Abstract
Oxygen deprivation is a major cause of cellular damage and death. Here we demonstrate that Caenorhabditis elegans embryos, which can survive both in anoxia (<0.001 kPa O2) by entering into suspended animation and in mild hypoxia (0.25-1 kPa O2) through a hypoxia-inducible factor 1-mediated response, cannot survive in intermediate concentrations of oxygen, between 0.01 and 0.1 kPa O2. Moreover, we show that carbon monoxide can protect C. elegans embryos against hypoxic damage in this sensitive range. Carbon monoxide can also rescue the hypoxia-sensitive mutant hif-1(ia04) from lethality in hypoxia. This work defines the oxygen tensions over which hypoxic damage occurs in C. elegans embryos and demonstrates that carbon monoxide can prevent this damage by inducing suspended animation.
Oxygen metabolism is a fundamental requirement for life in aerobic metazoans. Aerobic respiration accounts for the vast majority of energy production in most animals and also serves to maintain the redox potential necessary to carry out important cellular reactions. In hypoxia, decreased oxygen availability results in inefficient transfer of electrons to molecular oxygen in the final step of the electron transport chain. This inefficiency results in both a decrease in aerobic energy production and an increase in the production of damaging free radicals, mainly caused by the premature release of electrons at complex III and the formation of 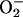 by cytochrome oxidase (1). Limited energy supplies and free radical damage can interfere with essential cellular processes, such as protein synthesis and maintenance of membrane potentials (2), and will ultimately lead to cell death.
by cytochrome oxidase (1). Limited energy supplies and free radical damage can interfere with essential cellular processes, such as protein synthesis and maintenance of membrane potentials (2), and will ultimately lead to cell death.
Hypoxia is a common natural stress, and several well conserved responses exist that facilitate cellular adaptation to hypoxic environments. To compensate for the decrease in the capacity for aerobic energy production in hypoxia, the cell must either increase anaerobic energy production or decrease energy demand (2). Examples of both of these responses are common in metazoans, and the particular response used depends, in general, on the amount of oxygen available to the cell.
In mild hypoxia, oxidative phosphorylation is still partially active, so some aerobic energy production is possible. The cellular response to this situation, which is mediated in part by hypoxia-inducible transcription factor 1 (HIF-1), is to supplement the reduced aerobic energy production by up-regulating genes involved in anaerobic energy production, such as glycolytic enzymes and glucose transporters (3, 4). This response also promotes the up-regulation of antioxidants such as catalase and superoxide dismutase, which guard against free radical-induced damage. As a result, the cell is able to maintain near normoxic levels of activity in mild hypoxia.
In an extreme form of hypoxia, which we call anoxia and define here as <0.001 kPa of O2, oxidative phosphorylation ceases and thus the capacity to generate energy is drastically reduced. To survive in this environment, the cell must decrease energy demand by reducing cellular activity (5). For example, in turtle hepatocytes deprived of oxygen, a directed effort by the cell to limit activities such as protein synthesis, ion channel activity, and anabolic pathways results in a 94% reduction in demand for ATP (2). In zebrafish (Danio rerio) embryos, exposure to anoxia leads to a complete arrest of the heartbeat, movement, cell cycle progression, and developmental progression (6). Similarly, C. elegans responds to anoxia by entering into suspended animation, in which all observable movement, including cell division and developmental progression, ceases (7, 8). C. elegans can remain suspended for 24 h or more and, upon return to normoxia, will recover with high viability. This response allows C. elegans to survive the hypoxic stress by reducing the rate of energetically expensive processes and preventing the occurrence of damaging, irrevocable events such as aneuploidy (7, 9).
One recently discovered response is the hypoxia-induced generation of carbon monoxide by heme oxygenase 1 (10). Endogenously produced carbon monoxide can activate signaling cascades that mitigate hypoxic damage through antiapoptotic (11) and antiinflammatory (12) activity, and similar cytoprotective effects can be achieved in transplant models by perfusion with exogenous carbon monoxide (13, 14). At higher concentrations, carbon monoxide competes with oxygen for binding to iron-containing proteins, such as mitochondrial cytochromes (15), although the cytoprotective effect that this activity may have in hypoxia has not been investigated.
Despite the existence of these sophisticated defense mechanisms that protect against hypoxic damage, hypoxia is still often a damaging stress. For example, mammals have both heme oxygenase 1 and HIF-1, and some evidence suggests that suspended animation is possible in mammals as well (16, 17). Yet hypoxic damage caused by trauma such as heart attack, stroke, or blood loss is a major cause of death. Our understanding of the limitations of the two fundamental strategies for surviving hypoxic stress, remaining animated or suspending animation, is hampered by the fact that it has been based on studies in a variety of systems under a variety of conditions. To better examine the relationship between these responses, we used C. elegans, which has both the ability to adapt to mild hypoxia through a HIF-1-mediated response and the ability to enter into suspended animation in response to anoxia. We found that the ranges of hypoxia that are protected by the HIF-1-mediated response and the suspended animation response do not overlap; there is an intermediate range of oxygen tensions, between the ranges protected by each response, that is lethal to C. elegans embryos. We also demonstrate that high concentrations of carbon monoxide can protect against hypoxia-induced lethality, both in wild-type C. elegans in the presence of this intermediate range of hypoxia and in animals lacking a functional HIF-1 response in the presence of mild hypoxia by inducing suspended animation.
Materials and Methods
Environmental Chamber and Apparatus. Oxygen deprivation experiments were carried out by using a custom atmospheric chamber designed by W. Van Voorhies (New Mexico State University, Las Cruces) (8). The chamber is a 30-ml glass syringe (Fisher no. 14-825-10B) fitted with a custom steel stopper that is lined with two Viton O-rings to ensure a tight seal. The stopper is bored through and has a steel lure lock on the exterior face so that a hose carrying compressed gas can be attached. A defined gas mixture is delivered to the chamber at a constant pressure and flow rate from compressed tanks by passing first through a rotometer (flow-tube number 032-41ST; Aalborg, Aalborg, Denmark) or a mass flow controller (no. 810, Sierra Instruments, Monterey, CA) to monitor flow rate and then through a 500-ml gas washing bottle (Fisher no. K28220-5001) containing 250 ml of water to hydrate the gas. One-quarter-inch OD nylon (Cole-Palmer no. P-06489-06) or FEP (a fluoropolymer) (Cole-Palmer no. A-06450-05) tubing was used, and connections between tubing and the regulators and between the tubing and the rotometers were made with brass John-Guest-type fittings (Byrne Gas, Seattle). All other connections were made with either microflow quick-connect fittings (Cole-Palmer no. A-06363-57, no. A-06363-52) or standard lure fittings (Cole-Palmer no. A-06359-37 and no. A-06359-17).
Viability of Nematodes in Hypoxia (Fig. 1). Bristol strain N2 was continuously maintained at 20°C with care taken to ensure that the population did not starve. Log-phase, adult C. elegans were picked into a drop of sterile water containing 100 μg/ml ampicillin, 15 μg/ml tetracycline, and 200 μg/ml streptomycin on a glass plate. Adults were chopped with a razor blade, and two-cell embryos were picked with a mouth pipet. Thirty to 60 two-cell embryos were transferred to a small glass boat (custom-made to fit atmospheric chambers; Avalon Glass Works, Seattle) filled with 3 ml of 1% agarose in M9. Boats were placed into a humid chamber for 2 h to allow the embryos to age and then placed into the environmental chamber. The environmental chambers were continuously perfused at room temperature with pure N2 (grade 4.5), 100 ppm O2/N2, 500 ppm O2/N2, 1,000 ppm O2/N2, or 5,000 ppm O2/N2 at 70 ml/min for 24 h. After exposure, agarose chunks containing the embryos were cut out of the boat and placed with embryos facing up onto a medium-sized nematode growth medium plate seeded with Escherichia coli (OP50). Embryos were scored for hatching 24 h after exposure, and hatched L1s were transferred to the surface of the nematode growth medium plate and followed to adulthood. Animals that could not be accounted for were not included in the total. All gases were supplied by Byrne Gas. The pure N2 was guaranteed to contain <10 ppm impurities, and all O2/N2 mixtures were certified to ±2% of the O2 content (e.g., 100 ppm O2/N2 was certified to contain between 98 and 102 ppm O2). Parts per million to kPa conversion was based on 1 million parts = 101 kPa at 1 atmosphere.
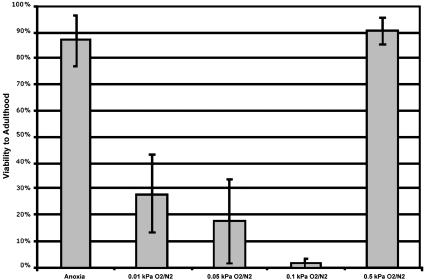
Fig. 1.
Discontinuity of survivability in hypoxia. Viabilities to adulthood were assayed after 24 h of anoxia (pure N2), intermediate hypoxia (0.01, 0.05, or 0.1 kPa O2), or mild hypoxia (0.5 kPa O2) in wild-type embryos. All data points are the result of at least three independent experiments, and worms that could not be accounted for were not included in the total.
Viability of Nematodes in Carbon Monoxide-Based Atmospheres. Thirty to 60 embryos were harvested from continuously maintained Bristol N2 and hif-2(ia04) strains as described above. Environmental chambers were continuously perfused at room temperature with pure CO (grade chemically pure) or 500 ppm O2/CO at 70 ml/min for 24 h. To achieve 2,500 ppm O2/CO or 2,500 ppm O2/N2, 5,000 ppm O2/N2 was mixed at a 1:1 ratio with either pure CO or pure N2 by using two mass flow controllers (Sierra Instruments no. 810) to precisely monitor flow. Each gas was delivered into a three-way valve (Cole-Palmer no. A-30600-23) at 50 ml/min, and the resulting mixture was then passed through a gas washing bottle and into an environmental chamber throughout the 24-h exposure. All gases were supplied by Byrne Gas. The 500-ppm O2/CO mixture was certified to ±2% of the oxygen content and contained 7,000 ppm N2 to ensure a consistent O2/CO ratio throughout the use of the tank.
Cell Biological Analysis. To determine the extent of developmental progression in nitrogen-based atmospheres (Figs. 2 and 3 and Table 1), two-cell embryos were exposed to various degrees of hypoxia as described above and were either immediately photographed or photographed after a 12-h recovery period in a humid chamber.
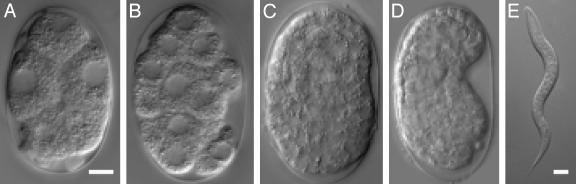
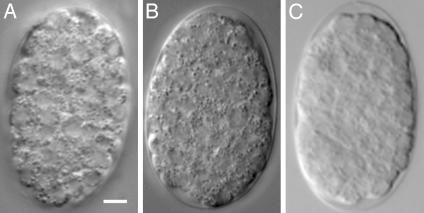
Fig. 2.
Developmental progression in hypoxia. Wild-type two-cell embryos were exposed to anoxia (<0.001 kPa O2) (A), 0.01 kPa O2 (B), 0.05 kPa O2 (C), 0.1 kPa O2 (D), or 0.5 kPa O2 (E) for 24 h and photographed immediately after exposure. White scale bars represent 5 μm.
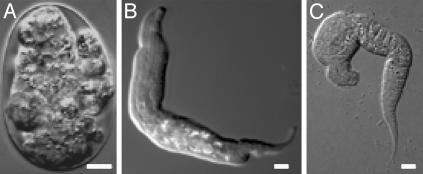
Fig. 3.
Terminal arrest after exposure to lethal oxygen tensions. A representative embryo or larva after a 24-h exposure to 0.01 kPa O2 (A), 0.05 kPa O2 (B), or 0.1 kPa O2 (C) followed by a 12-h recovery in normoxia. White scale bars represent 5 μm.
Table 1. Quantitation of developmental progression in hypoxia.
| Atmosphere | Embryos within range ± SD, % | Range of embryogenesis, min after two-cell stage | n |
|---|---|---|---|
| >0.001 kPa O2/N2 | 100 ± 0.0 | 20-40 min | 35 |
| 0.01 kPa O2/N2 | 92.9 ± 6.0 | 40-80 min | 115 |
| 0.05 kPa O2/N2 | 97.7 ± 2.0 | 100-140 min | 108 |
| 0.1 kPa O2/N2 | 91.4 ± 1.3 | 300-340 min | 60 |
To determine whether embryos arrested in carbon monoxide-based atmospheres (Fig. 3), two-cell embryos were aged in room air for 2 h and were either photographed immediately or put into 100% carbon monoxide or 0.05 kPa O2/CO for 24 h and photographed immediately after the exposure.
In all cases, differential interference contrast microscopy was done by placing embryos under a coverslip on a thin 1% agarose pad and viewing on a Zeiss Axioskop. Photographs were taken with rs image and photoshop (Adobe Systems, San Jose, CA) software.
Results
HIF-1 has been reported to be required in C. elegans in mild hypoxia [0.5 kPa O2 (7) and 1 kPa O2 (18)], and suspended animation is known to be possible in anoxia (<0.001 kPa O2) (7). To precisely define the ranges in which each of these responses are active, we determined the viability of wild-type C. elegans embryos after exposure to various oxygen tensions between mild hypoxia and anoxia for 24 h. Embryos exposed to anoxia entered suspended animation as previously reported and thus survived the exposure with high viability. Embryos in 0.5 kPa O2 remained animated throughout the exposure and also survived with high viability. However, we found to our surprise that embryos exposed to an intermediate range of oxygen tensions between mild hypoxia and anoxia (0.1 to 0.01 kPa O2) did not survive (Fig. 1).
Embryos did not hatch during exposure to this intermediate range of hypoxia, indicating that they did not successfully execute the HIF-1-mediated response. To determine whether they appeared suspended, we examined the possibility that embryos in this intermediate range arrested embryogenesis during the exposure. We found that embryos in lethal oxygen tensions did not arrest embryogenesis and that increased amounts of oxygen correlated with an increase in the extent of developmental progression in the embryo (Fig. 2 and Table 1). Upon reoxygenation, the majority of these embryos failed to hatch, and many of those that did hatch arrested as abnormal L1s (Fig. 3). These data show that this intermediate range of hypoxia is a unique stress in which oxygen levels are neither sufficiently high to facilitate continued animation nor sufficiently low to induce suspended animation.
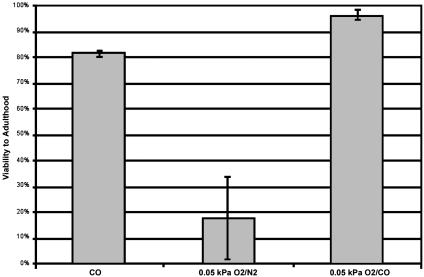
Based on these findings, we hypothesized that if carbon monoxide, a competitive inhibitor of oxygen binding, could induce suspended animation in the presence of low levels of oxygen, it would provide protection against this lethal range of hypoxia. To examine this possibility, we first determined the viability of C. elegans embryos in various concentrations of carbon monoxide. We found that, despite the toxic effects that high levels of carbon monoxide can have in some systems, C. elegans embryos are remarkably tolerant to a wide range of carbon monoxide tensions (data not shown). In fact, C. elegans embryos can withstand a continuous exposure to 101 kPa CO (100% CO) for 24 h with high viability (81.5% survival to adulthood; Fig. 5). Notably, in 101 kPa CO, embryos did not progress through embryogenesis during the exposure, indicating that they entered into suspended animation (Fig. 4 A and B). To test whether carbon monoxide could protect embryos in the presence of lethal oxygen tensions, we determined the viability of embryos exposed to 0.05 kPa O2 balanced with carbon monoxide. We found that, in contrast to embryos exposed to 0.05 kPa O2 balanced with N2 (most of which do not survive), these embryos recovered with 96.2% viability to adulthood (Fig. 5). Moreover, like embryos treated with 101 kPa CO, embryos in 0.05 kPa O2 balanced with carbon monoxide arrested embryogenesis, indicating that they entered into suspended animation (Fig. 4C). Therefore, carbon monoxide can protect against hypoxic damage in the presence of lethal oxygen tensions by inducing suspended animation.
Fig. 5.
Carbon monoxide protects against hypoxia. Viabilities to adulthood were assayed after exposure to 24 h of pure carbon monoxide, 0.05 kPa O2/N2, or 0.05 kPa O2/CO in wild-type embryos. All data points are the result of at least three independent experiments, and worms that could not be accounted for were not included in the total.
Fig. 4.
Carbon monoxide induces suspended animation. Shown is a representative embryo before exposure (A), after a 24-h exposure to pure carbon monoxide (B), and after a 24-h exposure to 0.05 kPa O2/CO (C). The white scale bar represents 5 μm.
To further examine the range of oxygen tensions that can be protected by excess carbon monoxide, we used embryos lacking HIF-1 function (the hif-1(ia04) strain) to ask whether protection against hypoxic damage was also possible in mild hypoxia. After testing various oxygen tensions between 0.1 and 1 kPa O2 balanced with nitrogen, we found that the maximal requirement for HIF-1 was in 0.25 kPa O2 balanced with nitrogen. In this atmosphere, wild-type embryos progress normally through development and exhibit high viability, but hif-1(ia04) embryos do not complete embryogenesis and exhibit 100% lethality (Table 2). We therefore examined whether carbon monoxide could protect hif-1(ia04) embryos in 0.25 kPa O2. In 0.25 kPa O2 balanced with carbon monoxide, both wild-type and hif-1(ia04) embryos entered into suspended animation and survived the exposure with high viabilities (78.7% and 84.0% survival to adulthood, respectively) (Table 2). Thus, the induction of suspended animation by carbon monoxide is possible at oxygen tensions as high as 0.25 kPa O2, and carbon monoxide can protect against mild hypoxia, even in the absence of HIF-1 function.
Table 2. Carbon monoxide protects hif-1 embryos against mild hypoxia.
| Survival ± SD, % (n)
|
||
|---|---|---|
| Atmosphere | Wild type | hif-1 (ia04) |
| 0.25 kPa O2N2 | 94.2 ± 1.2 (49) | 0.0 ± 0.0 (68) |
| 0.25 kPa O2/CO | 78.7 ± 21.9 (109) | 83.9 ± 13.8 (108) |
Discussion
Both remaining animated during hypoxic exposure through the aid of HIF-1 and arresting by means of the suspended animation response are independent, effective ways of protecting against hypoxic damage at particular oxygen tensions. We report here that in C. elegans, a system in which both responses exist, hypoxia can still be lethal, because the ranges of oxygen tensions over which each response provides protection do not overlap. Indeed, a 10-fold range of intermediate oxygen tensions exists in which there is no effective protection mechanism. In several species, oxygen becomes significantly limiting for respiration between 0.005 and 0.15 kPa O2 (19), which is remarkably similar to the lethal range for C. elegans embryos (0.01-0.1 kPa O2). Thus, our results support a model in which hypoxic damage stems from the consequences of inefficient mitochondrial activity, such as limited energy supplies and free radical formation. Although we might expect such mitochondrial inefficiencies to be detrimental across all of the ranges of hypoxia tested, perhaps because the inhibition of mitochondrial activity in mild hypoxia is relatively minor, the HIF-1-mediated response can compensate in this range without becoming overwhelmed. Likewise, in anoxia it may be that, although energy availability is drastically reduced, other effects of mitochondrial inefficiency, such as the formation of damaging free radicals, are also reduced because mitochondrial activity is at a minimum. Intermediate levels of hypoxia, however, may be damaging because there is enough oxygen to support some mitochondrial activity but not enough to allow complete, HIF-1-assisted animation.
We have demonstrated that excess carbon monoxide can protect against hypoxic damage by inducing suspended animation in oxygen tensions as high as 0.25 kPa O2, an oxygen tension that would otherwise promote animation. The mechanism by which the suspended animation response is activated is not clear, although it most likely involves the sensing of oxygen by an oxygen-binding molecule. High levels of carbon monoxide may induce suspended animation in hypoxia by directly interfering with oxygen sensing through competitive inhibition. It will be interesting to examine whether carbon monoxide accumulates naturally in hypoxic tissues in sufficient concentrations to induce local regions of suspended animation. It will also be interesting to examine the potential therapeutic benefits of administering high levels of carbon monoxide in certain blood-free situations such as organ transplantation.
Several studies have demonstrated that the induction of suspended animation in mammals is possible for a few hours at a time through a procedure in which exsanguination is followed by an aortic flush with cold saline (16, 17). Because these animals are blood-free while in suspension and thus would not be subject to the toxic effects of irreversible carbon monoxide binding to hemoglobin, it is intriguing to consider the possibility that the suspended animation procedure in these mammals might be enhanced by concurrent perfusion with carbon monoxide to ensure that low levels of residual oxygen do not induce damage.
Acknowledgments
We thank W. Van Voorhies for generous technical assistance with environmental chambers and gas manipulations; J. Goldmark, B. Buchwitz, D. Miller, L. B. Roth, and W. Van Voorhies for critical reading of the manuscript; and T. Stiernagle for providing nematode strains. Funding for this work was provided by National Institutes of Health Grant GM48435 (to M.B.R.). T.G.N. is supported by National Research Service Award T32 GM07270.
Abbreviation: HIF-1, hypoxia-inducible factor 1.
References
- 1.Semenza, G. L. (1999) Cell 98, 281-284. [DOI] [PubMed] [Google Scholar]
- 2.Hochachka, P. W., Buck, L. T., Doll, C. J. & Land, S. C. (1996) Proc. Natl. Acad. Sci. USA 93, 9493-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza, G. L. (2001) Cell 107, 1-3. [DOI] [PubMed] [Google Scholar]
- 4.Guillemin, K. & Krasnow, M. A. (1997) Cell 89, 9-12. [DOI] [PubMed] [Google Scholar]
- 5.Hochachka, P. W. & Lutz, P. L. (2001) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 130, 435-459. [DOI] [PubMed] [Google Scholar]
- 6.Padilla, P. A. & Roth, M. B. (2001) Proc. Natl. Acad. Sci. USA 98, 7331-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padilla, P. A., Nystul, T. G., Zager, R. A., Johnson, A. C. & Roth, M. B. (2002) Mol. Biol. Cell 13, 1473-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Voorhies, W. A. & Ward, S. (2000) J. Exp. Biol. 203, 2467-2478. [DOI] [PubMed] [Google Scholar]
- 9.Nystul, T. G., Goldmark, J. P., Padilla, P. A. & Roth, M. B. (2003) Science 302, 1038-1041. [DOI] [PubMed] [Google Scholar]
- 10.Dulak, J. & Jozkowicz, A. (2003) Acta Biochim. Pol. 50, 31-47. [PubMed] [Google Scholar]
- 11.Brouard, S., Otterbein, L. E., Anrather, J., Tobiasch, E., Bach, F. H., Choi, A. M. & Soares, M. P. (2000) J. Exp. Med. 192, 1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otterbein, L. E., Bach, F. H., Alam, J., Soares, M., Tao Lu, H., Wysk, M., Davis, R. J., Flavell, R. A. & Choi, A. M. (2000) Nat. Med. 6, 422-428. [DOI] [PubMed] [Google Scholar]
- 13.Otterbein, L. E., Zuckerbraun, B. S., Haga, M., Liu, F., Song, R., Usheva, A., Stachulak, C., Bodyak, N., Smith, R. N., Csizmadia, E., et al. (2003) Nat. Med. 9, 183-190. [DOI] [PubMed] [Google Scholar]
- 14.Amersi, F., Shen, X. D., Anselmo, D., Melinek, J., Iyer, S., Southard, D. J., Katori, M., Volk, H. D., Busuttil, R. W., Buelow, R. & Kupiec-Weglinski, J. W. (2002) Hepatology 35, 815-823. [DOI] [PubMed] [Google Scholar]
- 15.Gorman, D., Drewry, A., Huang, Y. L. & Sames, C. (2003) Toxicology 187, 25-38. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy, R., Safar, P., Tisherman, S. A., Basford, R., Bruttig, S. P., Capone, A., Dubick, M. A., Ernster, L., Hattler, B. G., Jr., Hochachka, P., et al. (1996) Crit. Care Med. 24, S24-S47. [PubMed] [Google Scholar]
- 17.Alam, H. B., Bowyer, M. W., Koustova, E., Gushchin, V., Anderson, D., Stanton, K., Kreishman, P., Cryer, C. M., Hancock, T. & Rhee, P. (2002) Surgery 132, 278-288. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, H., Guo, R. & Powell-Coffman, J. A. (2001) Proc. Natl. Acad. Sci. USA 98, 7916-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnaiger, E. (2001) Respir. Physiol. 128, 277-297. [DOI] [PubMed] [Google Scholar]