Abstract
Background
Mycoplasma pneumoniae (MP) pneumonia is a self-limiting disease, but some patients complain of progressive pneumonia, despite of appropriate antibiotic treatment. We aimed to introduce the role of immune-modulators (corticosteroid and/or intravenous immunoglobulin, IVIG) treatment for childhood MP pneumonia based on previous our experiences.
Materials and Methods
A retrospective case series analysis for 183 children with MP pneumonia was performed. MP pneumonia patients were diagnosed by two Immunoglobulin M (IgM) tests: the micro-particle agglutination method (≥1:40) and the cold agglutination test (≥1:4), and were examined twice at the initial admission and at discharge. Among 183 MP pneumonia patients, 90 patients with persistent fever for over 48 hours after admission or those with severe respiratory symptoms and signs received additional prednisolone (82 patients, 1 mg/kg/day) or intravenous methylprednisolone (8 patients, 5-10 mg/kg/day) with antibiotics. Four patients with aggravated clinical symptoms and chest radiographic findings after corticosteroid treatment received IVIG (1 g/kg/day, 1-2 doses).
Results
Mean age of 183 patients was 5.5 ± 3.2 years (6 months-15 years), and the male: female ratio was 1.1:1 (96:87). Fifty-seven patients (31%) were seroconverters and 126 seropositive patients showed increased diagnostic IgM antibody titres during admission (over 4 folds). The majority of the patients who received corticosteroids (86/90 cases) showed rapid defervescence within 48 hours with improved clinical symptoms, regardless of the used antibiotics. Also, 4 patients who received additional IVIG improved both clinically and radiographically within 2 days without adverse reaction.
Conclusions
In the era of macrolide-resistant MP strains, early additional immune-modulator therapy with antibiotics might prevent from the disease progression and reduce the disease morbidity without adverse reaction.
Keywords: Pneumonia, Mycoplasma, Corticosteroid, Immunoglobulin, Intravenous, Children
Introduction
Mycoplasma pneumoniae (MP) pneumonia is one of the most common agents of community-acquired pneumonia in previously healthy children and young adults [1]. In Korea, a 3- to 4-year cycle of nationwide MP pneumonia epidemics have been observed from the mid-1980s to the present [2]. Recently, macrolide-resistant MP (MRMP) strains are increasing in East Asian countries, including Japan, China and Korea [3, 4, 5]. However, the clinical implication of effective antibiotics against MRMP in children has not been defined [6].
Although MP pneumonia is usually a benign self-limiting disease, some patients complain of progressive severe pneumonia that is not responsive to antibiotic treatment. For these severely affected patients, additional corticosteroids are effective for the rapid improvement of clinical symptoms and chest radiographic findings in childhood and adult patients [7, 8, 9]. The rapid response to immune-modulators (corticosteroids) has suggested that acute lung injury in MP infections is associated with a hyperactive immune reaction of the host against the insults from MP infection, in addition to the role of MP replication in the host [1, 7, 8, 9].
We previously experienced that short-term use of oral prednisolone (1 mg/kg) was very effective for antibiotic non-responsive patients during the past two nationwide MP epidemics [7, 10]. In the recent 2011 epidemic, we used early corticosteroid treatment for MP pneumonia patients who had respiratory distress at presentation or during admission, or for those who had a persistent fever for ≥48 hours after admission. Among them, four patients did not respond to this therapy. Therefore, we attempted intravenous immunoglobulin (IVIG) as another immune-modulator and we obtained a favourable result as expected [1].
Although this study was not a case-controlled study for the effect of immune-modulators, in this study, we wanted to introduce the effect of additional immune-modulators on prevention from disease progression and reduction of disease morbidity, especially for severe MP pneumonia patients, by comparing our data with the respective data from other studies. We also introduce first cases of IVIG treatment for severe MP pneumonia, and briefly review the rationale of immunotherapy on MP pneumonia.
Materials and Methods
During the recent nationwide MP pneumonia epidemic in Korea, we treated 341 pneumonia patients at The Catholic University of Korea Daejeon St. Mary's Hospital, from June 1, 2011 to December 31, 2011. Among them, 183 MP pneumonia patients were selected by two Immunoglobulin M (IgM) tests: the micro-particle agglutination method (Serodia-Myco II, Fujirebio, Japan, ≥1:40) and the cold agglutination test (≥1:4). Patients were examined twice during the initial admission into the hospital and then during discharge. Patients with seroconversion or a 4-fold or greater increase in MP IgM titer with corresponding cold agglutination were defined as positive for MP pneumonia [10]. The patients who were examined by single testing (123 cases) regardless positivity of the results, or those who did not increased or decreased titres at second testing (35 cases) were excluded in this study, because of the high possibility of recent past infection. All patients had pneumonic lesions on initial chest radiography. The medical records and chest radiographic findings of 183 patients were retrospectively analyzed.
The majority of patients were treated initially with a macrolide (clarithromycin) and a broad-spectrum antibiotic (amoxacilin/ clavulanate or cefuroxime). We have experienced the effectiveness of additional corticosteroid treatment on severe MP pneumonia during previous nationwide epidemics in 2003 and 2006-2007 [7, 10]. In the recent 2011 epidemic, we planed to use the corticosteroids (prednisolone or intravenous methyprednisolone) earlier than in previous epidemics (persistent fever for ≥72 hours after admission) for rapid recovery of MP pneumonia patients. As for the indication of early corticosteroids, the patients who showed respiratory distress such as wheezing or tachypnea, requiring oxygen supply initially or during hospital stay (16 cases) or those who had persistent fever for ≥48 hours after admission with antibiotics (74 cases), received oral prednisolone (82 cases, 1 mg/kg/d, 2-3 days, tapering and stopping within a week) or intravenous methyprednisolone for severe patients with respiratory distress (8 cases, initially 5-10 mg/kg/day, tapering and stopping within a week). Patients with aggravated clinical symptoms and chest radiographic findings after corticosteroid treatment received IVIG (1 g/kg for 6 hours, 1-2 days). We also retrospectively analyzed the medical records and chest radiographic findings of 4 previously healthy children who were treated with IVIG.
1. Ethics
The written informed consents were obtained by caregivers of all children for their clinical records to be used in this study, and the study was approved by the Institutional Review Board of the Catholic University of Korea, Daejeon St Mary's Hospital.
2. Statistical analysis
All calculations were performed with SPSS ver. 14.0 (SPSS Inc.,Chicago, IL, USA). Comparisons between groups were performed using the Student's t-test for continuous variables, and chi-square test or Fisher's exact test for categorical variables. The data were expressed as mean ± standard deviation (SD) for continuous variables or as percentage of a specific group for categorical variables. All P-values were two-tailed, and a P-value of < 0.05 was considered statistically significant.
Results
1. Demographic and clinical characteristics of MP pneumonia patients
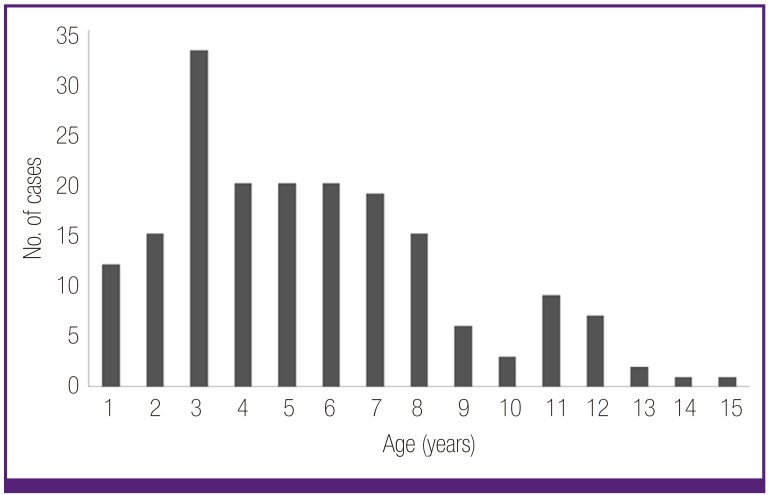
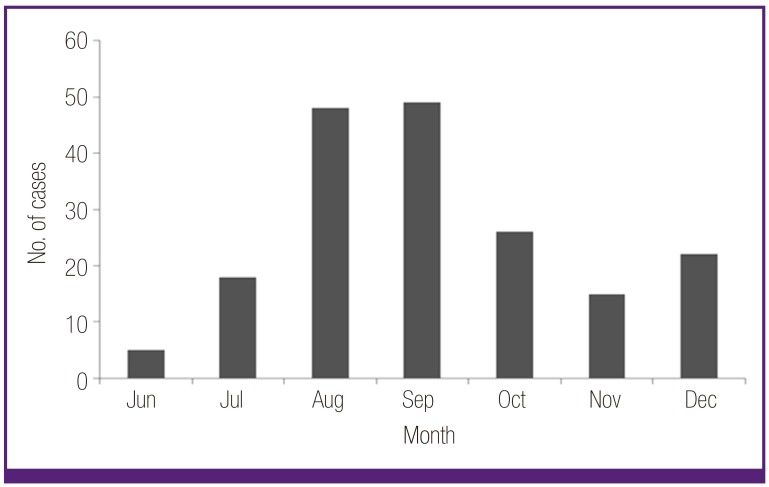
The mean age of 183 MP patients was 5.5 ± 3.2 years (ranged from 6 months to 15 years), and the male-to-female ratio was 1.1:1 (96:87). The age distribution of the subjects is shown in Figure 1, and the peak of cases was observed in summer and autumn season in Figure 2. In pneumonia patterns, 68 patients showed the bronchopneumonia, and 115 patients showed the segmental/lobar pneumonia according to our classification criteria [10]. Fifty-seven patients (31%) were seroconverters (from negative to positive cut-off values) and 126 seropositive patients showed increased diagnostic IgM antibody titres during admission. Polymerase chain reaction (PCR) assay for viral antigens of 16 respiratory viruses was checked in 79 patients; 7 patients were positive viral antigens (3 rhinovirus, 2 respiratory syncitial virus (RSV), 1 influenza B, and 1 rhinovirus and RSV).
Figure 1.
Age distribution of Mycoplasma pneumoniae pneumonia patients.
Figure 2.
Monthly cases of Mycoplasma pneumoniae pneumonia patients.
We analyzed the clinical and laboratory parameters of the 90 children treated with, and 93 patients treated without corticosteroids. The mean age (P = 0.01) were different between the groups. As expected, there were significant differences in the patients with fever duration ≥48 hours after admission, the patients with respiratory distresses, the patients with disease progression, and hospitalization stay between the groups. However, there were no differences in total fever duration and no patients experienced acute respiratory distress syndrome (ARDS) and intensive care unit (ICU) care. Most of the laboratory findings, including leukocyte count, C-reactive protein (CRP) level and PCR positivity of viruses showed no differences. However, there was a trend of higher coinfection rate in the corticosteroid group (Table 1).
Table 1.
Clinical and laboratory findings of the patients treated with steroids and without steroids
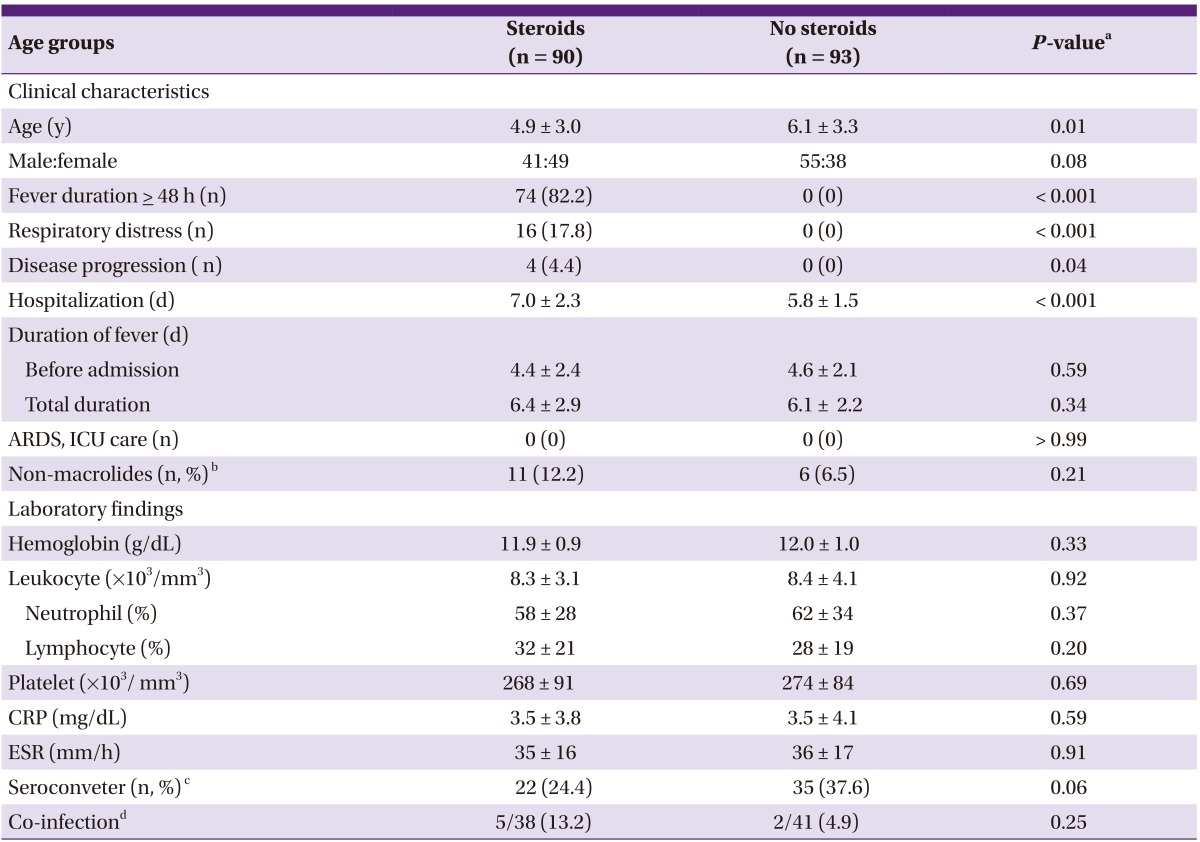
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
aP-values were obtained using Student's t-test for continuous variables, and Chi-square test or Fisher's exact test for categorical variables, as appropriate.
bNumber of patients who received only amoxicillin/calvunate or cefuroxime.
cNumber of patients who showed negative on the first examination, and converted to positive on the second examination (Serodia Myco II).
dNumber of patient who had positive PCR for respiratory viral antigens.
2. Treatment of MP pneumonia patients
Since we have believed that the pathogenesis of MP pneumonia is more responsible for the host excessive immune reaction than the pathogen induced cytopathy, in this epidemic we tried to use various antibiotics during study period. However, the study design was not prospective and the cases of each used antibiotic were uneven. Nine patients were treated with only a macrolide (clarithromycin), and 157 patients were treated initially with a chrarithromycin and a broad-spectrum antibiotic (amoxacilin/clavulanate or cefuroxime), and 18 patients were treated only amoxacilin/clavulanate (12 cases) or cefuroxime (6 cases). Among 18 patients with non-macrolide antibiotics, 11 patients received prednisolone because of prolonged fever, but no patients had a progression of the disease. Three patients received additional ciprofloxacin during clinical course. As previously mentioned, 90 patients received corticosteroids (82 prednisolone and 8 intravenous methyprednisolone), among them 4 patients received IVIG (1 g/kg for 6 hours, 1-2 days). The majority of the patients who received corticosteroids (86/90 cases), showed defervescence within 48 h with improved clinical symptoms, regardless of the used antibiotics including non-macrolides. Also, 4 patients who received additional IVIG improved both clinically and radiographically within 2 days. There were no patients with adverse reaction.
3. Clinical and laboratory findings of IVIG treated patients
All four patients had symptoms and signs indicative of lower respiratory tract infection upon admission, including fever (>38℃) and cough, with pneumonia infiltrates on chest radiographs. The case 1 infant (8 months of age, male) showed fever and respiratory distress with wheezing and tachypnea at presentation, and received intravenous methylprednisolone (5 mg/kg/day, divided 2 dose) with antibiotics at the first hospital day (HD). Fever was abolished on next day, but no clinical improvement with increased peribroncial infiltrations was observed during 2 days after steroid treatment, IVIG (1 g/kg/day) was given on the third and fourth HD. The patients of cases 2 (6 years of age, male) and case 3 (6 years of age, male) complained of fever and cough with 3 to 4 days in duration, and received prednisolone (1 mg/kg/day) treatment on third HD because of persistent fever and aggravated cough. Since the patients showed no clinical improvement and aggravated chest radiographic findings during prednisolone treatment, two doses of IVIG (1 g/kg/day, for 2 days) were given, because of no defervescence with one dose IVIG. The fourth patient (10 years of age, female) was treated with clarithromycin and cefuroxime initially, and added ciprofloxacin on third HD. However, the patient's clinical symptoms and chest radiographic findings were worse, intravenous methyprednisone (10 mg/kg/day, 6th HD) and one dose IVIG (1g/kg/day, 7th HD) were infused (Table 2). Serial chest radiographs of the patients (case 2 and case 4) were presented in Figures 3 and 4. Laboratory findings of the 4 patients are presented in Table 3. Two patients were seroconverted, and two patients were increased MP antibody titres. In the PCR study for respiratory viruses, infant patient was coinfected with RSV type 1 (Table 3). All 4 patients showed clinical and radiological improvement within 2 days as previously presented.
Table 2.
Clinical findings of IVIG treated patients
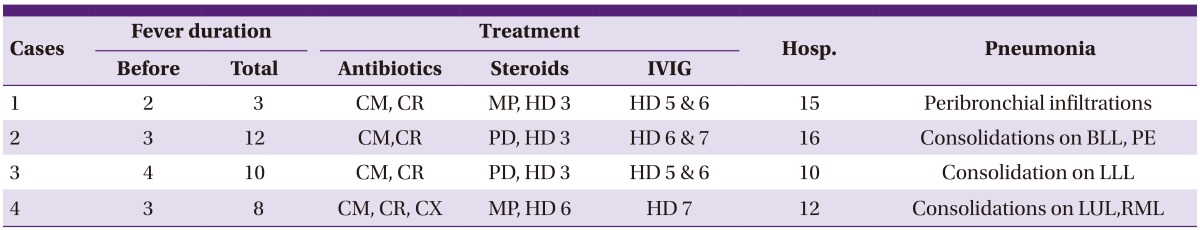
Hosp., hospitalization (days); CM, clarithromycin; CR, cefuroxime; MP, methyprednisolon; HD, hospital day; PD, prednisolone; BLL, both lower lung fields; PE, pleural effusion, LLL, left lower lung field; CX, ciprofloxacin; LUL, left upper lung field; RML, right middle lung field.
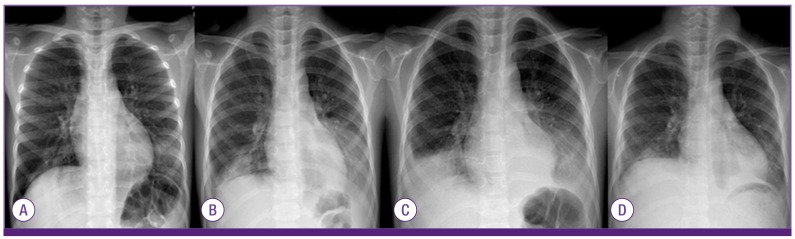
Figure 3.
The chest radiographs of the case 2 that had progressive pneumonia during hospitalization; on admission (A), 1 day after prednisolone treatment (1 mg/kg/day, 4th HD) (B), before IVIG treatment (6th HD) (C), and 5 days after IVIG treatment (1 g/kg/day for 2 days, 12th HD) (D).
HD, hospital day; IVIG, intravenous immunoglobulin.
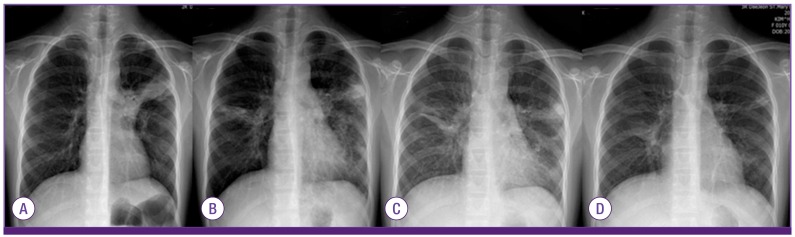
Figure 4.
The chest radiographs of the case 4 that also showed progressive pneumonia during hospitalization; on admission (A), 3 days after additional ciprofloxacin treatment (6th HD) (B), and 1 day after methylprednisolone (10 mg/kg) and IVIG (1g/kg, one dose, 7th HD) (C), and 5 days after both treatment (12th HD) (D).
HD, hospital day; IVIG, intravenous immunoglobulin.
Table 3.
Laboratory findings of IVIG treated patients
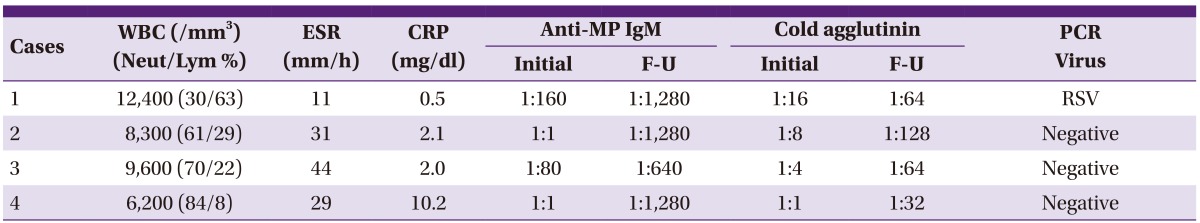
IVIG, intravenous immunoglobulin; WBC, white blood cells; Neut/Lym, neutrophil/lymphocyte differential; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; anti-MP IgM, anti-Mycoplasma pneumoniae IgM antibodies; F-U, follow-up; PCR, polymerase chain reaction; RSV, respiratory syncitial virus.
Discussion
MP pneumonia is a self-limiting disease. Although sensitive antibiotics can induce rapid defervescence in MRMP pneumonia patients [11, 12], antibiotics' role on progressive pneumonia and extrapulonary manifestations, such as encephalopathy and hemolytic anemia, is still controversial [13]. Recently in neighbouring countries to Korea such as Japan and China, very high prevalence of MRMP strains, ranging over 80% in children, has been reported [3, 4, 5, 11, 12]. An investigation group in Korea reported that 63% of the isolated MP strains were MRMP strains in the 2011 nationwide epidemic [5]. It was reported that the patients affected with MRMP strains had a prolonged fever duration (mean 9-10 days) with macrolide therapy, compared to the patients with macrolide-sensitive MP strains (mean 5.5-7 days) [14, 15]. In 2011 epidemic in Korea, many pediatricians faced difficulty of the non-responsiveness of antibiotics and progressive pneumonia on some severely affected pneumonia patients (personal communications), and one study group reported that mean total fever duration of severe MP pneumonia patients who received methyprednisolone pulse therapy was 11.8 days [16]. Although MP pneumonia is self-limited, prolonged fever with protracted clinical course is troublesome on patients, parents and doctors who are worried about disease progression or complications with long morbidity. Thus our treatment policy for patients with fever duration of ≥48 hours after hospitalization was thought to be adequate, and we showed that early use of immune-modulators (corticosteroids and IVIG) with conventional antibiotic treatment prevented disease progression and induced rapid improvement, as shown previous studies [7, 10]. The fever duration of the corticosteroid group in this series (mean 6.4 days) was shorter than that in the MRMP infected patients treated without corticosteroids (9-10 days) in previous studies in Japan [14, 15], and that in lately corticosteroid treated patients in this epidemic in Korea (11.8 days) [16]. Furthermore, it was not longer than that in the control group in which all patients showed a defervescence within 2 days (6.1 days), despite of the steroid treated group's possession of severe cases with respiratory distress and the cases of possibly prolonged fever with clinical deterioration. Especially, no patients had progressive pneumonia after immune-modulator treatment despite of a large MP series. These findings suggest that importance of early control of the disease. Although patients infected with MRMP had the prolonged fever duration, few cases showed apparent treatment failure with macrolides [14, 15], suggesting the self-limited nature of the diseases. In addition, some severely affected patients treated with MP-sensitive antibiotics (tetracyclines or fluoroquinolones) in the early stage of the disease can have progressive severe pneumonia [17, 18], as well as the case 4 in this series. Okada et al reported that the antibiotic change from macrolides to tetracyclins (minocyclines or doxycyclines) for MRMP pneumonia patients with prolonged fever over 3 days or worsened pneumonia induced rapid defervescence within 24 hours, but 44% of these patients received corticosteroids for 1-2 days [11]. It has also been reported that empiric antibiotic coverage of atypical pathogens for community-acquired pneumonia have shown no benefit of clinical efficacy in hospitalized children and adults with pneumonia [19, 20]. At present time, both tetracyclines and fluoroquinolones are not recommended for use in children younger than 8 years due to complications such as teeth discolouration and potential cartilage damage [6], despite the majority of MP pneumonia patients under 8 years of age in this series.
The immunopathogenesis of MP pneumonia is still unknown. MP pneumonia patients show a variety of clinical severity from mild pneumonia to fatal progressive pneumonia, but the host immune response against the insults from MP infection may be similar. The rapid response to immune-modulators (corticosteroids) and the limited effectiveness of antibiotics on some severely affected patients have suggested that acute lung injury in MP infections is associated with a hyperactive immune reaction of the host, rather than a direct cytopathic effect of MP on respiratory cells [1, 7, 8, 9, 21]. It is also postulated that substances originated from injured lung cells can induce further inflammation with corresponding immune cells if released into systemic circulation or near local lesions. Therefore, early control of initial lung injury from hyperactive immune reactions may be essential for the prevention of further lung injury and a reduction of morbidity in MP pneumonia as well as other respiratory viral pneumonias such as influenza pneumonia. The beneficial effect of corticosteroids may act on immune cells, especially abnormally hyperactivated non-specific T cells, by inducing apoptosis [21, 22]. Although high-dose and prolonged use of corticosteroids are known to induce side effects such as immune suppression, medium-dose and short-term use of our policy (prednisolone 1 mg/kg/day for 2-3 days, tapering and stopping within 7 days) may not cause serious effect on immune system. Since the effect of corticosteroids is dependent on the dose administered and the severity of affected patients with excess immune substances, a higher dose may be needed for more severe patients in early stage of the disease, as suggested based on this study. In addition, initial IVIG treatment may be helpful for patients who have difficulty with the use of corticosteroids.
In the present study, we found that high-dose IVIG played an important role to prevent from the disease progression for the patients with severe inflammation. Four IVIG-treated patients who remained febrile with aggravated pneumonia lesions after corticosteroids showed clinical and radiological improvement within 2 days, as shown in figures. We also previously experienced the effect of IVIG on a severe influenza pneumonia case with corticosteroid treatment [23]. The immune-modulation mechanisms of IVIG is unknown, but various mode of actions, including Fc receptor blockage, neutralization of etiologic agents and the suppression of activated immune cells, have been proposed [24]. Because high-dose IVIG has the action of systemic protein modulation in vivo [25], IVIG may act on the proteins that are involved in protein cascade pathways in the inflammatory process. Although many investigators have reported the beneficial effects of corticosteroids on severe antibiotic non-responsive MP pneumonia patients [7, 8, 9, 10, 16, 17, 18] and experimental animals [26, 27], there are few reports regarding the effects of IVIG on MP pneumonia. Yun et al reported that IVIG therapy was beneficial for patients with pneumonia, including MP pneumonia, who did not respond to initial antibiotic therapy [28]. Some investigators have used IVIG for extrapulmonary manifestations of MP infection, including encephalopathy, rhabdomyolysis and hemolytic anemia [29, 30].
Single serologic test for diagnosis of MP pneumonia is problematic because of a lack of IgM antibodies at presentation (false negative) and a longer existence of IgM antibodies in epidemics (false positive) [1, 13]. Thus, our patient-selection policy in this study could help to overcome the early diagnostic problem and reduce false-positive and false negative cases as much as possible during epidemic periods.
We previously reported that the severity of MP pneumonia is associated with IgM negativity in the early stages of MP pneumonia in older children [10]. This finding suggests that recovery of lung injury may begin at the stage of the appearance of specific antibodies during the self-limiting inflammatory process, and specific antibodies and specific T cells against pathogens may not be related to lung injury in the early stage of disease. We also found that young children (<2 years of age) had a milder clinical course with milder pneumonic lesions compared to older children [10]. In this series, the patients treated with corticosteroids were younger, and coinfection rate with viruses tended higher than the patients treated without corticosteroids. It is in part explained that we used corticosteroids early for patients who showed wheezing and respiratory distress at presentation and coinfection with a viral agent might be responsible for the severe symptoms of the lower respiratory tract infection. Nevertheless, the patients treated immune modulators showed a rapid clinical improvement, including virus coinfected patients.
There are some limitations in this study. We could not confirm the strains in this series were MRMP strains. Although this retrospective work was not a case-control study, we showed the effectiveness of immune-modulators through the comparison with the results from other study groups. We used various antibiotics in this series, but we did not aim to deny the important role of antibiotics on MP pneumonia. Antibiotics may affect on not only MP but also normal flora that can possibly be helpful for replication of MP in the host, because of fastidious growing nature of MPs in the culture system without feeding cells [1]. For some severe patients, we used the immune-modulators prior to a definitive diagnosis of MP pneumonia. Given the large outbreak of pneumonia patients in epidemic period (summer season), the relatively mild clinical condition against chest radiographic finding and the normal or low leukocyte count with low lymphocyte differential, we could diagnose early MP pneumonia and tried the preemptive treatments for possibly progressive MP pneumonia.
In conclusion, additional immune-modulator therapy for MP pneumonia patients, including severe or potentially progressive pneumonia patients, showed rapid clinical and chest radiographic improvement without side effects. To our knowledge, this study may contain the first report regarding high-dose IVIG treatment for severe MP pneumonia in children. In the future, controlled clinical studies for suitable antibiotics with or without immune-modulators on children with severe MRMP infection are needed.
Acknowledgment
No fund in this study. The authors thank Drs. Sun-Mi Huh, Min-Chae Kim, Seung-Woo Keum and other doctors for patient care during study period.
References
- 1.Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther. 2008;6:509–521. doi: 10.1586/14787210.6.4.509. [DOI] [PubMed] [Google Scholar]
- 2.Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008;56:326–331. doi: 10.1016/j.jinf.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Morozumi M, Takahashi T, Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J Infect Chemother. 2010;16:78–86. doi: 10.1007/s10156-009-0021-4. [DOI] [PubMed] [Google Scholar]
- 4.Cao B, Zhao CJ, Yin YD, Zhao F, Song SF, Bai L, Zhang JZ, Liu YM, Zhang YY, Wang H, Wang C. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infections in China. Clin Infect Dis. 2010;51:189–194. doi: 10.1086/653535. [DOI] [PubMed] [Google Scholar]
- 5.Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, Kang HM, Lee J, Ahn YM, Kang YH, Lee JH. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg Infect Dis. 2013;19:1281–1284. doi: 10.3201/eid1908.121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother. 2013;68:506–511. doi: 10.1093/jac/dks457. [DOI] [PubMed] [Google Scholar]
- 7.Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, Lee BC. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2006;41:263–268. doi: 10.1002/ppul.20374. [DOI] [PubMed] [Google Scholar]
- 8.Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect. 2008;57:223–228. doi: 10.1016/j.jinf.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyashita N, Obase Y, Ouchi K, Kawasaki K, Kawai Y, Kobayashi Y, Oka M. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol. 2007;56:1625–1629. doi: 10.1099/jmm.0.47119-0. [DOI] [PubMed] [Google Scholar]
- 10.Youn YS, Lee KY, Hwang JY, Rhim JW, Kang JH, Lee JS, Kim JC. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010;10:48. doi: 10.1186/1471-2431-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–1649. doi: 10.1093/cid/cis784. [DOI] [PubMed] [Google Scholar]
- 12.Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, Saito A, Kondo E, Teranishi H, Ogita S, Tanaka T, Kawasaki K, Nakano T, Terada K, Ouchi K. Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother. 2013;57:2252–2258. doi: 10.1128/AAC.00048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkinson TP, Balish MF, Waits KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–973. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Yamazaki T, Narita M, Okazaki N, Suzuki I, Andoh T, Matsuoka M, Kenri T, Arakawa Y, Sasaki T. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother. 2006;50:709–712. doi: 10.1128/AAC.50.2.709-712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara K, Morozumi M, Okada T, Matsushima T, Komiyama O, Okada T, Matsushima T, Komiyama O, Shoji M, Ebihara T, Ubukata K, Sato Y, Akita H, Sunakawa K, Iwata S. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother. 2009;15:380–383. doi: 10.1007/s10156-009-0715-7. [DOI] [PubMed] [Google Scholar]
- 16.You SY, Jwa HJ, Yang EA, Kil HR, Lee JH. Effects of methylprednisolone pulse therapy on refractory Mycoplasma pneumoniae pneumonia in children. Allergy Asthma Immunol Res. 2014;6:22–26. doi: 10.4168/aair.2014.6.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Lee KY, Kim MS, Youn YS, Hwang JY, Rhim JW, Kang JH, Lee JS. Corticosteroid treatment in siblings affected with severe Mycoplasma pneumoniae pneumonia. Infect Chemother. 2009;41:190–195. [Google Scholar]
- 18.Takei T, Morozumi M, Ozaki H, Fujita H, Ubukata K, Kobayashi I, Kadota K, Miyamae T, Yokota S, Iwata S, Takahashi T. Clinical features of Mycoplasma pneumoniae infections in the 2010 epidemic season: report of two cases with unusual presentations. Pediatr Neonatol. 2013;54:402–405. doi: 10.1016/j.pedneo.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Mulholland S, Gavranich JB, Chang AB. Antibiotics for community acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database Sys Rev. 2010;7:CD004875. doi: 10.1002/14651858.CD004875.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Eliakim-Raz N, Robenshtok E, Shefet D, Grafter-Gvili A, Vidal L, Paul M, Leibovici L. Empiric antibiotic coverage of atypical pathogens for community-acquired pneumonia in hospitalized adults. Cochrane Database Syst Rev. 2012;9:CD004418. doi: 10.1002/14651858.CD004418.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youn YS, Lee KY. Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2012;55:42–47. doi: 10.3345/kjp.2012.55.2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KY, Rhim JW, Kang JH. Hyperactive immune cells (T cells) may be responsible for acute lung injury in influenza infections: a need for early immune-modulators for severe cases. Med Hypotheses. 2011;76:64–69. doi: 10.1016/j.mehy.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhim JW, Lee KY, Youn YS, Kang JH, Kim JC. Epidemiological and clinical characteristics of childhood pandemic 2009 H1N1 virus infection: an observational cohort study. BMC Infect Dis. 2011;11:225. doi: 10.1186/1471-2334-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee KY, Lee JS. Immunoglobulin G has a role for systemic protein modulation in vivo: a new concept of protein homeostasis. Med Hypotheses. 2006;67:848–855. doi: 10.1016/j.mehy.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Tagliabue C, Salvatore CM, Techasaensiri C, Mejias A, Torres JP, Katz K, Gomez AM, Esposito S, Principi N, Hardy RD. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J Infect Dis. 2012;198:1180–1188. doi: 10.1086/591915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirao S, Wada H, Nakagaki K, Saraya T, Kurai D, Mikura S, Yasutake T, Higaki M, Yokoyama T, Ishii H, Nakata K, Aakashi T, Kamiya S, Goto H. Inflammation provoked by Mycoplasma pneumoniae extract: implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunol Med Microbiol. 2011;62:182–189. doi: 10.1111/j.1574-695X.2011.00799.x. [DOI] [PubMed] [Google Scholar]
- 28.Yun HJ, Kim YH, Hyun MC. The effect of immunoglobulin as adjuvant therapy in pediatric patients with antibiotic ineffective pneumonia. Pediatr Allergy Respir Dis. 2010;20:17–22. [Google Scholar]
- 29.Attilakos A, Palaiologou P, Lagona E, Voutsioti A, Dinopoulos A. Mycoplasma pneumoniae encephalopathy: recovery after intravenous immunoglobulin. Pediatr Neurol. 2008;38:357–359. doi: 10.1016/j.pediatrneurol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Weng WC, Peng SS, Wang SB, Chou YT, Lee WT. Mycoplasma pneumonia-associated transverse myelitis and rhabdomyolysis. Pediatr Neurol. 2009;40:128–130. doi: 10.1016/j.pediatrneurol.2008.10.009. [DOI] [PubMed] [Google Scholar]