Abstract
BACKGROUND
Multi-detector computed tomography (CT) has emerged as a modality for the non-invasive assessment of coronary artery disease (CAD). Prior studies have selected patients for evaluation and have excluded many of the “real-world” patients commonly encountered in daily practice. We compared 64-detector-CT (64-CT) to conventional coronary angiography (CA) to investigate the accuracy of 64-CT in determining significant coronary stenoses in a “real-world” clinical population.
METHODS
A total of 1,818 consecutive patients referred for 64-CT were evaluated. CT angiography was performed using the GE LightSpeed VCT (GE® Healthcare). Forty-one patients in whom 64-CT results prompted CA investigation were further evaluated, and results of the two diagnostic modalities were compared.
RESULTS
A total of 164 coronary arteries and 410 coronary segments were evaluated in 41 patients (30 men, 11 women, age 39–85 years) who were identified by 64-CT to have significant coronary stenoses and who thereafter underwent CA. The overall per-vessel sensitivity, specificity, positive predictive value, negative predictive value, and accuracy at the 50% stenosis level were 86%, 84%, 65%, 95%, and 85%, respectively, and 77%, 93%, 61%, 97%, and 91%, respectively, in the per-segment analysis at the 50% stenosis level.
CONCLUSION
64-CT is an accurate imaging tool that allows a non-invasive assessment of significant CAD with a high diagnostic accuracy in a “real-world” population of patients. The sensitivity and specificity that we noted are not as high as those in prior reports, but we evaluated a population of patients that is typically encountered in clinical practice and therefore see more “real-world” results.
Keywords: coronary artery disease, 64-detector coronary computed tomography, coronary angiography
Background
The non-invasive evaluation of patients with suspected coronary artery disease (CAD) is traditionally undertaken utilizing myocardial perfusion imaging. In the assessment of patients with an intermediate pretest likelihood of CAD, non-invasive imaging plays an important role in risk stratification and selection of further treatment strategies. The detection of CAD by non-invasive imaging has been based on assessment of the significant coronary stenoses through visualization of inducible ischemia on stress testing. When significant inducible ischemia is documented, these patients are usually referred for invasive coronary angiography (CA). CA is currently felt to be the gold standard for the identification of coronary atherosclerotic stenoses. CA, however, is invasive, and carries a small but definite risk of morbidity and mortality.1,2 The newly emerging and ever-advancing non-invasive method of coronary computed tomography (CT) allows for the evaluation of coronary stenoses in terms of plaque burden and plaque characterization and is therefore of immense value for the evaluation of patients with and at risk for CAD.1–6
64-Detector coronary computed tomographic angiography (64-CT) has emerged rapidly because of the technical improvements in spatial (0.6 mm) and temporal (85–160 milliseconds) resolution. These improvements result in high-quality images obtained in a single breath-hold of about 10 seconds. The use of this fast, high resolution, contrast enhanced imaging modality can provide adequate quantification of the degree and nature of coronary artery stenoses in patients suspected of having CAD. However, thus far, data regarding the diagnostic accuracy of 64-CT1,2,7–16 and 16-detector CT17–23 have been obtained in very specific patient populations that have been specifically selected to represent the most ideal patients to undergo 64-CT, and those studies excluded many commonly encountered patients in daily practice. These highly selected patients do not represent a “real-world” clinical population of patients. These prior studies excluded many patients with irregular heart rates (atrial fibrillation, atrial premature contractions, ventricular premature contractions), many patients with contraindications to beta-blockers where they could have employed other rate-control methods, and many patients with prior history of CAD. Accordingly, the aim of the present study was to compare 64-CT to conventional CA to investigate the accuracy of 64-detector CT in determining significant coronary stenoses in a “real-world” clinical population at a busy tertiary care center performing a large number of coronary CT angiography during the initial phase of time when 64-CT angiography became available. Today, even more advanced spatial and temporal resolution is obtained utilizing newer scanners with greater detectors (320 detectors) and faster temporal resolution (dual-source). Yet the point noted is that even the more recent studies that evaluate these newer advanced technologies will select specific patients and therefore may affect the true diagnostic yield in a real-world population, which is not so highly and specifically selected.
Methods
Patient population
A total of 1,818 consecutive outpatients and inpatients undergoing 64-CT were evaluated. Of these patients, those in whom results of 64-CT prompted further evaluation by CA were identified and studied. An exemption from the requirement of IRB review for this study protocol was granted by the North Shore LIJ Institutional Review Board, and the research was conducted in accordance with the principles of the Declaration of Helsinki.
All patients were >18 years of age, not pregnant (urine B-HCG negative for females of childbearing potential), able to receive intravenous medications, and able to give informed consent. Patients with hemodynamic instability or evidence of renal insufficiency (serum creatinine ≥1.5 mg/dL) were excluded. Patients taking the oral antihyperglycemic metformin were asked to hold that medication starting on the day of the CT scan and to continue to hold the medication until two days after the study. Patients with known or suspected allergy to iodine or contrast agents were also excluded. This study population is felt to be “real-world” because it encompasses a large number of patients with a diverse range of indications and a number of patients with criteria felt not to be ideal in all cases for cardiac CT scanning.
64-Detector CT scanning technique
Upon presentation to the CT scanner, patients with heart rates ≥70/minute received an intravenous dose of 5 mg of metoprolol 5–10 minutes before the scan, unless they had known overt heart failure, significant ECG atrioventricular conduction abnormalities (second-degree AV block or more severe), or bronchospastic disease. Further doses (up to a total of 25 mg, 5 minutes apart) were administered if heart rate control (<70/minute) was not achieved, and as long as blood pressure allowed (systolic blood pressure was ≥100 mm Hg). In patients with significant bronchospastic disease or other contraindications to beta-blockers, calcium channel blockade was used for rate control using verapamil intravenously at a dose of 5 mg over 2 minutes repeated up to four times (total dose of 20 mg) every 5 minutes till achieving rate response and as long as blood pressure allowed (systolic blood pressure was ≥100 mm Hg). Mean heart rate at the time of the scan was 58.5 ± 6.2/minute and when a patient required rate control, they generally required one or two doses.
All patients were scanned with a 64-CT scanner (GE LightSpeed VCT, GE® Healthcare). Angiographic scan parameters included the following: number of slices per rotation – 64; individual detector slice width of 0.625 mm; and 12.5 cm spatial coverage in 5 seconds at a gantry rotation speed of 330 milliseconds. After the patient was advanced into the scanner bore, the first acquisition consisted of a localizer image of the chest. The second acquisition was a non-contrast scan for calcium scoring performed with scanning parameters including gantry rotation time 330 milliseconds, tube voltage 120 kVp, tube current 225 mA, prospective gating at 70% of the R–R interval, and collimation 64 × 0.625 mm. The third acquisition consists of a test bolus scan performed using a bolus of 20 cc of non-ionic iodinated contrast material (Omnipaque [iohexol], GE® Healthcare, Amersham Health). Segmental images were then obtained at 1 image/second over the aortic root. The scan was continued till a threshold of 100 Hounsfield units (HU) was reached in a region of interest positioned in the ascending aorta. This allowed graphical estimation of the timing needed for acquisition timing of the coronary angiogram. The final acquisition was the contrast-enhanced angiogram. Patients were asked to breathe deeply and then hold their breath at end-inspiration. Iohexol was administered according to the following protocol: 50 cc at a rate of 5 cc/second, followed by 20 cc at a rate of 3.5 cc/second, followed by a chaser bolus of normal saline 50 cc at a rate of 5 cc/second. The imaging parameters for this scan were rotation time 330 milliseconds, tube voltage 120 kVp, and collimation 64 × 0.625 mm. Image reconstruction was performed at 10% increments through the R–R cardiac cycle. After image acquisition, images were transferred to a GE® AW workstation for analysis.
Image analysis of 64-CT
The CT dataset was analyzed by two independent experienced readers blinded to the patient clinical data. Differences in interpretation were resolved by consensus. Using the AHA coronary artery scoring system,24 the interpreters identified and classified the coronary arteries. Stenoses were graded, based upon comparison of the region of maximal luminal narrowing with a normal appearing adjacent segment. All segments were evaluated regardless of the size of the coronary artery. A significant stenosis was one judged to be greater than 50% luminal narrowing. Any structure with a density of 130 HU or more that could be visualized separately from the contrast-enhanced coronary lumen, assigned to the coronary artery wall, and identified in at least two independent planes was defined as a calcified atherosclerotic plaque.
For the analysis of the coronaries, the original axial dataset pre- (for calcium scoring) and post-contrast was examined. Further, curved multiplanar reconstructions (Fig. 1) were also employed for analysis. The results of 64-CT where there were no significant or obstructive stenoses were further classified as “normal” or as having non-obstructive CAD when ath-erosclerotic lesions <50% of luminal diameter were present. Those arteries with lesions ≥50% were classified as obstructive. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were determined on a per-vessel and per-segment basis using CA as the gold standard for significant stenoses of both ≥50% and ≥70%. The segments of the coronaries were divided as follows: the left main was one segment, and the remaining arteries (left anterior descending, left circumflex, and right coronary) were each divided into equal thirds of proximal, middle, and distal portions. This method was employed so as to allow for the greatest possible diagnostic yield to our studies as compared to prior studies, which strictly adhered to documenting for example proximal versus middle left anterior descending artery (LAD) based on visualization of the first septal branch. It is clear that the visualization of the first septal is sometimes difficult, and this will therefore limit the evaluation if restrictive definitions initially derived for conventional CA24 are employed.
Figure 1.

(A) Three-dimensional reconstruction (“volume rendered”) revealing the presence of proximal left anterior descending artery high grade long segment stenosis (arrow). (B) Curved multiplanar reconstruction in the same patient reveals the high grade stenosis consisting of predominantly fatty plaque (arrow). (C) Invasive CA confirms the stenosis (arrow).
Catheter-based coronary angiography
Conventional CA was performed according to standard clinical protocols. Invasive coronary angiograms were evaluated off-line by two independent, blinded investigators. For coronary artery lesions, the mean diameter reduction was determined in typically two projections. All coronary arteries were imaged for diagnostic purposes at the discretion of the performing angiographer. CA was performed using standard diagnostic techniques. Arteries were identified by AHA system24 as present or absent and stenosis if present accordingly. If a stenosis was noted, it was quantified into a percent stenosis. A stenosis was determined to be significant if it was visually judged to be at least ≥50% of the maximal luminal diameter.
Statistical analysis
Statistical analysis was performed using SPSS version 11.5 (SPPS Corporation). Continuous variables were described as mean values ± standard deviation. Sensitivity, specificity, PPV, and NPV for 64-CT CA for detecting significant stenoses in all coronary arteries were calculated with CA regarded as the standard of reference. Comparisons between patient groups were performed using the Student’s t-test. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 41 patients (30 men, 11 women, age 39–85 years) were identified by 64-CT to have significant coronary stenoses and thereafter underwent CA. CA was performed on average within 10.5 ± 8 days of the 64-CT. The patient demographics (Table 1) included typical patients commonly encountered in a clinical practice. Hypertension, hypercholesterolemia, and smoking history were present in 56%, 63%, and 27% of our patients, respectively. Sixteen (39%) of our patients had a body mass index (BMI) ≥25 kg/m2 and nine (22%) were obese with a BMI ≥30 kg/m2. Ten percent of our patients referred from CT to undergo CA had a history of atrial fibrillation (5% were in atrial fibrillation during the CT exam), and 17% had a prior history of CAD (Fig. 2) although none of them had prior stenting or coronary artery bypass surgery. The most common indication for 64-CT was chest pain followed by an abnormal stress test (Table 2).
Table 1.
Patient characteristics (N=41).
| Mean age (yrs ± SD) | 62.4 ± 1.1 |
| Male | 30 (73%) |
| Female | 11 (27%) |
| Mean height (cm ± SD) | 173 ± 7.8 |
| Mean weight (kg ± SD) | 83 ± 23.3 |
| Mean Body Mass Index (BMI; kg/m2 ± SD) | 27.5 ± 6.2 |
| BMI≥25 kg/m2 | 16 (39%) |
| BMI≥30 kg/m2 (obese) | 9 (22%) |
| Symptomatic | 33 (80%) |
| Asymptomatic | 8 (20%) |
| Hypertension | 23 (56%) |
| Diabetes mellitus | 3 (7%) |
| Current smoking | 11 (27%) |
| Hypercholesterolemia | 26 (63%) |
| Prior history of coronary artery disease | 7 (17%) |
| Prior history of atrial fibrillation/flutter | 4 (10%) |
| Atrial fibrillation during the CT exam | 2 (5%) |
| Calcium score>1000 units | 12 (29%) |
| Mean heart rate during CT (beats/min ± SD) | 58.5 ± 6.2 |
Figure 2.

(A) Curved multiplanar reconstruction of proximal left anterior descending artery in a patient with a prior history of angioplasty reveals a mixed (fatty and calcified) plaque with an area of significant stenosis (arrow) just distal to a septal perforator (asterisk). (B) Invasive CA confirms the stenosis (arrow) just distal to the septal perforator (asterisk), however, provides no information about the mixed nature of the plaque.
Table 2.
Indications for 64-CT scan.
| Chest pain | 14 (34%) |
| Dyspnea | 7 (17%) |
| Abnormal stress test | 12 (29%) |
| Symptoms + strong family history | 5 (12%) |
| Other | 3 (7%) |
Effect of calcium score
The overall population’s mean calcium (agatston) score was 789 units with large variation from zero up to 2800 units. Twelve (29%) patients had a calcium score above 1000 units. For those ≥60 years old, the mean calcium score was 1187 ± 1205 vs 226 ± 240 for those <60 years old (P = 0.009). There was a trend toward less agreement between 64-CT and CA results with higher calcium scores (11 of the 28 [39.3%] subjects whose CT agree with CA results had a total calcium score >400 versus 9 out of 13 [69.2%] subjects with a total calcium score >400 whose results disagreed at the 70% stenosis cut-off; P = 0.10). Therefore, more patients with higher calcium scores had disagreement between the 64-CT result and the CA result showing a limitation of 64-CT with higher calcium scores. There was also a trend toward higher calcium scores in patients erroneously noted (false positives) to have significant stenoses (≥50%) by 64-CT (P = 0.059). Higher calcium scores caused stenoses to be “over-called” during interpretation leading to increased “false positives.” Despite this, there were still cases of severe calcification (Fig. 3), where some diagnostic information could be derived.
Figure 3.
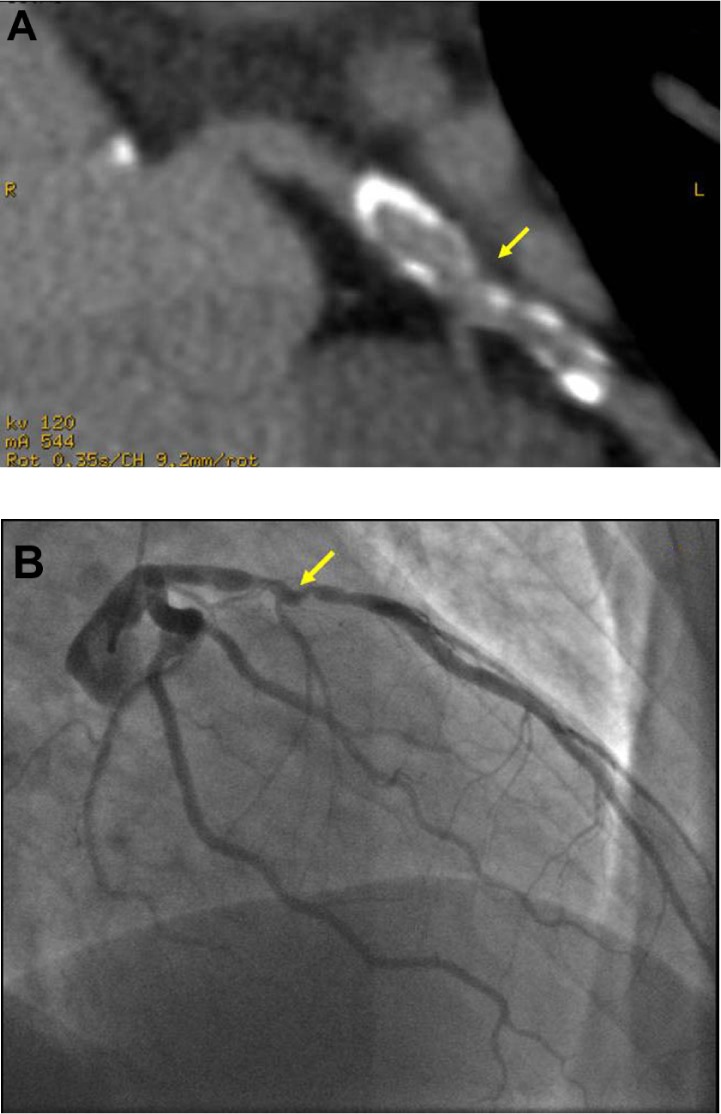
(A) Oblique tomographic view of the left circumflex coronary artery of a patient with a calcium score of 1377 agatston units reveals a severely calcified vessel generally precluding assessment for stenosis. Despite this, there is an area of narrowing noted by the arrow, which was found on invasive CA (B) to reveal a significant stenosis (arrow) just before the marginal branch.
Comparison of 64-CT to the standard of CA (Table 3)
Table 3.
Sensitivity, specificity, and positive and negative predictive values of 64-CT compared to the gold standard of conventional angiography at the 50% and 70% stenosis levels.
| SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) | ACCURACY (%) | |
|---|---|---|---|---|---|
| Per-vessel analysis | |||||
| 50% Stenosis cut-off | 86 | 84 | 65 | 95 | 85 |
| 70% Stenosis cut-off | 60 | 94 | 72 | 91 | 88 |
| Per-segment analysis | |||||
| 50% Stenosis cut-off | 77 | 93 | 61 | 97 | 91 |
| 70% Stenosis cut-off | 54 | 98 | 73 | 95 | 94 |
| Per-patient analysis | |||||
| 50% Stenosis cut-off | 90 | 35 | 75 | 63 | 66 |
| 70% Stenosis cut-off | 64 | 57 | 50 | 70 | 61 |
A total of 164 coronary arteries and 410 coronary segments were evaluated in 41 patients at the 50% and 70% coronary stenosis levels. None of the coronary segments needed to be excluded from analysis. The overall per-vessel sensitivity, specificity, PPV, NPV, and accuracy at the 50% stenosis level were 86%, 84%, 65%, 95%, and 85%, respectively. The overall per-segment sensitivity, specificity, PPV, NPV, and accuracy at the 50% stenosis level were 77%, 93%, 61%, 97%, and 91%, respectively. Values at the 70% level showed a similar trend with as expected a slightly lower sensitivity and slightly higher specificity (Table 3). The per-patient sensitivity, specificity, PPV, NPV, and accuracy at the 50% stenosis level were 90%, 35%, 75%, 63%, and 66%, respectively. The per-patient sensitivity, specificity, PPV, NPV, and accuracy at the 70% stenosis level were 64%, 57%, 50%, 70%, and 61%, respectively.
Discussion
Advances over prior technologies and studies
The development and use of 16-slice CT already constituted an important step forward in non-invasive CA, and although the ability to identify high-grade coronary stenoses was reported from several groups, this technology was affected by numerous limitations.17–23 One of these limitations is spatial resolution, which limits the reliable identification of coronary lesions to the major coronary branches with a diameter of at least 2 mm. Furthermore, it is consistently reported that because of the limited temporal resolution, motion artifacts can only be avoided in patients with heart rates of <65 beats/minute.5,7,23 Those limitations probably contributed to the fact that all existing prior studies of 16-slice CT studies focused on the detection of high-grade stenosis only and, until now, no attempt was directed toward a CT-derived stenosis quantification. In comparison to 16-slice CT scanning, 64-CT has increased slices per gantry rotation (64 vs 16) and faster gantry speed (330 milliseconds/rotation vs 375) and really advanced the field of coronary CT scanning to the point of making it a more mainstream diagnostic tool, and of course newer current technologies only continue to expand on the presence of coronary CT scanning in the cardiology diagnostic armamentarium. These advances translate into better spatial resolution because of thinner detectors (0.4 vs 0.75 mm) and greater temporal resolution (160 vs 188 milliseconds). The increase in resolution also enhances differentiation between non-calcified plaque and calcified plaque.1–4
Despite these technologic advances, major trials of coronary CT scanning have continued to limit their patient populations to only ideal candidate patients. These trials exclude many patients commonly encountered in daily clinical practice such as diabetics, those with a large body habitus, those with atrial fibrillation or premature contractions that may distort images because of inappropriate gating, and/or those with high calcium scores. If these patients are excluded, a large proportion of the “real-world” patients encountered everyday in clinical practice are not included in the evaluation. This means that the numbers for sensitivity, specificity, and accuracy reported by these trials do not apply to this large proportion of patients. Our study is the first to allow for the evaluation of all patients commonly presenting to a busy clinical practice in a tertiary care center that performs over a thousand coronary CT studies per year.
Comparison of 64-CT with the “gold standard” (Table 4)
Table 4.
Visual representation of the data noted in table 3 of 64-CT compared to the gold standard of conventional angiography at the 50% and 70% stenosis levels.

|
Prior studies demonstrate the high diagnostic accuracy of 64-CT, but they exclude a large proportion of patients who are precisely the patients that should undergo coronary CT based on appropriateness criteria.25 Leschka et al.8 presented the first study exploring the diagnostic performance of 64-slice CT CA. They evaluated all coronary segments ≥1.5 mm and reported a high sensitivity and specificity for detecting significant lesions using a 64-CT. They excluded patients with severe renal insufficiency (creatinine level >120 μmol/L), hemodynamic instability, atrial fibrillation, inability to follow breath-hold commands, previous percutaneous transluminal coronary stent placement, and previous bypass surgery. Their overall sensitivity for classifying stenoses was 94%, specificity was 97%, PPV was 87%, and NPV was 99%.8
Raff et al.1 studied 70 consecutive patients. They excluded patients with irregular heart rate, patients at risk for iodinated contrast agents (congestive heart failure, dye allergy, or elevated serum creatinine >1.5 mg/dL), or contraindications to beta-blocking drugs. They found that of the 1,065 coronary segments identified, 935 (88%) could be evaluated, and the specificity, sensitivity, PPV, and NPV for the presence of significant stenoses identified by 64-CT in comparison to CA were: by segment (n = 935), 86%, 95%, 66%, and 98%, respectively; by artery (n = 279), 91%, 92%, 80%, and 97%, respectively; by patient (n = 70), 95%, 90%, 93%, and 93%, respectively.1
Mollet et al.2 studied 52 patients (34 men; mean age, 59.6 ± 12.1 years) with atypical chest pain, stable or unstable angina pectoris, or non–ST-segment elevation myocardial infarction scheduled for diagnostic conventional CA. No patients with previous percutaneous coronary intervention, coronary bypass surgery, presence of arrhythmia, impaired renal function (serum creatinine >120 mmol/L), or known contrast allergy were included. They evaluated all coronary segments, regardless of size. Lesions with ≥50% luminal narrowing were considered significant stenoses. Invasive CA demonstrated the absence of significant disease in 25% (13 of 52), single-vessel disease in 31% (16 of 52), and multivessel disease in 45% (23 of 52) of patients. Ninety-four significant stenoses were present in the remaining 51 patients. Sensitivity, specificity, PPV, and NPV of CT for detecting significant stenoses on a segment-by-segment analysis were 99% (93 of 94; 95% CI, 94–99), 95% (601 of 631; 95% CI, 93–96), 76% (93 of 123; 95% CI, 67–89), and 99% (601 of 602; 95% CI, 99–100), respectively.2
Leber et al.7 studied 59 patients scheduled for CA because of stable angina pectoris. Contrast-enhanced 64-slice CT was performed before the invasive angiogram. A further subset of 18 patients had intravascular ultrasound (IVUS) of 32 vessels performed as part of the catheterization procedure. Patients with atrial fibrillation, previous bypass surgery, previous stenting of >1 vessel, an unstable clinical condition, or a contraindication to the administration of contrast agent were excluded. The overall correlation between the degree of stenosis detected by quantitative CA and that detected with 64-CT was r = 0.54. Sensitivity for the detection of stenosis <50%, stenosis >50%, and stenosis >75% was 79%, 73%, and 80%, respectively, and specificity was 97%. In comparison with IVUS, 46 of 55 (84%) lesions were identified correctly. Plaque and lumen areas derived by CT correlated well with IVUS, however, the results were limited by the insufficient ability of CT to exactly quantify the degree of stenosis despite excluding patients with atrial fibrillation, stenting, and bypass surgery.7
Other investigations9–16 excluded a similar cohort of “real-world” patients including those with irregular heart rhythms, contraindications to beta-blockers, and prior history of CAD. Pugliese et al.9 excluded those with presence of arrhythmias, previous bypass graft surgery, or percutaneous coronary intervention. Similarly, Ropers et al.10, Ghostine et al.13, Ong et al.14, and Busch et al.15 excluded all patients with irregular heart rates and history of CAD. Schuijf et al.16 excluded any patient with known CAD, defined as a history of myocardial infarction or coronary revascularization and/or presence of one or more angiographically documented coronary stenoses with ≥50% luminal diameter. Meijboom et al.26 excluded patients with a history of arrhythmia and contraindications to beta-blockers. The studies by Fine et al.11 and Ehara et al.12 were unique evaluations in that they included accuracy for patients with a history of coronary disease (including bypass surgery and stents) in their calculations. More recently, results of the Coronary Evaluation Using Multi-detector Spiral Computed Tomography Angiography using 64 Detectors (CorE-64 trial),27 the first multicenter trial assessing 64-detector CT scanning, reported a sensitivity of 85% and a specificity of 90% compared with the gold standard of invasive angiography in a group of 291 patients. This trial excluded patients with prior CAD, those with arrhythmias, and those with high calcium scores >600 agatston units.27
Real-world cardiac CT findings
Our results are consistent with and extend the findings of previously published CT coronary angiographic studies employing 16- and 64-slice scanners. In aggregate, these studies document that non-invasive coronary CT imaging can accurately determine the presence or absence of significant coronary lesions in highly selected patient populations. Our study documents and extends the utility of this technique to “real-world” patients encountered in daily practice. Furthermore, in contrast to prior studies, our results show that our sensitivity, specificity, and accuracy in the evaluation of these patients, especially in the per-patient analysis, are not as high as the results obtained when a highly selected group of patients is chosen, and that our accuracy can be tempered by higher calcium scores, motion artifacts, and less than ideal heart rate and rhythm control even though we were able to evaluate all coronary segments compared to prior studies (Table 5).
Table 5.
Accuracy for the detection of coronary stenoses (≥50%, per segment) using 64-CT and the selected patient groups included.
| AUTHOR | SCANNER | PER SEGMENT | HISTORY OF CAD, PRIOR CORONARY INTERVENTION, AND/OR CABG | ATRIAL FIBRILLATION/IRREGULAR RHYTHM | CONTRAINDICATION TO BETA-BLOCKERS | ALL VESSEL SEGMENTS/SIZES EVALUATED | |
|---|---|---|---|---|---|---|---|
| SENSITIVITY (%) | SPECIFICITY (%) | ||||||
| Raff et al. 2005 | Sensation 64 | 86 | 95 | − | − | − | − |
| Mollet et al. 2005 | Sensation 64 | 99 | 95 | − | − | − | − |
| Leber et al. 2005 | Sensation 64 | 73 | 97 | + | − | + | + |
| Leschka et al. 2005 | Sensation 64 | 94 | 97 | − | − | − | − |
| Pugliese et al. 2006 | Sensation 64 | 99 | 96 | − | − | − | + |
| Ropers et al. 2006 | Sensation 64 | 93 | 97 | − | − | − | − |
| Fine et al. 2006 | Sensation 64 | 95 | 96 | + | − | − | − |
| Ehara et al. 2006 | Sensation 64 | 90 | 94 | + | − | + | − |
| Ghostine et al. 2006 | Sensation 64 | 72 | 99 | − | − | + | − |
| Ong et al. 2006 | Sensation 64 | 80 | 93 | − | − | − | − |
| Busch et al. 2006 | Sensation 64 | 82 | 95 | − | − | − | − |
| Schuijf et al. 2006 | Sensation 64 | 85 | 97 | − | − | − | + |
| Meijboom et al. 2006 | Sensation 64 | 94 | 98 | + | − | − | + |
| Miller et al. 2007 | Acquilion 64 | 85 | 90 | − | − | − | − |
| Makaryus, et al. Current study | GE Lightspeed VCT 64 | 77 | 93 | + | + | + | + |
Notes: +, patients with this condition were studied. −, patients with this condition were not studied.
Our data further noted a trend as a prior report suggested1 toward decreased accuracy with greater calcium scores. A final and important point is the small number of patients (41) out of a total of 1,818 patients who were found by 64-CT to have significant obstructions necessitating CA. While some observers may consider this inappropriate use of 64-CT in too low a pretest probability group, we view it as an advance and the reason 64-CT should be employed to guide patients away from the catheterization lab and prevent the unnecessary use of and risk associated with invasive CA, which thousands of patients unnecessarily experience every year with the false negative rate with invasive CA reported as high as 25–30%. We stress, however, that we do not condone or advocate the overuse or inappropriate use of coronary CT, but are simply highlighting the fact that some patients may be inappropriately excluded from obtaining a coronary CT because of stringent exclusions (ie, high calcium score, atrial fibrillation etc.), which in experienced hands may be mitigated to the point of obtaining a diagnostic and useful study.
Our study further points to the fact that the published studies exclude a large proportion of patients encountered in everyday clinical practice. If we examine the traditionally held notion of excluding patients with calcium score of ≥1000 units, 29% of our patient population would be excluded. If we use the exclusion of patients with a history of atrial fibrillation, 10% would have been excluded. If we combine these two criteria of history of atrial fibrillation and calcium score >1000, more than a third (37%) of our patient population would be excluded from undergoing a cardiac CT. This represents a large portion of our patient population who may benefit from the diagnostic abilities of 64-CT.
Study limitations
A drawback of our analysis and recent coronary 64-CT and CA comparison studies is the selection bias of referring only low-risk patients to undergo 64-CT, and referring higher risk patients directly to CA. Although this may be a prudent means of evaluating and treating patients, it limits the assessment of the accuracy of 64-CT for the detection of CAD. Further to this point, for the most part, it is rare (and likely unnecessary) for a patient with a normal 64-CT to be referred for CA. This limits our ability to assess the true NPV on a patient-by-patient basis. Although our study did not use quantitative CA and stenoses were semi-quantitatively assessed with both CA and 64-CT, this method of stenosis evaluation showed a high degree of agreement in patients with observed stenoses on 64-CT and CA. Further, while variability may be introduced with a “visual” semi-quantitative estimate of stenosis whether on 64-CT or CA, the truth is that an “experienced” visual estimate is what thousands of operators employ everyday in clinical practice and this further speaks to our point of the “real-world” nature of our study. Next, the radiation exposure inherent with CT may be a limitation of the technique. The most effective ways to reduce radiation exposure are ECG tube current modulation (we applied this technique in all patients to reduce radiation exposure) and newly developed step-and-shoot prospective techniques. The present study was also limited to small numbers of patients in a single center that underwent both 64-CT and CA. Finally, 64-CT and CA were not performed simultaneously, but the median delay between the two tests was small with regard to the natural progression of CAD.28,29 Finally, newer techniques utilizing functional means (such as fractional flow reserve)30 allow us to better define the hemodynamic significance of a stenosis, which anatomic coronary analysis cannot provide; however, there is still a major place for the anatomic quantification of coronary stenoses and in this area, coronary CT angiography is a robust tool.
Conclusions
64-CT is an accurate imaging tool that allows a non-invasive assessment of anatomically significant CAD with a high diagnostic accuracy in real-world patients. Our sensitivity and specificity, although not as high as those in prior reports, still reveal strong accuracy compared to commonly employed non-invasive modalities for the evaluation of CAD. The appeal of 64-CT compared with CA is that it is non-invasive, avoiding catheter-associated risks.
Our patient population results were obtained in patients with a wide spectrum of clinical settings and circumstances and therefore make our results more applicable to everyday practice. While we do not advocate the careless acquisition of 64-CT imaging in all less than ideal patients, we at the same time do not feel it is appropriate to just deny a patient this imaging technique without properly examining the special situation and making an informed decision on a case-by-case basis. We examined patients with a history of irregular heart rates, a wide range of coronary calcification, a wide range of body habitus, and all vessel segments without restrictions on size. The high NPV makes 64-CT an excellent diagnostic tool for the evaluation of low to intermediate risk patients who are symptomatic, or asymptomatic high-risk patients. A normal 64-CT rules out disease in these patients with high certainty (85–91%). It is this patient population, therefore, that should be targeted, as appropriateness criteria recommend,25 to undergo 64-CT in the assessment of CAD. With this advancing technology, especially with newer scanners that allow further advances in technology, coronary CT now commands a robust and evolving role in the diagnosis of anatomic CAD and suggests that this non-invasive technique can now be considered a viable alternative to invasive CA in selected patients.
Footnotes
Author Contributions
Conceived and designed the experiments: ANM, LB. Analyzed the data: ANM, SH, LL, JNM. Wrote the first draft of the manuscript: ANM, JNM. Contributed to the writing of the manuscript: ANM, SH, LL, JNM. Agree with manuscript results and conclusions: ANM, SH, LL, JNM, LB. Jointly developed the structure and arguments for the paper: ANM, SH, LL, JNM, LB. Made critical revisions and approved final version: ANM, SH, LL, JNM, LB. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Thomas E Vanhecke, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Raff G, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 2.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 3.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664–6. doi: 10.1161/01.cir.0000055738.31551.a9. [DOI] [PubMed] [Google Scholar]
- 4.Kuettner A, Trabold T, Schroeder S, et al. Noninvasive detection of coronary lesions using 16-detector multislice spiral computed tomography technology: initial clinical results. J Am Coll Cardiol. 2004;44:1230–7. doi: 10.1016/j.jacc.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 5.Achenbach S, Giesler T, Ropers D, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically gated, multislice spiral computed tomography. Circulation. 2001;103:2535–8. doi: 10.1161/01.cir.103.21.2535. [DOI] [PubMed] [Google Scholar]
- 6.Knez A, Becker CR, Leber A, et al. Usefulness of multislice spiral computed tomography angiography for determination of coronary artery stenoses. Am J Cardiol. 2001;88:1191–4. doi: 10.1016/s0002-9149(01)02060-4. [DOI] [PubMed] [Google Scholar]
- 7.Leber AW, Knez A, Ziegler FV, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography; a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 8.Leschka S, Alkadhi H, Plass A, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26:1482–7. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 9.Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575–82. doi: 10.1007/s00330-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 10.Ropers D, Rixe J, Anders K, et al. Usefulness of multidetector row spiral computed tomography with 64 × 0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97:343–8. doi: 10.1016/j.amjcard.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Fine JJ, Hopkins CB, Ruff N, Newton FC. Comparison of accuracy of 64-slice cardiovascular computed tomography with coronary angiography in patients with suspected coronary artery disease. Am J Cardiol. 2006;97:173–4. doi: 10.1016/j.amjcard.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Ehara M, Surmely JF, Kawai M, et al. Diagnostic accuracy of 64-slice computed tomography for detecting angiographically significant coronary artery stenosis in an unselected consecutive patient population: comparison with conventional invasive angiography. Circ J. 2006;70:564–71. doi: 10.1253/circj.70.564. [DOI] [PubMed] [Google Scholar]
- 13.Ghostine S, Caussin C, Daoud B, et al. Non-invasive detection of coronary artery disease in patients with left bundle branch block using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1929–34. doi: 10.1016/j.jacc.2006.04.103. [DOI] [PubMed] [Google Scholar]
- 14.Ong TK, Chin SP, Liew CK, et al. Accuracy of 64-row multidetector computed tomography in detecting coronary artery disease in 134 symptomatic patients: influence of calcification. Am Heart J. 2006;151:e1–6. doi: 10.1016/j.ahj.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Busch S, Johnson TR, Nikolaou K, et al. Visual and automatic grading of coronary artery stenoses with 64-slice CT angiography in reference to invasive angiography. Eur Radiol. 2007 Jun;17(6):1445–51. doi: 10.1007/s00330-006-0512-y. [DOI] [PubMed] [Google Scholar]
- 16.Schuijf JD, Wijns W, Jukema JW, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48:2508–14. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 17.Vogl TJ, Abolmaali ND, Diebold T, et al. Techniques for the detection of coronary atherosclerosis: multi-detector row CT coronary angiography. Radiology. 2002;223:212–20. doi: 10.1148/radiol.2231010515. [DOI] [PubMed] [Google Scholar]
- 18.Kopp AF, Schroeder S, Kuettner A, et al. Non-invasive coronary angiography with high resolution multidetector-row computed tomography. Results in 102 patients. Eur Heart J. 2002;23:1714–25. doi: 10.1053/euhj.2002.3264. [DOI] [PubMed] [Google Scholar]
- 19.Mollet NR, Cademartiri F, Nieman K, et al. Multislice spiral CT coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43:2265–70. doi: 10.1016/j.jacc.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Kuettner A, Kopp AF, Schroeder S, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with angiographically proven coronary artery disease. J Am Coll Cardiol. 2004;43:831–9. doi: 10.1016/j.jacc.2003.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Mollet NR, Cademartiri F, Krestin GP, et al. Improved diagnostic accuracy with 16-row multislice CT coronary angiography. J Am Coll Cardiol. 2005;45:128–32. doi: 10.1016/j.jacc.2004.09.074. [DOI] [PubMed] [Google Scholar]
- 22.Martuscelli E, Romagnoli A, D’Eliseo A, et al. Accuracy of thin-slice computed tomography in the detection of coronary stenoses. Eur Heart J. 2004;25:1043–8. doi: 10.1016/j.ehj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002;106:2051–4. doi: 10.1161/01.cir.0000037222.58317.3d. [DOI] [PubMed] [Google Scholar]
- 24.Austen W, Edwards J, Frye R, et al. A reporting system on patients evaluated for coronary artery disease: report of the Ad Hoc Committee for grading of coronary artery disease, council on cardiovascular surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol. 2010;56(22):1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Meijboom WB, Mollet NR, Van Mieghem CA, et al. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48(8):1658–65. doi: 10.1016/j.jacc.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 27.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009 Apr;19(4):816–28. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–6. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 29.Mercado N, Maier W, Boersma E, et al. Clinical and angiographic outcome of patients with mild coronary lesions treated with balloon angioplasty or coronary stenting. Implications for mechanical plaque sealing. Eur Heart J. 2003;24:541–51. doi: 10.1016/s0195-668x(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 30.Crystal GJ, Klein LW. Fractional flow reserve: historical background, physiological basis, advantages and limitations, and potential gender differences. Curr Cardiol Rev. 2014 Oct 20; doi: 10.2174/1573403X10666141020113318. [DOI] [PMC free article] [PubMed] [Google Scholar]


