Abstract
Purpose
Targeting a single pathway in pancreatic adenocarcinoma (PC) is unlikely to impact its natural history. We tested the hypothesis that simulataneous targeting of the EGFR and IGF-1R pathways would significantly improve progression free survival (PFS) by abrogating reciprocal signaling that promote drug resistance.
Methods
This was a phase Ib/II study testing cixutumumab, combined with erlotinib and gemcitabine (G) in patients with untreated metastatic PC. The control arm was erlotinib plus G. The primary endpoint was PFS. Eligibility included performance status 0/1 and normal fasting blood glucose. Polymorphisms in genes involved in G metabolism and in EGFR pathway were also studied.
Results
The Phase I results (n=10) established the safety of cixutumumab 6 mg/kg IV/week, erlotinib 100 mg/day orally and G 1000 mg/m2 IV D 1, 8, and 15 of a 28-day cycle. In the RP2 portion (116 eligible patients, median age 63) the median PFS and overall survival (OS) were 3.6 and 6.7 months on the cixutumumab arm, and 3.6 and 7.0 on the control arm. Major grade 3 and 4 toxicities were (cixutumumab/control) elevation of transaminases (12%/6%), fatigue (16%/12%), gastrointestinal (35%/28%), neutropenia (21%/10%), and thrombocytopenia (16%/7%). Grade 3/4 hyperglycemia was seen in 16% of patients on cixutumumab. Grade 3 or 4 skin toxicity was similar in both arms of the study (< 5%). No significant differences in PFS by genotype were seen for any of the polymorphisms.
Conclusion
Adding the IGF-1R inhibitor, cixutumumab to erlotinib and G did not lead to longer PFS or OS in metastatic PC.
INTRODUCTION
Survival of patients with pancreatic adenocarcinoma (PC) remains very poor because of the presence of metastatic disease in the majority of patients at the time of diagnosis.1 Its marked resistance to conventional therapies characterizes the disease and, unfortunately, a number of targeted agents have failed to demonstrate activity in PC patients.
Epidermal growth factor receptor (EGFR) and insulin like growth factor-1 receptor (IGF-1R) mediated signaling have widely been considered attractive targets for anti-cancer therapy.2,3 These pathways regulate cell proliferation, survival, angiogenesis and invasion.4,5,6 Further, there is pre-clinical evidence that aberrations in these pathways play a role in tumor maintenance of PC.7,8 A phase III trial of the tyrosine kinase inhibitor erlotinib added to gemcitabine versus erlotinib alone resulted in an improvement of 12 days in median survival time (6.24 vs. 5.9 months) in favor of erlotinib with a hazard ratio of 0.82 (95% CI, 0.69 to 0.99; P=.038).9 However, in another phase III trial when added to gemcitabine, the anti-EGFR monoclonal antibody cetuximab failed to provide any benefit compared to gemcitabine alone‥10 A recent randomized phase II trial of gemcitabine plus AMG479, a monoclonal antibody which targets the IGF-1R showed improvement in overall survival (hazard ratio of 0.67 [95% CI 0.41–1.04]; P =0.12) when compared to gemcitabine alone.11
Unlike other cancers, PC’s lack the activating mutations in the EGFR that would select patients who may benefit from tyrosine kinase inhibitors.12 There is ample evidence to indicate that blockade of a single receptor tyrosine kinase is insufficient to produce enough inhibition of the downstream signaling to translate into a meaningful clinical benefit. The redundancy and cross talk between signaling pathways is at least partly responsible for the failure of targeted therapies in patients with cancer.13,14
The rationale for this study was pre-clinical studies suggesting that simultaneous targeting of the EGFR and IGF-R pathways resulted in more effective growth inhibition and induction of apoptosis in various cancer cell lines.15–19 Experimental findings suggested that inhibiting either receptor alone resulted in reciprocal activation of the downstream pathways that are shared by both receptors, which may explain resistance to either drug when administered alone. Cixutumumab is a fully human IgG1/λ monoclonal antibody targeting IGF-1R with pre-clinical activity against pancreas cancer.20 The recommended dose of single agent for phase II studies was 6 mg/kg IV Q week. In this study, a phase Ib investigation of a cohort of patient to determine the optimal dose of cixutumumab in combination with erlotinib and gemcitabine was completed prior to the randomized phase II portion of the trial. The primary endpoint of the Phase II part of the trial was progression free survival, with overall survival and objective tumor as secondary endpoints. Polymorphisms in genes involved in gemcitabine metabolism, (ribonucleotide reductase subunit M1, deoxycytidine deaminase) and in EGFR-related pathway (EGF, EGFR, IGF1, FCGR2A/3A, IL-8) were selected for testing to explore any potential predictive or prognostic impact.
PATIENTS AND METHODS
Patients
Patients with metastatic histologically proven adenocarcinoma of the pancreas who were previously not treated with systemic therapy were eligible (ClinicalTrials.gov Identifier: NCT00617708). Patients were to have a Zubrod performance status (PS) of < 1, evaluable or measurable disease, and without major comorbidities that would preclude treatment with study medications. Patients were to have adequate organ function determined by the following parameters: AST/ALT < 2.5 times the upper limit of normal (ULN), bilirubin within the normal range, creatinine < 1.5 mg/dL, neutrophil count < 1,500/mm3, platelet count < 100,000/mm3, and fasting blood glucose within the normal limits. Patients with a history of diabetes mellitus were allowed entry into the study, provided it was well controlled. Patients who had received prior therapy with either gemcitabine or EGFR targeting agents were not eligible. All patients provided signed informed consent in accordance with institutional and federal guidelines.
Treatment
Patients received gemcitabine 1000 mg/m2 intravenously over 30 minutes administered once weekly for 3 weeks out of 4. Erlotinib 100 mg was administered orally once per day continuously. In the phase Ib portion of the study cixutumumab 6 mg/kg (starting dose level) was administered days 1, 8, 15, 22 of each 28 day cycle in addition to the gemcitabine and erlotinib. The starting dose of cixutumumab (6 mg/g) in the combination was determined to be sufficiently safe, and was used in the randomized phase II portion of the study. The doses of cixutumumab were planned to be reduced if sufficient adverse events occurred in the dose levels 1, 2, and 3 of 6 mg/g, 4 mg/kg, and 3 mg/kg, respectively. For the phase II, patients were randomly assigned to receive gemcitabine and erlotinib with or without cixutumumab. Standard antiemetics were used prior to the administration of gemcitabine. A treatment cycle was 28 days. Treatment was continued until disease progression, undue toxicities, or patient refusal.
Statistical Considerations
The primary endpoint for the phase II portion of this trial was progression-free survival (PFS), with overall survival (OS) as a secondary endpoint. Based on a type 1 error of 10% and 90% power, approximately 106 patients were needed to detect an improvement from 2 months to 3.3 months (corresponding to a 1.65 hazard ratio). This sample size also had an approximate 82% power to detect a 1.6 hazard ratio for OS (corresponding to an improvement from median of 6 months to median of 9.6 months). PFS was calculated from date of registration to date of first documentation of progression or symptomatic deterioration (as defined in above), or death due to any cause. Patients last known to be alive and progression free were censored at date of last contact. OS was measured from date of registration to date of death due to any cause. Patients last known to be alive were censored at date of last contact. The log rank test was used for the comparison of treatment arms.
On-Study Evaluations
Patients were evaluated by history and physical examination at baseline and at each clinic visit (approximately at 4-week intervals). Zubrod performance status was determined at each visit. At the beginning of each cycle, patients underwent evaluation of serum biochemistry including blood fasting blood glucose. Cross sectional imaging with either a computerized tomographic (CT) scan or magnetic resonance imaging (MRI) was performed every 8 weeks.
Gemcitabine metabolism and EGFR pathway polymorphisms
Eighty-nine out of 114 eligible patients’ genomic DNA was extracted from peripheral WBC using the QIAamp kit (Qiagen, Valencia, CA, USA). The samples were tested using polymerase chain reaction (PCR) restriction fragment length polymorphism (RFLP) technique. Briefly, forward and reverse primers were used for PCR amplification; PCR products were digested by restriction enzymes (New England Biolab, Ipswich, MA, USA); alleles were separated on 4% NuSieve ethidium bromide stained agarose gel and/or samples were analyzed by direct sequencing.
RESULTS
Patient Characteristics (Table 1)
Table 1.
Characteristics of 116 eligible patients with metastatic pancreatic cancer who were accrued on the phase II portion of the study.
| Cixutumumab Gemcitabine + Erlotinib |
Gemcitabine + Erlotinib | |
|---|---|---|
| N | 57 | 59 |
| Median age | 63 | 64 |
| Females | 60% | 41% |
| ECOG PS 0/1 (%) | 40/60 | 45/55 |
| Liver metastases (%) | 80% | 68% |
| Prior radical pancreatic surgery | 14% | 12% |
| Pre-existing diabetes mellitus | 25% | 32% |
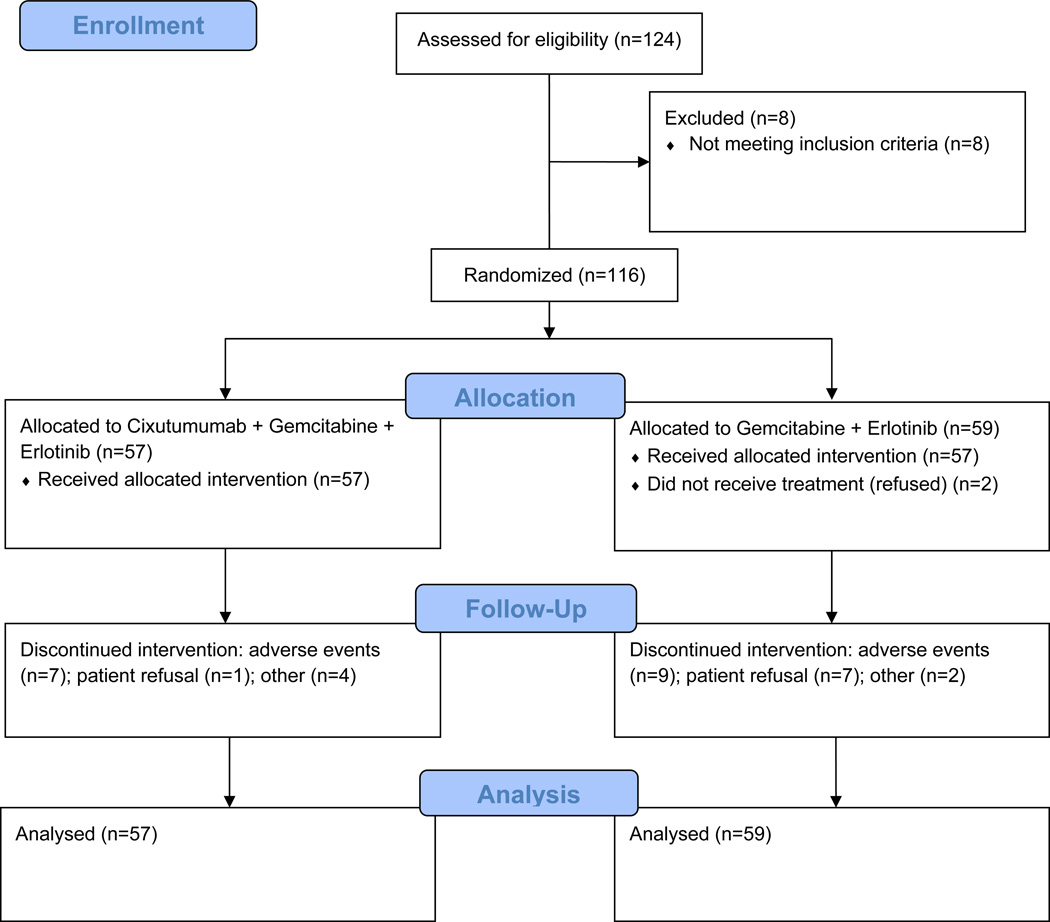
A total of 134 patients with metastatic pancreatic adenocarcinoma were registered on study between March 1, 2008 and September 1, 2010. Ten evaluable patients were accrued onto the phase I b portion of the study. In the randomized phase II part of the study 124 patients were enrolled. Eight patients were considered ineligible due to inadequate hematologic function (2), inadequate coagulation function (2), inadequate hepatic function (2), poor performance status (1) and lack of documented metastatic disease (1). The median age of eligible patients was 63 years. On the cixutumumab arm, 60% of patients were female versus 42% on control arm. Both arms of the phase II study were balanced with respect to known prognostic factors. Two eligible patients on the control arm did not receive protocol treatment, and thus were not evaluable for adverse event assessment. They are included in the efficacy analyses according to the intent-to-treat principle.
Determination of the Recommended Phase II Dose of Cixutumumab
Ten patients were treated in the phase Ib portion of the study. All patients received cixutumumab at a dose of 6 mg/kg (dose level 1) in combination with gemcitabine and erlotinib. There was only one dose limiting toxicity in a patient who experienced an infusion-related allergic reaction. It was therefore decided that the dose of cixutumumab at 6 mg/kg represented a safe dose to proceed with the randomized phase II portion of the study.
Efficacy Parameters
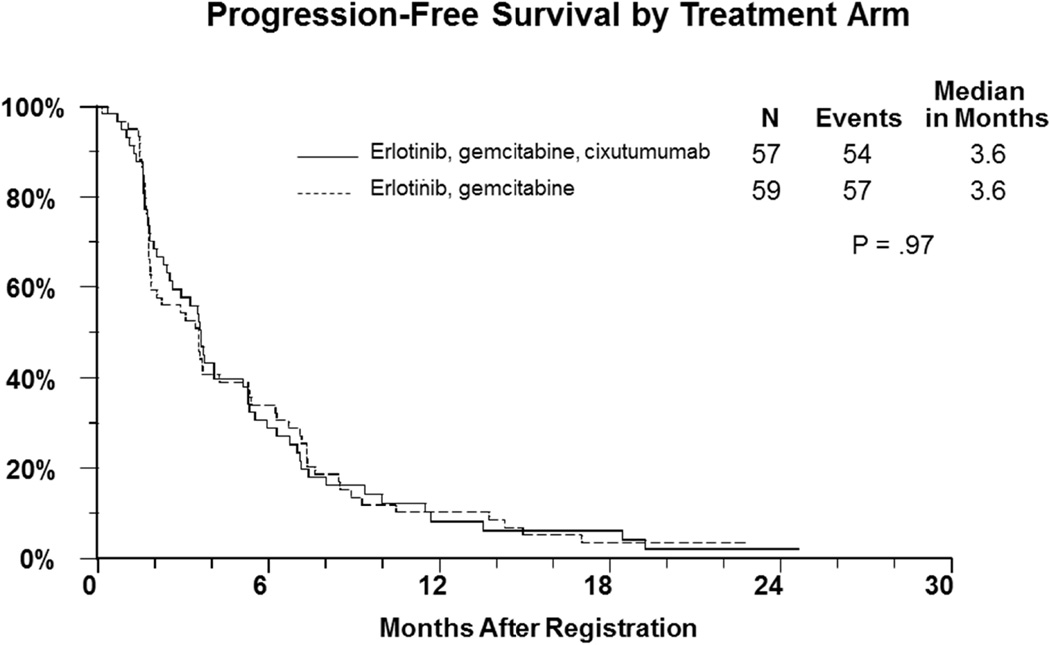
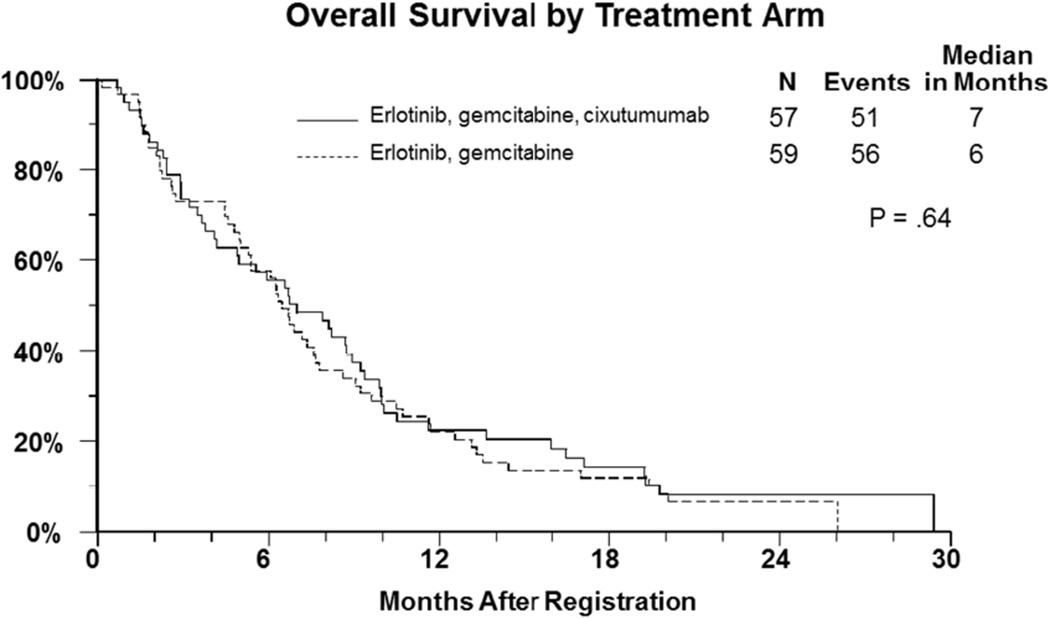
One hundred and sixteen patients who participated in the randomized phase II part of the study were assessed for outcome. The median progression free survival in both arms of the study was 3.6 months (Figure-1) (HR= 1.0, 95% CI: 0.68–1.44, p=0.97). The median overall survival in patients who revived gemcitabine, erlotinib, and cixutumumab was 7 months versus 6.7 months in those who received gemcitabine and erlotinib (HR=1.1, 95% CI=0.75–1.61, p=0.64) (Figure-2). There were 7 objective responses (2 confirmed) in the cixutumumab arm, and 9 (3 confirmed) in the control arm.
Figure 1.
Kaplan Meier curves for progression free survival (primary endpoint) of patients with metastatic pancreatic cancer treated with gemcitabine plus erlotinib with with or without cixutumumab.
Figure 2.
Kaplan Meier curves for overall survival (secondary endpoint) of patients with metastatic pancreatic cancer treated with gemcitabine plus erlotinib with or without cixutumumab.
Toxicity Profile (Table 2)
Table 2.
Selected toxicities (percent) in 114 patients with metastatic pancreatic cancer who participated in the randomized phase II portion of the study. Data presented are in percentages using the CTCAE 4.0.
| Cixutumumab Gemcitabine + Erlotinib |
Gemcitabine + Erlotinib | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | 57 | 57 | ||||||||
| Grade | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Neutropenia | 1.8 | 10.5 | 28 | 8.8 | 0 | 8.8 | 5.3 | 17.5 | 0 | 0 |
| Thrombocytopenia | 26.3 | 21.1 | 24.6 | 3.5 | 0 | 36.8 | 12.3 | 10.5 | 1.8 | 0 |
| Anemia | 22.8 | 31.6 | 14 | 0 | 0 | 21.1 | 24.6 | 14.0 | 0 | 0 |
| Hyperglycemia | 8.8 | 8.8 | 22.8 | 5.3 | 0 | 14 | 3.5 | 0 | 1.8 | 0 |
| Fatigue | 14.0 | 35.1 | 22.8 | 5.3 | 0 | 24.6 | 24.6 | 21.1 | 0 | 0 |
| Nausea | 26.3 | 28 | 15.8 | 0 | 0 | 28 | 15.8 | 10.5 | 0 | 0 |
| Vomiting | 19.3 | 14 | 8.8 | 0 | 0 | 26.3 | 12.3 | 1.8 | 0 | 0 |
| Skin rash | 19.3 | 24.6 | 5.3 | 0 | 0 | 5.3 | 31.6 | 3.5 | 0 | 0 |
| Diarrhea | 33.3 | 15.8 | 5.3 | 0 | 0 | 30 | 7 | 3.5 | 0 | 0 |
| Hypophosphatemia | 0 | 1.8 | 0 | 0 | 0 | 0 | 1.8 | 0 | 0 | 0 |
| ALT | 22.8 | 12.3 | 15.8 | 0 | 0 | 15.8 | 12.3 | 7 | 0 | 0 |
| AST | 21.1 | 21.1 | 10.5 | 0 | 0 | 17.5 | 17.5 | 5.3 | 0 | 0 |
NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 was used for reporting of adverse events. Patients (n = 114) who received any drug medication in the randomized phase II part of the study were eligible for toxicity evaluation. The median number of cycles administered were 3 and 2 in the experimental versus control arms, respectively. The most common adverse events in both arms of the study were elevation of transaminases, fatigue, gastrointestinal and hematologic. In patients who were assigned cixutumumab, the most common grade 3 or 4 toxicity was hyperglycemia (Table 2). Hyperglycemia grades 1–4 was seen in 45% of patients treated with cixutumumab, of which 28% were grade 3 or 4. Nineteen percent of patients on the control arm had hyperglycemia, one patient with grade 4. No patients discontinued from treatment on protocol because of hyperglycemia.
Gemcitabine metabolism and EGFR pathway polymorphisms
Patients in this molecular correlates study were similar to the overall dataset in terms of demographics and outcome. No significant differences in PFS by genotype were seen for any of the 14 SNPs in this dataset. The only nominally significant difference is in IGF1, where among control arm patients the hazard ratio (95% CI) comparing GG vs. AA/AG is 2.00 (1.06,3.78), p=0.033. Among patients on the cixutumumab arm, it is 0.87 (0.44,1.73), p=0.69. This is the only SNP with some hint of interaction with treatment arm, although the evidence is pretty weak, with p=0.066.
DISCUSSION
The demonstration of benefit from systemic therapy in advanced pancreatic cancer remains very challenging. Multiple trials testing conventional cytotoxic drugs and even targeted agents have failed to show a major impact on the natural history of this disease.2 Erlotinib, an oral EGFR kinase inhibitor was associated with marginal benefit when combined with gemcitabine in patients with advanced disease. However, cetuximab, a monoclonal antibody against EGFR failed to demonstrate any benefit in a similar patient population. Collectively, these results suggest that in unselected patients, targeting the EGFR pathway alone has a very small impact in patients with Stage IV pancreatic cancer. Possible explanations for this include the high frequency of oncogenic KRAS mutations present in pancreatic cancer, de novo resistance to anti-EGFR drugs and the absence of activating mutations of the receptor in patients with pancreatic cancer.
Another recognized mechanism of resistance to targeting the EGFR pathway is signaling through the IGF-1R driven pathway and vice versa. Pre-clinical work supports the simultaneous blockade of both receptors to achieve more effective inhibition of cell proliferation and survival by abrogating downstream signaling shared by both receptors.21–27 The effectiveness of dual blockade is thought to be a consequence of the inhibition of reciprocal downstream signaling through PI3K/AKT/mTOR and RAS/RAF/MEK/ERK pathways that occurs when either receptor is individually blocked by a single targeted therapy.
This study failed to demonstrate any benefit with the addition of cixutumumab to the combination of erlotinib and gemcitabine in patients with metastatic adenocarcinoma of the pancreas based on any of the proposed efficacy parameters. Progression free survival, the primary endpoint of this study, was identical in both arms. The dose of cixutumumab was the of maximum tolerated single dose of the drug established in previous phase I studies. There was no increase in toxicity with the addition of the experimental drug that would have reduced exposure to treatment relative to the control arm of the study. In particular, the incidence and severity of hyperglycemia was not dose limiting, with 27% of patients experiencing a grade 3 or 4 toxicity.
A potential explanation for the lack of benefit when targeting either EGFR or IGF-1R remains the high frequency of downstream KRAS mutations in patients with pancreatic cancer. Such mutations result in increased signaling by the KRAS gene product that may not be responsive to blockade of the upstream EGFR or IGF-1R. Given the very low frequency (20% or less) of wild type KRAS genotype in pancreatic cancer, it would be very difficult to test with a reasonable degree of certainty the influence of the KRAS mutation status on the outcome of this study.28 Moreover, the identical outcome of the primary endpoint in the two study arms makes further molecular exploration of archived tumoral material from study participants less likely to produce a subgroup that exhibits a meaningful association between a certain molecular profile and treatment outcome. In this study, the strategy of targeting two cell surface receptor molecules did not demonstrate the validity of such a treatment approach. A possible explanation would be the inability of this strategy to overcome growth and survival promoting signals from downstream mutations involving molecules other than KRAS, such as those in the PI3K/AKT axis.29 Other mechanisms of resistance to the EGFR blockade may include the epithelial to mesenchymal transformation (EMT) and the activation of signaling pathways, such as c-MET driven pathway, that would entail different combination therapies.30–33 The newly activated S1115 study to be conducted by SWOG, will test inhibition of KRAS effector pathways by dual targeting of MEK and Akt in patients with metastatic pancreatic cancer (ClinicalTrials.gov Identifier: NCT01658943).
The effect of single nucleotide polymorphisms of genes associated with gemcitabine metabolism and EGFR-related pathway with clinical outcome were explored in this study34–38. None of the 14 single nucleotide polymorphisms tested was significantly associated with PFS in both treatment arms. A false negative result cannot be excluded given the limited number of patients in the study, which is further limited by stratifying both treatment arms. Further larger biomarker embedded clinical trial needed to confirm the predict/prognostic role of these polymorphisms.
The outcome of this study provides further evidence of the difficulty in successfully developing targeted therapies in patients with pancreatic cancer in the absence of molecular determinants of response and/or resistance to the tested drug(s). At this time, there are no biomarkers that predict benefit from drugs targeting EGFR or IGF-1R pathways and the role of KRAS mutations remains poorly defined.39,40 Therefore, future testing of combinations of targeted agents will require a better selection of targets and must be based on stricter pre-clinical testing in multiple pancreatic cancer tumor models.
Fig. 1.
CONSORT Flow Diagram for Phase II Portion of SWOG S0727
Acknowledgments
SUPPORT: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA35176, CA45808, CA58882, CA35178, CA14028, CA76448, CA45807, CA46441, CA46368, CA27057, CA86780, CA52654, CA073590, CA42777, CA20319, CA76462, CA11083, CA45450, CA46113, CA58861, CA74647, CA45461, CA95860, CA139519, CA76447, CA128567, CA67575, CA22433, CA37981 and CA58723
Footnotes
Results presented in part at the 46th and 48th Annual Meetings of the American Society of Clinical Oncology (ASCO) (June 4–8, 2010 and June 1–5, 2012, Chicago, Illinois), and at the 2010 and 2012 ASCO Gastrointestinal Cancers Symposiums (January 22–24, 2010, Orlando, FL and January 19–21, 2012, San Francisco, CA)
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST:
Consultant or advisory role: Philip A. Philip (self/compensated), Lilly and Genentech
Honoraria: Syma Iqbal (self) from Genentech; Philip A. Philip (self), Lilly and Genentech
Research funding: Syma Iqbal (self) from Genentech; Philip A. Philip (self), Lilly
None: Jacqueline Benedetti, Charles Blanke, Rakesh Gaur, Bryan Goldman, Heinz-Josef Lenz, Andrew Lowy, Ramesh Ramanathan, Takeru Wakatsuki, Robert Whitehead
AUTHOR CONTRIBUTIONS:
Conception and design: Jacqueline Benedetti, Charles Blanke, Bryan Goldman, Heinz-Josef Lenz, Andrew Lowy, Philip A. Philip, Ramesh Ramanathan, Robert Whitehead
Collection and assembly of data: Jacqueline Benedetti, Charles Blanke, Rakesh Gaur, Bryan Goldman, Syma Iqbal, Heinz-Josef Lenz, Philip A. Philip, Robert Whitehead
Data analysis and interpretation: Jacqueline Benedetti, Charles Blanke, Heinz-Josef Lenz, Philip A. Philip, Takeru Wakatsuki
Manuscript writing: Jacqueline Benedetti, Charles Blanke, Rakesh Gaur, Bryan Goldman, Syma Iqbal, Heinz-Josef Lenz, Andrew Lowy, Philip A. Philip, Ramesh Ramanathan, Takeru Wakatsuki, Robert Whitehead
Final approval of manuscript: Jacqueline Benedetti, Charles Blanke, Rakesh Gaur, Bryan Goldman, Syma Iqbal, Heinz-Josef Lenz, Andrew Lowy, Philip A. Philip, Ramesh Ramanathan, Takeru Wakatsuki, Robert Whitehead
Administrative Support: Philip A. Philip
Provision of study material or patients: Heinz-Josef Lenz, Ramesh Ramanathan,
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Philip PA. Novel targets for pancreatic cancer therapy. Surg Oncol Clin N Am. 2010;19(2):419–429. doi: 10.1016/j.soc.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Rieder S, Michalski CW, Friess H, Kleeff J. Insulin-like growth factor signaling as a therapeutic target in pancreatic cancer. Anticancer Agents Med Chem. 2011;11(5):427–433. doi: 10.2174/187152011795677454. [DOI] [PubMed] [Google Scholar]
- 4.Ardito CM, Grüner BM, Takeuchi KK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22(3):304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardito CM, Grüner BM, Takeuchi KK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Res. 2012;72(10):2554–2564. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelber JA, Reno T, Kaushal S, et al. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res. 2012;72(10):2554–2564. doi: 10.1158/0008-5472.CAN-11-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collisson EA, Trejo CL, Silva JM, et al. A Central Role for RAF->MEK->ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2012;2(8):685–693. doi: 10.1158/2159-8290.CD-11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valsecchi ME, McDonald M, Brody JR, et al. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118(14):3484–3493. doi: 10.1002/cncr.26661. [DOI] [PubMed] [Google Scholar]
- 9.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 10.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindler HL, Richards DA, Garbot LE, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834–2842. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Jang KT, Ki CS, et al. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109(8):1561–1569. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 13.Jameson MJ, Beckler AD, Taniguchi LE, et al. Activation of the insulin-like growth factor-1 receptor induces resistance to epidermal growth factor receptor antagonism in head and neck squamous carcinoma cells. Mol Cancer Ther. 2011;10(11):2124–2134. doi: 10.1158/1535-7163.MCT-11-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Veeken J, Oliveira S, Schiffelers RM, et al. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009;(6):748–760. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 15.Tandon R, Kapoor S, Vali S, et al. Dual epidermal growth factor receptor (EGFR)/insulin-like growth factor-1 receptor (IGF-1R) inhibitor: a novel approach for overcoming resistance in anticancer treatment. Eur J Pharmacol. 2011;667(1–3):56–65. doi: 10.1016/j.ejphar.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 16.Qi HW, Shen Z, Fan LH. Combined inhibition of insulin-like growth factor-1 receptor enhances the effects of gefitinib in a human non-small cell lung cancer resistant cell line. Exp Ther Med. 2011;2(6):1091–1095. doi: 10.3892/etm.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurbin A, Wislez M, Busser B, et al. Insulin-like growth factor-1 receptor inhibition overcomes gefitinib resistance in mucinous lung adenocarcinoma. J Pathol. 2011;225(1):83–95. doi: 10.1002/path.2897. [DOI] [PubMed] [Google Scholar]
- 18.Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoël MJ, et al. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res. 2009;15(17):5445–5456. doi: 10.1158/1078-0432.CCR-08-2980. [DOI] [PubMed] [Google Scholar]
- 19.Kaulfuss S, Burfeind P, Gaedcke J, Scharf JG. Dual silencing of insulin-like growth factor-I receptor and epidermal growth factor receptor in colorectal cancer cells is associated with decreased proliferation and enhanced apoptosis. Mol Cancer Ther. 2009;8(4):821–833. doi: 10.1158/1535-7163.MCT-09-0058. [DOI] [PubMed] [Google Scholar]
- 20.Rowinsky EK, Schwartz JD, Zojwalla N, et al. Blockade of insulin-like growth factor type-1 receptor with cixutumumab (IMC-A12): a novel approach to treatment for multiple cancers. Curr Drug Targets. 2011;12(14):2016–2033. doi: 10.2174/138945011798829401. [DOI] [PubMed] [Google Scholar]
- 21.Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signaling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 22.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson RI, Hutcheson IR, Knowlden JM, et al. Nonendocrine pathways and endocrine resistance: observations with antiestrogens and signal transduction inhibitors in combination. Clin Cancer Res. 2004;10:346S–354S. doi: 10.1158/1078-0432.ccr-031206. [DOI] [PubMed] [Google Scholar]
- 24.Camirand A, Zakikhani M, Young F, Pollak M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005;7:R570–R579. doi: 10.1186/bcr1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 26.Jones HE, Gee JM, Taylor KM, et al. Development of strategies for the use of anti-growth factor treatments. Endocr Relat Cancer. 2005;2(Suppl 1):S173–S182. doi: 10.1677/erc.1.01004. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 28.Schultz NA, Roslind A, Christensen IJ, et al. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas. 2012;41(5):759–766. doi: 10.1097/MPA.0b013e31823cd9df. [DOI] [PubMed] [Google Scholar]
- 29.Shin DH, Min HY, El-Naggar AK, et al. Akt/mTOR counteract the antitumor activities of cixutumumab, an anti-insulin-like growth factor I receptor monoclonal antibody. Mol Cancer Ther. 2011;10(12):2437–2448. doi: 10.1158/1535-7163.MCT-11-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung JH, Rho JK, Xu X, et al. Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer. 2011;73(2):176–182. doi: 10.1016/j.lungcan.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Wu JJ, Hynes M, Dosch J, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218–2227. doi: 10.1053/j.gastro.2011.08.009. e5. [DOI] [PubMed] [Google Scholar]
- 33.Bauer TW, Somcio RJ, Fan F, et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther. 2006;5(7):1676–1682. doi: 10.1158/1535-7163.MCT-05-0175. [DOI] [PubMed] [Google Scholar]
- 34.Lurje G, Nagashima F, Zhang W, et al. Polymorphisms in cyclooxygenase-2 and epidermal growth factor receptor are associated with progression-free survival independent of K-ras in metastatic colorectal cancer patients treated with single-agent cetuximab. Clin Cancer Res. 2008;14:7884–7895. doi: 10.1158/1078-0432.CCR-07-5165. [DOI] [PubMed] [Google Scholar]
- 35.Nakahira S, Nakamori S, Tsujie M, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. International journal of cancer. Journal International du Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Park DJ, Lu B, et al. Epidermal growth factor receptor gene polymorphisms predict pelvic recurrence in patients with rectal cancer treated with chemoradiation. Clin Cancer Res. 2005;11:600–605. [PubMed] [Google Scholar]
- 38.Zhang W, Stoehlmacher J, Park DJ, et al. Gene polymorphisms of epidermal growth factor receptor and its downstream effector, interleukin-8, predict oxaliplatin efficacy in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2005;5:124–131. doi: 10.3816/ccc.2005.n.025. [DOI] [PubMed] [Google Scholar]
- 39.da Cunha Santos G, Dhani N, Tu D, et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA 3. Cancer. 2010;116(24):5599–5607. doi: 10.1002/cncr.25393. [DOI] [PubMed] [Google Scholar]
- 40.Ii M, Li H, Adachi Y, et al. The efficacy of IGF-I receptor monoclonal antibody against human gastrointestinal carcinomas is independent of k-ras mutation status. Clin Cancer Res. 2011;17(15):5048–5059. doi: 10.1158/1078-0432.CCR-10-3131. [DOI] [PubMed] [Google Scholar]