho-kinase and eNOS in the rat corpus cavernosum. Confocal microscopic images of Rho-kinase (a and d; green), eNOS (b and e; red), and an overlay image of Rho-kinase and eNOS(c and f; yellow) in control rat corpus cavernosum demonstrating that Rho-kinase and eNOS are coexpressed in the endothelium (white arrow) are shown. Photomicrograph is representative of three experiments.
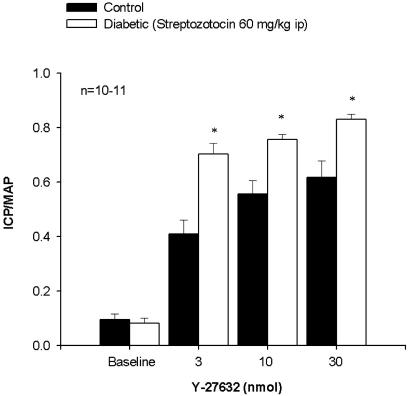
Effect of Y-27632 on Erectile Responses in Control and STZ-Diabetic Rats. Direct intracavernosal injection of the Rho-kinase inhibitor, Y-27632 (3-30 nmol), caused a dose-dependent increase in ICP/MAP in both the control and STZ-diabetic rat (Fig. 2). There was a significantly greater increase in ICP/MAP in response to Y-27632 in the STZ-diabetic rat at all doses studied (Fig. 2). Local administration of Y-27632 into the corpora had no significant effect on MAP (data not shown).
Fig. 2.
In vivo erectile responses to Y-27632 in control and STZ-diabetic rats. Shown is a bar graph demonstrating the increase in ICP in control and STZ-diabetic rats in response to intracavernous injection of the Rho-kinase inhibitor Y-27632 (3-30 nmol). n, number of experiments; *, P < 0.05, response significantly different to control.
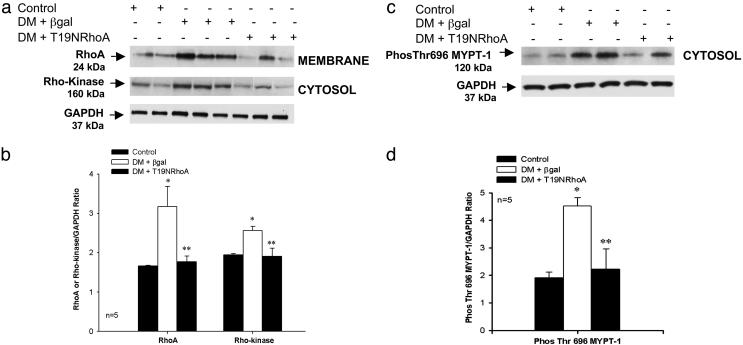
Western Blot Analysis of RhoA, Rho-Kinase, MYPT-1 Phosphorylation, eNOS, and nNOS. Corporal RhoA (membrane) and Rho-kinase (cytosol) protein levels were measured in control and 7 days after intracavernosal administration of A AVCMVβgal or AAVCMVT19NRhoA in STZ-diabetic rats (Fig. 3). Corporal RhoA and Rho-kinase protein levels were significantly higher in the STZ-diabetic rats transfected with AAVCMVβgal (lanes 3-5) when compared to control rats (Fig. 3 a and b, lanes 1 and 2). Seven days after transfection with AAVCMVT19NRhoA, corporal RhoA and Rho-kinase protein levels were significantly reduced in STZ-diabetic rats (Fig. 3 a and b, lanes 6-8) when compared to STZ-diabetic rats transfected with AAVCMVβgal (Fig. 3 a and b, lanes 3-5). When RhoA and Rho-kinase levels were analyzed by densitometry and expressed as the ratio of RhoA or Rho-kinase divided by GAPDH, RhoA and Rho-kinase protein levels were significantly elevated in the STZ-diabetic rats transfected with AAVCMVβgal but lower in AAVCMVT19NRhoA-transfected STZ-diabetic rats (Fig. 3b).
Fig. 3.
Effect of diabetes on corporal RhoA, Rho-kinase, and MYPT-1 protein expression. (a) Western blot analysis demonstrating corporal expression of RhoA, Rho-kinase, and GAPDH protein in control (lanes 1 and 2) and 7 days after gene transfer of AAVCMVβgal (lanes 3-5) or AAVCMVT19NRhoA (lanes 6-8) in STZ-diabetic (DM) rats. (b) Densitometry analysis of RhoA and Rho-kinase protein. (c) Western blot analysis demonstrating corporal expression of Phos-Thr-696 MYPT-1 in control (lanes 1 and 2) and 7 days after gene transfer of AAVCMVβgal (lanes 3 and 4) or AAVCMVT19NRhoA (lanes 5 and 6) in STZ-diabetic rats. (d) Densitometry analysis of Phos-Thr-696 MYPT-1. *, P < 0.05 when compared to control; **, P < 0.05 when compared to STZ-diabetic tissue transfected with AAVCMVβgal.
Rho-kinase phosphorylates the MYPT-1 subunit of MLC phosphatase at various sites. Phosphorylation of the Thr-696 residue of this subunit by Rho-kinase is positively correlated with the inhibition of MLC phosphatase activity and an increase in smooth muscle tone, and serves as a marker for Rho-kinase activity (7). To measure Rho-kinase activity, we measured corporal MYPT-1 phosphorylation (cytosol) by using Western blot analysis in control and STZ-diabetic rats (Fig. 3c). MYPT-1 phosphorylation at Thr-696 was found to be increased in corporal tissue isolated from the STZ-diabetic rats transfected with AAVCMVβgal (Fig. 3 c and d, lanes 3 and 4) when compared to control rats (Fig. 3 c and d, lanes 1 and 2). Seven days after transfection with AAVCMVT19NRhoA, corporal MYPT-1 phosphorylation at Thr-696 was significantly reduced in STZ-diabetic rats (Fig. 3 c and d, lanes 5 and 6) when compared to AAVCMVβgal-transfected STZ-diabetic rats (Fig. 3 c and d, lanes 3 and 4).
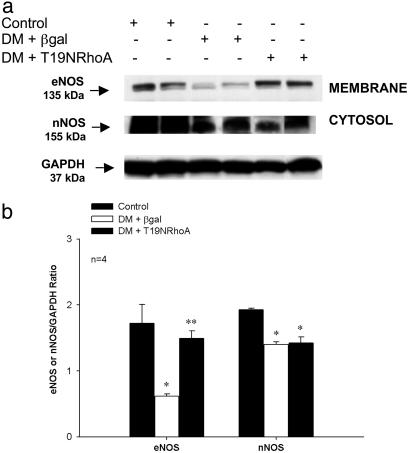
Corporal eNOS and nNOS protein levels were significantly lower in the AAVCMVβgal-transfected STZ-diabetic rats (Fig. 4a, lanes 3 and 4) when compared to control (Fig. 4a, lanes 1 and 2). Seven days after transfection with AAVCMVT19NRhoA, corporal eNOS protein levels were significantly elevated in STZ-diabetic rats (Fig. 4a, lanes 5 and 6) when compared to AAVCMVβgal-transfected STZ-diabetic rats (Fig. 4a, lanes 3 and 4). When nNOS and eNOS levels were analyzed by densitometry, eNOS protein levels were significantly higher in STZ-diabetic rats after transfection with AAVCMVT19NRhoA when compared to eNOS protein levels in AAVCMVβgal-transfected STZ-diabetic rats (Fig. 4b). There was no significant change in corporal nNOS protein expression after AAVCMVT19NRhoA transfection in the STZ-diabetic rat and was still significantly lower than control (Fig. 4b).
Fig. 4.
Effect of diabetes on corporal eNOS and nNOS protein expression. (a) Western blot analysis demonstrating corporal expression of eNOS, nNOS, and GAPDH protein in control (lanes 1 and 2) and 7 days after gene transfer of AAVCMVβgal (lanes 3 and 4) or AAVCMVT19NRhoA (lanes 5 and 6) in STZ-diabetic rats. (b) Densitometry analysis of eNOS and nNOS protein. *, P < 0.05 when compared to control; **, P < 0.05 when compared to STZ-diabetic tissue transfected with AAVCMVβgal.
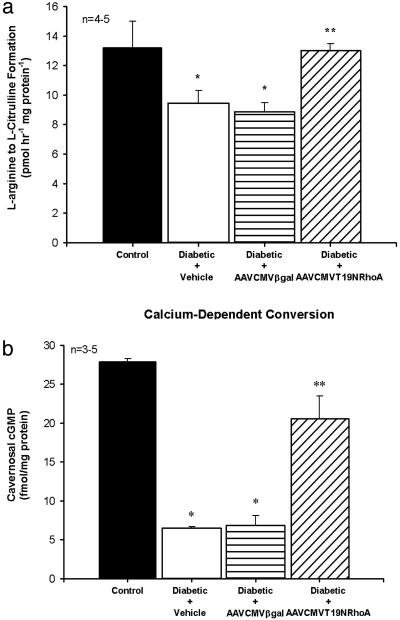
Cavernosal NOS Activity and cGMP Levels. Corporal tissue constitutive NOS activity and cGMP concentrations were measured in control and in STZ-diabetic rats 7 days after treatment with vehicle or transfection with AAVCMVβgal and AAVCMVT19NRhoA. Corporal constitutive NOS activity and cGMP levels were significantly lower in the STZ-diabetic rats treated with vehicle or transfected with AAVCMVβgal when compared to control (Fig. 5). Gene transfer of AAVCMVT19NRhoA to the diabetic penis resulted in corporal constitutive NOS activity and cGMP concentrations that were significantly higher when compared to STZ-diabetic rats treated with vehicle or AAVCMVβgal (Fig. 5).
Fig. 5.
Effect of diabetes on corporal NOS activity and cGMP levels. (a) NOS activity as measured by calcium-dependent conversion of l-arginine to l-citrulline. (b) cGMP levels in control and STZ-diabetic rats corporal tissue after intracavernosal administration of vehicle, AAVCMVβgal, and AAVT19NRhoA. n, number of tissue samples; *, P < 0.05 when compared to control; **, P < 0.05 when compared to STZ-diabetic tissue treated with vehicle or AAVCMVβgal.
Effect of AAVT19NRhoA on Erectile Responses in the Diabetic Rat. Baseline ICP was significantly (P < 0.05) elevated in AAVCMVT19NRhoA-treated (17.3 ± 1.2 mmHg; 1 mmHg = 133 Pa) versus AAVCMVβgal-treated (7.4 ± 0.7 mmHg) and control (9.0 ± 1.1 mmHg) animals; however, baseline MAP did not significantly differ between the three groups (data not shown). These data suggest that RhoA/Rho-kinase decrease corporal perfusion as result of heightened corporal vasoconstrictor tone and that inhibition of RhoA relaxes corporal smooth muscle and increases ICP.
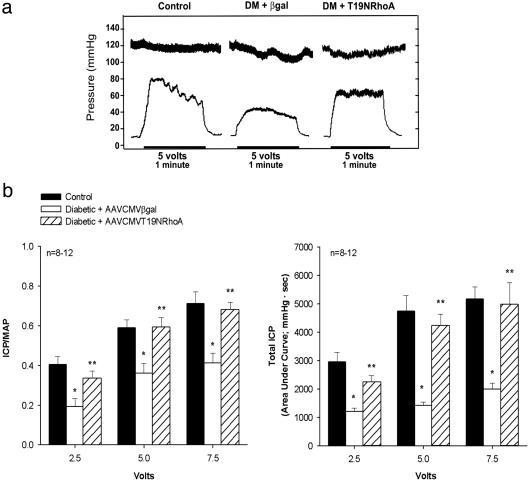
The effect of CNS on erectile function in vivo was measured to evaluate the functional consequence of inhibition of RhoA via adeno-associated viral gene transfer of the dominant-negative RhoA mutant to the corpora of STZ-diabetic rats. Two months after the induction of diabetes with STZ and 7 days after transfection with AAVCMVβgal or AAVCMVT19NRhoA in STZ-diabetic rats, the cavernosal nerve-induced erectile response was measured (Fig. 6). Voltage-dependent cavernosal nerve-induced erectile response [ICP/MAP and total ICP (area of under the erectile curve)] was significantly lower in STZ-diabetic rats transfected with the reporter gene when compared to control rats (Fig. 6). In contrast, the rats transfected with AAVCMVT19NRhoA had significantly greater increases in ICP/MAP and total ICP in response to CNS; this was similar to the response measured in control rats (Fig. 6).
Fig. 6.
Influence of in vivo gene transfer of the dominant-negative RhoA mutant on neurogenic-mediated erectile responses. (a) Representative ICP tracing showing responses to CNS at the 5-V setting for 1 min in control (Left) and STZ-diabetic (DM) rats transfected with AAVCMVβgal (Center) or AAVCMVT19NRhoA (Right). (b) Bar graph depicting the voltage-dependent erectile response (ICP/MAP) and total ICP (area under the erectile curve in mmHg/sec) in response to CNS in control and STZ-diabetic rats transfected with AAVCMVβgal or AAVCMVT19NRhoA. In vivo experiments were conducted 7 days after transfection with adeno-associated viruses. n, number of experiments; *, P < 0.05, response significantly different compared to control; **, P < 0.05, response significantly different compared to STZ-diabetic rats transfected with AAVCMVβgal.
Discussion
In this study, we examined the role of the RhoA/Rho-kinase pathway in the maintenance of penile vascular tone and its role in impairing corporal NO synthesis as observed in STZ-diabetic rats. The results demonstrate that RhoA/Rho-kinase is up-regulated in cavernosal tissue isolated from the penis of STZ-diabetic rats. Moreover, these data identify a physiologic role for RhoA/Rho-kinase in modulating erectile function in vivo by reducing endothelial NOS protein and activity, thus decreasing penile cGMP levels. Additionally, this study demonstrates that the Rho-kinase inhibitor, Y-27632, can induce a greater erectile response when administered intracavernosally to the diabetic rat, suggesting that Rho-kinase activity is increased in the diabetic corpus cavernosum. Confocal microscopy demonstrated that Rho-kinase and eNOS are coexpressed in the endothelial lining of the corpus cavernosum of the rat. Adeno-associated viral gene transfer of the dominant-negative RhoA mutant to the diabetic penis decreased RhoA/Rho-kinase protein and restored erectile function in vivo because of an increase in penile eNOS protein/activity and elevated cGMP levels. Furthermore, these data using gene transfer provide evidence for a role of the RhoA/Rho-kinase signaling pathway in mediating at least a component of diabetes-related endothelial and erectile dysfunction in the corporal vasculature. These findings suggest that RhoA/Rho-kinase may influence erectile function through attenuation of endothelial-derived NO formation in the penis.
The mechanisms governing diabetic erectile failure are dependent on a number of factors, including penile endothelial dysfunction, neuropathy, oxidative stress, and structural changes (12). Such alterations in the corporal vasculature may underlie the high prevalence (>50%) of ED in diabetic men (24). A major factor contributing to diabetic ED is a reduction in the number of nitrergic NOS nerve fibers, NOS activity, and impaired endothelial- and neurogenic-mediated corporal smooth muscle relaxation (4, 20, 22). Studies have demonstrated that chemically induced and genetic diabetic animals (both type 1 and 2) have significant diminishment in penile eNOS/nNOS and cGMP levels at a time when erectile responses are impaired (20, 21, 23, 25). The RhoA/Rho-kinase signaling pathway has been shown to be an important signal transduction mechanism mediating vascular endothelial dysfunction (26). However, little is known about the role of RhoA/Rho-kinase in mediating diabetes-related ED.
Studies in vascular smooth muscle have demonstrated that sustained contraction is G protein-dependent, and involves activation of RhoA and its translocation to the plasma membrane (27). Previous investigations have demonstrated the importance of the calcium-sensitizing enzyme, Rho-kinase, in the maintenance of penile detumescence and vasoconstriction of the corporal vasculature (9). Both Rho-kinase isoforms, Rho-kinase-α and Rho-kinase-β, are found in corporal tissue (15, 28, 29). Direct intracavernous injection of the Rho-kinase inhibitor, Y-27632, results in a sustained increase in ICP and penile erection independent of NO/cGMP, demonstrating the importance of this signaling pathway in the maintenance of the penis in the contracted (flaccid) state (9). Recently, endothelin-1-induced contraction of corporal smooth muscle was shown to be mediated by an up-regulation of Rho-kinase-β protein in alloxan-induced diabetic rabbit corporal tissue, suggesting that increased sensitivity and force generation of corporal smooth muscle is largely dependent on the RhoA/Rho-kinase pathway (15). Additionally, β3 receptor-mediated corporal smooth muscle relaxation involves inhibition of RhoA/Rho-kinase (30). The results of the present study demonstrate that RhoA, Rho-kinase-α, and Rho-kinase activity as determined by phosphorylated Thr-696 residue of Rho-kinase (MYPT-1) are up-regulated in corporal tissue of STZ-diabetic rats. Additionally, agonist-induced erectile responses of Y-27632 are enhanced, suggesting that RhoA/Rho-kinase-mediated Ca2+ sensitization contributes to intracavernous-pressure regulation in vivo and that RhoA/Rho-kinase signaling is augmented in diabetes. These data indicate increased susceptibility of the corporal vasculature in diabetic rats to Rho-kinase inhibition, which may be due to greater responsiveness and binding of the enzyme to Y-2763, or greater resting tone due to increases in Rho-kinase resulting in greater erectile response to Y-27632. An in vitro study examining the effect of Rho-kinase inhibitors on corporal smooth muscle relaxation in control and diabetic mice showed no change in the EC50 values (31). Differing results in the present study may be caused by different experimental settings (in vivo vs. in vitro) and species differences. Because Rho-kinase has been shown to increase phosphorylation of smooth muscle myosin either by deactivating myosin phosphatase or by direct phosphorylation of smooth muscle myosin, an up-regulation of the RhoA/Rho-kinase pathway may lead to heightened corporal smooth muscle tone in diabetes and lead to impaired erectile function.
cGMP/protein kinase G (PKG) and RhoA have counteracting effects in a number of cellular functions, in particular, smooth muscle contraction. Substantial evidence demonstrates that NO activation of cGMP/PKG inhibits RhoA activity by phosphorylation of RhoA at Ser-188, thus inhibiting its membrane translocation and activity (32, 33). Phosphorylation of RhoA reduces its ability to activate Rho-kinase. In this study, eNOS and Rho-kinase were coexpressed in the endothelial lining of corporal sinusoids. In the STZ-diabetic rat, where NO synthesis is impaired and cGMP reduced, RhoA and Rho-kinase activity are increased as a result of reduced phosphorylation of RhoA. Thus, in the diabetic rat where NO production is impaired and Rho-kinase activity increased, Rho-kinase inhibits myosin phosphatase and maintains corporal smooth muscle tone in a more contracted state, leading to ED by two distinct mechanisms. The impaired erectile response in the STZ-diabetic rat is associated with increased expression of RhoA/Rho-kinase at a time when eNOS expression/activity and cGMP levels were reduced. Gene transfer of the dominant-negative RhoA mutant to the diabetic corpora reduced corporal RhoA/Rho-kinase and restored eNOS expression/activity, which resulted in physiologically measurable changes in erectile function. Our findings support the hypothesis that ED related to diabetes is in part mediated through a decrease in NO synthesis and a subsequent increase in RhoA/Rho-kinase, further inhibiting the formation of endothelial-derived NO, corporal smooth muscle relaxation, and penile erection.
In conclusion, the results of the present study provide evidence, at both the molecular and functional level, for a biological role of RhoA/Rho-kinase regulating eNOS expression and function in the diabetic corpora cavernosum. These data suggest a possible mechanism by which diabetic men may develop ED resulting from decreased NO production and up-regulation of the RhoA/Rho-kinase signaling pathway in the corporal vasculature. Inhibition of RhoA/Rho-kinase by adeno-associated viral gene transfer of the dominant-negative RhoA mutant to the diabetic penis enhances penile eNOS expression, constitutive NOS activity, and cGMP levels, thereby restoring endothelial-derived NO vasodilation and erectile function. Hence, RhoA/Rho-kinase may represent a therapeutic target for the treatment of diabetes-related ED.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MLC, myosin light chain; NOS, NO synthase; eNOS, endothelial NOS; nNOS, neuronal NOS; ED, erectile dysfunction; STZ, streptozotocin; MAP, mean arterial pressure; ICP, intracavernosal pressure; CNS, cavernous nerve stimulation.
This work was supported by a Young Investigator Award from the International Society for Sexual and Impotence Research (to T.J.B. and H.C.C.), Juvenile Diabetes Research Foundation International (S.C.), and National Institutes of Health Grant HL62000 (to P.J.K.).
References
- 1.Rajfer, J., Aronson, W. J., Bush, P. A., Dorey, F. J. & Ignarro, L. J. (1992) N. Engl. J. Med. 326, 90-94. [DOI] [PubMed] [Google Scholar]
- 2.Burnett, A. L., Lowenstein, C. J., Bredt, D. S., Chang, T. S. & Snyder, S. H. (1992) Science 257, 401-403. [DOI] [PubMed] [Google Scholar]
- 3.Hurt, K. J., Musicki, B., Palese, M. A., Crone, J. K., Becker, R. E., Moriarity, J. L., Snyder, S. H. & Burnett, A. L. (2002) Proc. Natl. Acad. Sci. USA 99, 4061-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saenz de Tejada, I., Goldstein, I., Azadzoi, K., Krane, R. J. & Cohen, R. A. (1989) N. Engl. J. Med. 320, 1025-1030. [DOI] [PubMed] [Google Scholar]
- 5.Angulo, J., Cuevas, P., Fernandez, A., Gabancho, S., Allona, A., Marti, n-Morales, A., Moncada, I., Videla, S. & Tejada, I. S. (2003) Biochem. Biophys. Res. Commun. 312, 1202-1208. [DOI] [PubMed] [Google Scholar]
- 6.Chacko, S., Conti, M. A. & Adelstein, R. S. (1977) Proc. Natl. Acad. Sci. USA 74, 129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng, J., Ito, M., Ichikawa, K., Isaka, N., Nishikawa, M., Hartshorne, D. J. & Nakano, T. (1999) J. Biol. Chem. 274, 37385-37390. [DOI] [PubMed] [Google Scholar]
- 8.Gong, M. C., Gorenne, I., Read, P., Jia, T., Nakamoto, R. K., Somlyo, A. V. & Somlyo, A. P. (2001) Am. J. Physiol. 281, C257-C269. [DOI] [PubMed] [Google Scholar]
- 9.Chitaley, K., Wingard, C. J., Clinton Webb, R., Branam, H., Stopper, V. S., Lewis, R. W. & Mills, T. M. (2001) Nat. Med. 7, 119-122. [DOI] [PubMed] [Google Scholar]
- 10.Rees, R. W., Ralph, D. J., Royle, M., Moncada, S. & Cellek, S. (2001) Br. J. Pharmacol. 133, 455-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitaley, K., Bivalacqua, T. J., Champion, H. C., Usta, M. F., Hellstrom, W. J., Mills, T. M. & Webb, R. C. (2002) Biochem. Biophys. Res. Commun. 298, 427-432. [DOI] [PubMed] [Google Scholar]
- 12.Bivalacqua, T. J., Usta, M. F., Champion, H. C., Kadowitz, P. J. & Hellstrom, W. J. (2003) J. Androl. 24, Suppl., S17-S37. [DOI] [PubMed] [Google Scholar]
- 13.Laufs, U., Marra, D., Node, K. & Liao, J. K. (1999) J. Biol. Chem. 274, 21926-21931. [DOI] [PubMed] [Google Scholar]
- 14.Ming, X. F., Viswambharan, H., Barandier, C., Ruffieux, J., Kaibuchi, K., Rusconi, S. & Yang, Z. (2002) Mol. Cell. Biol. 22, 8467-8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, S., Hypolite, J. A., Changolkar, A., Wein, A. J., Chacko, S. & DiSanto, M. E. (2003) Int. J. Impot. Res. 15, 53-62. [DOI] [PubMed] [Google Scholar]
- 16.Qiu, R. G., Chen, J., McCormick, F. & Symons, M. (1995) Proc. Natl. Acad. Sci. USA 92, 11781-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder, R. O., Xiao, X. & Samulski, R. J. (1996) in Current Protocols in Human Genetics, eds. Dracopoli, N. C., Haines, J. L., Krof, B. R., Moir, D., Morton, C., Seidman, C., Seidman, J. & Smith, D. (Wiley, New York), pp. 12.11.11-12.12.23.
- 18.Champion, H. C., Bivalacqua, T. J., Hyman, A. L., Ignarro, L. J., Hellstrom, W. J. & Kadowitz, P. J. (1999) Proc. Natl. Acad. Sci. USA 96, 11648-11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bivalacqua, T. J., Armstrong, J. S., Biggerstaff, J., Abdel-Mageed, A. B., Kadowitz, P. J., Hellstrom, W. J. & Champion, H. C. (2003) Am. J. Physiol. 284, H1408-H1421. [DOI] [PubMed] [Google Scholar]
- 20.Cellek, S., Foxwell, N. A. & Moncada, S. (2003) Diabetes 52, 2353-2362. [DOI] [PubMed] [Google Scholar]
- 21.Akingba, A. G. & Burnett, A. L. (2001) Mol. Urol. 5, 189-197. [DOI] [PubMed] [Google Scholar]
- 22.Bivalacqua, T. J., Hellstrom, W. J., Kadowitz, P. J. & Champion, H. C. (2001) Biochem. Biophys. Res. Commun. 283, 923-927. [DOI] [PubMed] [Google Scholar]
- 23.Bivalacqua, T. J., Usta, M. F., Champion, H. C., Adams, D., Namara, D. B., Abdel-Mageed, A. B., Kadowitz, P. J. & Hellstrom, W. J. (2003) J. Urol. 169, 1911-1917. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, R. W. (2001) Urol. Clin. North Am. 28, 209-216. [DOI] [PubMed] [Google Scholar]
- 25.Jesmin, S., Sakuma, I., Salah-Eldin, A., Nonomura, K., Hattori, Y. & Kitabatake, A. (2003) J. Mol. Endocrinol. 31, 401-418. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa, H. (2002) J. Cardiovasc. Pharmacol. 39, 319-327. [DOI] [PubMed] [Google Scholar]
- 27.Somlyo, A. P. & Somlyo, A. V. (2003) Physiol. Rev. 83, 1325-1358. [DOI] [PubMed] [Google Scholar]
- 28.Wang, H., Eto, M., Steers, W. D., Somlyo, A. P. & Somlyo, A. V. (2002) J. Biol. Chem. 277, 30614-30621. [DOI] [PubMed] [Google Scholar]
- 29.Rees, R. W., Ziessen, T., Ralph, D. J., Kell, P., Moncada, S. & Cellek, S. (2002) Int. J. Impot. Res. 14, 1-7. [DOI] [PubMed] [Google Scholar]
- 30.Cirino, G., Sorrentino, R., Bianca, R., Popolo, A., Palmieri, A., Imbimbo, C., Fusco, F., Long, N., Tajana, G., Ignarro, L. J. & Mirone, V. (2003) Proc. Natl. Acad. Sci. USA 100, 5531-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buyukafsar, K. & Un, I. (2003) Eur. J. Pharmacol. 472, 235-238. [DOI] [PubMed] [Google Scholar]
- 32.Sawada, N., Itoh, H., Yamashita, J., Doi, K., Inoue, M., Masatsugu, K., Fukunaga, Y., Sakaguchi, S., Sone, M., Yamahara, K., et al. (2001) Biochem. Biophys. Res. Commun. 280, 798-805. [DOI] [PubMed] [Google Scholar]
- 33.Sauzeau, V., Le Jeune, H., Cario-Toumaniantz, C., Smolenski, A., Lohmann, S. M., Bertoglio, J., Chardin, P., Pacaud, P. & Loirand, G. (2000) J. Biol. Chem. 275, 21722-21729. [DOI] [PubMed] [Google Scholar]