Abstract
Although antibiotics whose epithelial lining fluid (ELF) concentrations are reported high tend to be preferred in treatment of pneumonia, measurement of ELF concentrations of antibiotics could be misled by contamination from lysis of ELF cells and technical errors of bronchoalveolar lavage (BAL). In this review, ELF concentrations of anti-methicillin resistant Staphylococcus aureus (MRSA) antibiotics were interpreted considering above confounding factors. An equation used to explain antibiotic diffusion into CSF (cerebrospinal fluid) was adopted: ELF/free serum concentration ratio = 0.96 + 0.091 × ln (partition coefficient / molecular weight1/2). Seven anti-MRSA antibiotics with reported ELF concentrations were fitted to this equation to see if their ELF concentrations were explainable by the penetration capacity only. Then, outliers were modeled under the assumption of varying contamination from lysed ELF cells (test range 0-10% of ELF volume). ELF concentrations of oritavancin, telavancin, tigecycline, and vancomycin were well described by the diffusion equation, with or without additional impact from cell lysis. For modestly high ELF/free serum concentration ratio of linezolid, technical errors of BAL should be excluded. Although teicoplanin and iclaprim showed high ELF/free serum ratios also, their protein binding levels need to be cleared for proper interpretation. At the moment, it appears very premature to use ELF concentrations of anti-MRSA antibiotics as a relevant guide for treatment of lung infections by MRSA.
Keywords: Epithelial lining fluid, Methicillin-resistant Staphylococcus aureus, Bronchoalveolar lavage, Protein binding
Introduction
In a review of our own [1], we raised the idea that various epithelial lining fluid (ELF)/serum concentration ratios of antibiotics might be understood with their protein binding, penetration capacity, potential contamination from intracellular concentrations, and possible technical errors of bronchoalveolar lavage (BAL). With an equation reflecting protein binding and penetration capacity through cellular barrier, low ELF concentrations of beta-lactams could be explained with poor penetration of their free fraction through the tightly linked alveolar epithelium. High ELF/free serum concentration ratios of macrolides, fluoroquinolones could be explained with lysis of ELF cells, mainly alveolar macrophages, which might occur during the process of measurement. There existed outliers not fitting to the model. However, other possible technical errors - overestimation of the volume of ELF due to prolonged dwelling time of BAL fluid, improper volume of instilled fluid, contamination of blood etc. - might explicate the gaps. Therefore, our model was believed to explain various ELF/serum concentration ratios of antibiotics, either they were lower or higher, without imagination of additional mechanisms such as active transport system in the alveolar epithelium, existence of which is doubtful.
In this review, we focused on ELF concentrations of anti-MRSA(methicillin-resistant Staphylococcus aureus) antibiotics. MRSA is one of the most prevalent pathogens of nosocomial pneumonia, and is also becoming an important pathogen causing community-acquired pneumonia. While just a few anti-MRSA antibiotics were included in our previous review, several other reports on the topic have been published recently.
Materials and Methods
1. Data Sources
For the evaluation, Medline (January 1993 to May 2014) was searched for BAL measuring concentrations of anti-MRSA antibiotics in ELF. Only human studies are included. Data on antibiotic concentrations measured simultaneously in serum, ELF, and alveolar macrophages were preferred. Under the criteria, the following 7 anti-MRSA antibiotics from a total of 9 publications were included in the evaluation: Iclaprim [2], Linezolid [3, 4, 5], Oritavancin [6], Teicoplanin [7], Telavancin [8], Tigecycline [9], and Vancomycin [6, 10, 11].
2. Steps simulating estimated ELF concentrations of antibiotics
1) Lipophilicity and diffusibility of antibiotics were considered. Unbound free antibiotics in serum are considered to freely equilibrate with the interstitial levels of antibiotics. However, because alveolar epithelial cells are linked by tight junctions, interstitial antibiotics must pass through the alveolar epithelial cells to reach ELF. Penetration of antibiotics through the cellular barrier is mainly influenced by lipophilicity and diffusibility of the drugs, which is similar to drug penetration through blood-CSF(cerebrospinal fluid) barrier. Thus, the following equation was adopted from a study evaluating drug entry into CSF through the blood-CSF barrier to express ELF-to-serum level ratios of antibiotics [12].
Celf/Cfs (or AUCelf/AUCfs) = 0.96 + 0.091 · ln (PC · MW-1/2) = K
Celf/Cfs, the ratio of ELF concentration/free serum concentration
A UCelf/AUCfs, the ratio of area under the curve (AUC) in ELF/free AUC in serum
PC, octanol/water partition coefficient
MW, molecular weight
Physical and chemical properties of the antibiotics investigated are presented at Table 1. Partition coefficient of teicoplanin was not found throughout publications that constant K expressing penetration capacity could not be calculated. Therefore, for the constant K of teicoplanin, CSF/free serum AUC ratio of the antibiotic from a study was adopted [13].
Table 1.
Physical and chemical properties of the antibiotics investigated
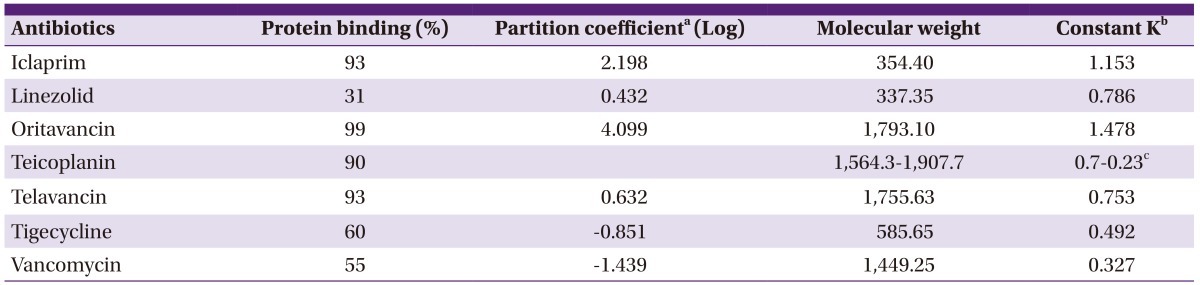
aSciFinder Scholar database (American Chemical Society 2007).
bConstant K = 0.96 + 0.091 · ln (partition coefficient / molecular weight1/2).
cCSF(cerebrospinal fluid)/free plasma area under the curve ratio adopted from a study evaluating CSF penetration of teicoplanin. 90% of protein binding was considered also (Reference 8).
2) Potential lysis of ELF cells was considered. Lysis of some fraction of ELF cells, consisting around 3-10% of ELF volume, would influence the measured ELF concentrations of antibiotic. In that case, original ELF concentrations without contamination from cellular component can be calculated as follows.
mCelf × (Velf + Vcell) = oCelf × Velf + Ccell × Vcell.
oCelf = mCelf × (1+ Vcell/Velf) - Ccell × (Vcell/Velf).
mCelf, measured ELF concentration
Velf, volume of ELF
Vcell, volume of lysed cells
oCelf, original ELF concentration
Ccell, intracellular concentration.
3) ELF concentrations measured by BAL were interpreted considering above 2 factors. The ratios of expected original Celf/Cfs (or AUCelf/AUCfs) divided by the constant K were plotted against the extent of cell lysis. For interpretation, as original Celf/(Cfs · K) (or original AUCelf/[AUCfs · K]) approached 1.0 without consideration of cell lysis, the ratio of ELF concentration/free serum concentration of an antibiotic were regarded to be explained on the basis of the penetration capacity of the antibiotic. Furthermore, as the equation approached 1.0 with a larger extent of cell lysis within 10% of ELF volume, we concluded that the measured ELF concentration might be explained by contamination of antibiotics from lysed cells.
Results
Antibiotic concentrations or their AUC in ELF, alveolar macrophage cells comparing to serum levels are presented at Table 2. In some studies, antibiotic concentrations in alveolar macrophages were not measured. Therefore, ratios of intracellular concentration to free plasma levels were adopted from a study evaluating antibiotic activities in human THP-1 macrophages (intracellular) versus those in culture medium (extracellular) by using a 0- to 24-h exposure time and a wide range of extracellular concentrations [14].
Table 2.
Antibiotic concentrations in ELF and alveolar macrophage cells comparing to serum levels
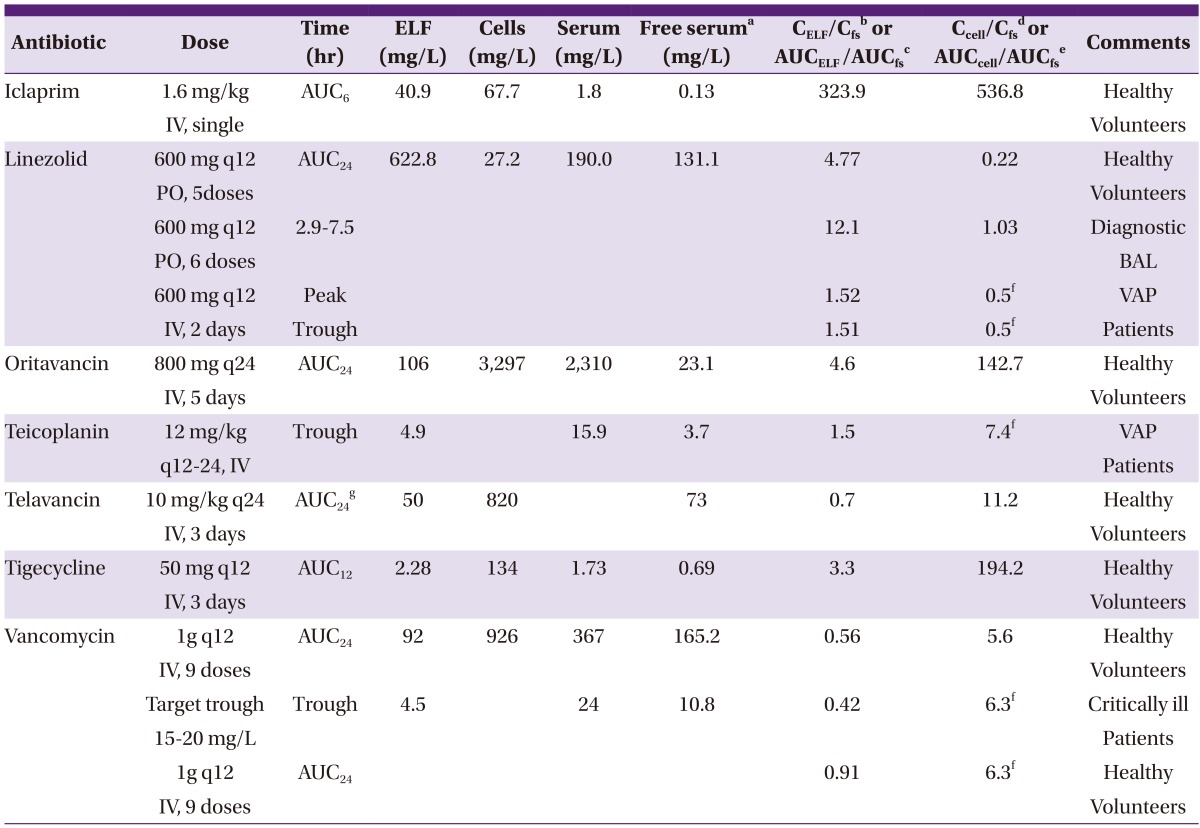
aFree serum concentrations or AUCs(area under the curve) were calculated from the protein binding levels of the corresponding antibiotics.
bCELF/Cfs, ratio of antibiotic concentrations in ELF comparing to their free serum levels.
cAUCELF/AUCfs, ratio of AUC of antibiotic concentrations in ELF comparing to their free serum AUC.
dCcell/Cfs, ratio of intracellular antibiotic concentrations comparing to their free serum levels.
eAUCcell/AUCfs, ratio of AUC of intracellular antibiotic concentrations comparing to their free serum AUC.
fRatios of intracellular concentration to free serum levels were adopted from a study evaluating antibiotic activities in human THP-1 macrophages versus those in culture medium (Reference 14).
gAUCs were calculated from approximate antibiotic levels retrieved from time-concentration graphs.
ELF, epithelial lining fluid; AUC, area under the curve; BAL, bronchoalveolar lavage; VAP, ventilator associated pneumonia; IV, intravenous; PO, by mouth(per os).
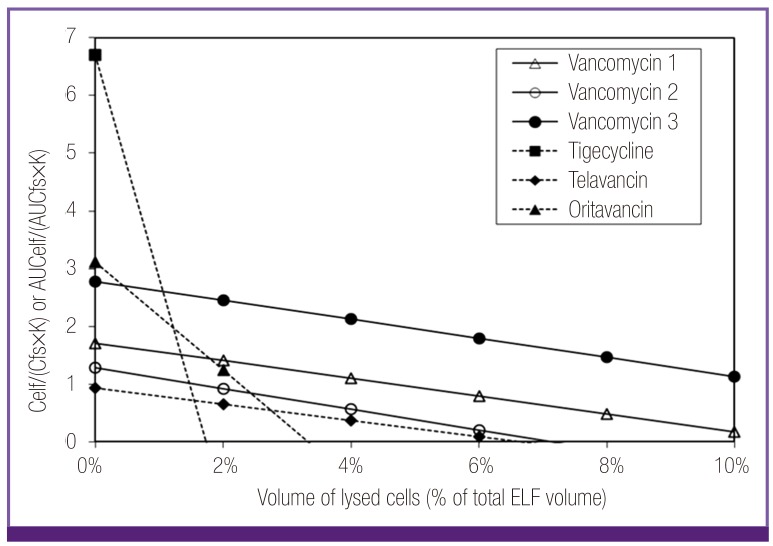
When the ELF/free serum concentration ratio of antibiotics were analyzed by the penetration capacity (constant K) and influence of possible cellular lysis, those of oritavancin, telavancin, tigecycline, and vancomycin could be explained by the penetration capacity with or without contamination of antibiotics from lysed cells (Fig. 1). Low ELF concentrations of telavancin and vancomycin were mainly explained by their poor penetration capacity, like beta-lactams, although a small percentage of cellular lysis was also needed for the full interpretation in case of vancomycin. High ELF concentrations of oritavancin and tigecycline were explicable with potential contamination from their high intracellular concentrations, similar to macrolides and fluoroquinolones.
Figure 1.
Plot of ELF/free serum concentration ratios of oritavancin, telavancin, tigecycline and vancomycin considering penetration capacity (constant K) and potential lysis of ELF cells. Low ratios of telavancin and vancomycin were considered to be related to their low penetration capacity. High ratios of oritavancin and tigecycline beyond their penetration capacity could be explained with contamination from lysed ELF cells within the range of the volume percentage of the cells (3-10%) in ELF.
Celf, ELF concentration; Cfs, free serum concentration; K, constant K=0.96 + 0.091·ln (partition coefficient · molecular weight-1/2); AUCelf, AUC in ELF; AUCfs, free AUC in serum; ELF, epithelial lining fluid; AUC, area under the curve.
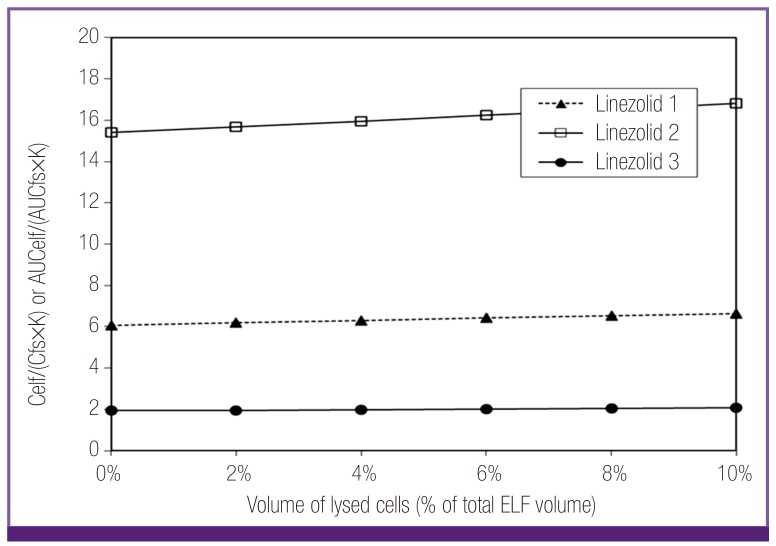
While the ELF/ free serum concentration ratio of linezolid were modestly high (1.5-12.1), neither penetration capacity nor cell lysis would account for this pattern (Fig. 2). However, it was considered that technical errors in the process of BAL could be involved in the high measured ELF concentrations of linezolid. The data showing comparable ELF concentration to free serum level of linezolid (Linezolid 3) were obtained from a study using mini-BAL rather than the traditional BAL. While the mini-BAL instilled 40 ml of saline instead of 200 ml, volume of instilled fluid during BAL or other possible errors of BAL might make the difference in interpretation of amount of solutes in ELF. In that sense, the modest high ELF concentrations of linezolid would be explainable within the range of error.
Figure 2.
Plot of ELF/free serum concentration ratios of linezolid considering penetration capacity (constant K) and potential lysis of ELF cells. The modest ratio of linezolid could not be explained with either penetration capacity nor cell lysis. By the difference of the ratios depending on different techniques (linezolid 1, 2 vs. linezolid 3) it was considered that technical errors might be involved in the process of BAL.
Celf, ELF concentration; Cfs, free serum concentration; K, constant K=0.96 + 0.091·ln (partition coefficient · molecular weight-1/2); AUCelf, AUC in ELF; AUCfs, free AUC in serum; ELF, epithelial lining fluid; AUC, area under the curve.
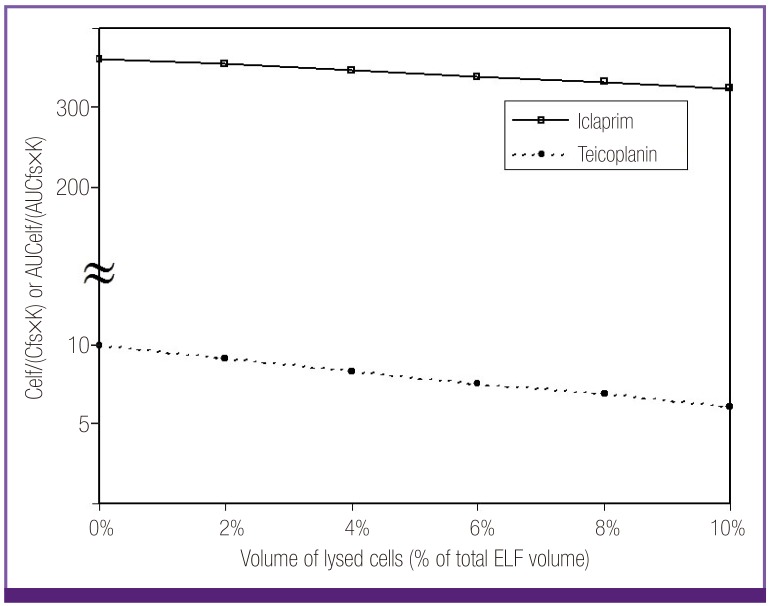
ELF/ free serum concentration ratio of iclaprim and teicoplanin also could not be explained with either penetration capacity or cell lysis (Fig. 3). While the ELF/free serum concentration ratio of teicoplanin were modest, the ratio of iclaprim was very high even after consideration of total cell lysis. However, in case of these 2 anti-MRSA antibiotics, their real free serum levels might be different from the calculated levels in this review. Studies on protein binding of teicoplanin and iclaprim have reported that decrease of antimicrobial activities of the antibiotics, represented by changes in MIC/MBC (minimal inhibitory concentration/minimal bactericidal concentration), were much less than those expected from their very high in vitro protein binding levels of 90% and 93%, respectively [15, 16]. Especially for iclaprim, there were no significant changes in MIC/MBC when culture media were mixed with human serum. If the protein binding is ignored based on the above studies, high ELF/free serum concentration ratio of teicoplanin and iclaprim are also explainable with the penetration capacity and cell lysis.
Figure 3.
Plot of ELF/free serum concentration ratios of iclaprim and teicoplanin considering penetration capacity (constant K) and potential lysis of ELF cells. The ratios could not be explained with either penetration capacity nor cell lysis. Protein binding levels of the antibiotics need to be cleared for the proper interpretation, for biologically measured levels of their protein binding were negligible despite the reported high in vitro protein binding rates.
Celf, ELF concentration; Cfs, free serum concentration; K, constant K=0.96 + 0.091·ln (partition coefficient · molecular weight-1/2); AUCelf, AUC in ELF; AUCfs, free AUC in serum; ELF, epithelial lining fluid; AUC, area under the curve.
Discussion
Our previous and current review proposed a model comprehending protein binding, penetration capacity through cellular barrier, and potential cellular contamination during process of BAL, which could explain various ELF/serum concentration ratios of antibiotics. However, even with consideration of other possible technical errors, high ELF concentration of an anti-MRSA antibiotic, iclaprim looked very exceptional. As stated above, we believe that the issue on huge discrepancy between in vitro protein binding levels and the biologically measured protein binding rates of iclaprim needs to be solved first to understand real physiology of the antibiotic through alveolar epithelial cells.
Protein binding rate is usually obtained in vitro at a fixed drug and protein concentration. However, this measurement may not be physiologic in many ways. First, binding of drugs to serum protein is a continuing dynamic of association and dissociation. The proportion of binding at steady state varies depending on association constant, drug concentration and protein concentration. Even with given association constant and protein concentration, proportion of binding can change continuously with changing total drug level in vivo [17]. Second, since the effect of protein binding is buffered by relatively large amounts of extravascular fluid, the percentage of protein binding in vitro measured at equilibrium does not contribute to the same extent in vivo situation [18, 19]. In addition, antibiotics bind to bacteria, interstitial and cellular associated proteins and intracellular substances, and these binding sites may compete with serum albumin for antibiotic binding [18]. Furthermore, inflammation of infected tissues is expected to alter passage of intravascular antibiotics into the tissues.
In case of iclaprim, whose MIC/MBC did not change after being mixed with albumin in spite of its very high in vitro protein binding, bacteria could be better binding sites of the antibiotic than albumin molecules, or iclaprim might be able to exert their antibiotic effect on bacteria even after binding to protein. The same phenomena could happen to teicoplanin although the degree would be less than iclaprim. At current, it is not known why biologically measured protein binding rates of iclaprim and teicoplanin are so different from in vitro protein binding levels. Assessment of effect of protein binding on iclaprim and teicoplanin should be cleared further for proper interpretation of their high ELF/calculated free serum concentration ratios.
On the other hand, another anti-MRSA antibiotic, daptomycin, has presented interesting data on the issue of alveolar site infection. Clinical study demonstrated it's inferior outcomes for community-acquired pneumonia (79.4%) compared to ceftriaxone (87.9%). Rat models found that daptomycin did not exhibit any activity against organisms of bronchially induced pneumonia while it killed the organisms predictably when these organisms were introduced hematogenously. And it was subsequently determined by in vitro study that pulmonary surfactant inhibited daptomycin activity [20]. On the basis of these studies, we may conclude that daptomycin is not effective in lung infection, by virtue of an MIC shift caused by the presence of surfactant.
However, we need to focus on the facts that daptomycin did have considerable clinical success rates (79.4%) for community-acquired pneumonia and was also effective for bacteremic pneumonia in animal studies. That might imply that daptomycin is still active in some deep site lung infections where surface contamination with surfactant may not be a player. In fact, it is questionable if ELF including surfactant represents the lung site of infection. Because lung infections disrupt the alveolar wall and invade the interstitial space, superficial areas like ELF should not represent the site of progressed lung infections. ELF concentration of antibiotics should have some meaning for superficial alveolar infections such as bronchitis, perhaps in the very early phase of community-acquired pneumonia, just like the bronchially induced rat pneumonias. The lower success rate of daptomycin in community-acquired pneumonia may also represent that superficial infection where inhibition by surfactant could hinder the activity of daptomycin.
Even considering the superficial alveolar infection, we believe that antibiotics with their high measured ELF concentrations should not necessarily be favored over other antibiotics with lower ELF penetration, because there exist many possibilities of error as our analyses shows. The differences could be just derived from penetration difference. Instead, interstitial concentrations of antibiotics would correlate better to the microbiological and clinical outcomes of the invasive lung infections. Microdialysis studies, measuring interstitial concentrations of antibiotics precluding contamination from vascular and cellular components of the tissue, have reported lung tissue concentrations of antibiotics, regardless of the classes, similar to free serum levels [21, 22, 23, 24].
Therefore, we are not convinced that antibiotics have different AUIC, representing the 24-h area under the concentration versus time curve (AUC)/MIC ratio, vs. response curves between blood and lung tissue. Our studies have presented the target value of AUIC ≥ 125 for ciprofloxacin [25] and AUIC >350 for vancomycin [26, 27], which was primarily derived from studies of nosocomial pneumonia, but seems to work just fine for bacteremias and other infection sites. We believe that is because there is equilibration between blood and lung tissue, producing similar exposure. Basically, lung is not known as a secretary organ. Because the MIC of the individual pathogen is the key driver of outcome, we believe that those antibiotics need studies based on microbial killing as well as clinical outcome if they wish to compare themselves appropriately.
Conclusion
ELF concentrations of most anti-MRSA antibiotics were explainable by their penetration capacity and the anticipated range of cell lysis in the performance of the measurement itself. For interpretation of high ELF concentration ratios of linezolid, iclaprim, and teicoplanin, technical errors of traditional BAL and protein binding based on biological activity need to be considered. In view of the technical and interpretive problems of ELF concentrations, it should be premature to use ELF concentrations of anti-MRSA antibiotics as a relevant guide for treatment of MRSA pneumonia, and the lung microdialysis experiments may offer an overall better correlation with microbiological outcomes than ELF concentrations.
Acknowlegement
This work was supported by Grant from Inje University, 2012 (20120848).
References
- 1.Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews J, Honeybourne D, Ashby J, Jevons G, Fraise A, Fry P, Warrington S, Hawser S, Wise R. Concentrations in plasma, epithelial lining fluid, alveolar macrophages and bronchial mucosa after a single intravenous dose of 1.6 mg/kg of iclaprim (AR-100) in healthy men. J Antimicrob Chemother. 2007;60:677–680. doi: 10.1093/jac/dkm242. [DOI] [PubMed] [Google Scholar]
- 3.Boselli E, Breilh D, Rimmelé T, Djabarouti S, Toutain J, Chassard D, Saux MC, Allaouchiche B. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit Care Med. 2005;33:1529–1533. doi: 10.1097/01.ccm.0000168206.59873.80. [DOI] [PubMed] [Google Scholar]
- 4.Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46:1475–1480. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honeybourne D, Tobin C, Jevons G, Andrews J, Wise R. Intrapulmonary penetration of linezolid. J Antimicrob Chemother. 2003;51:1431–1434. doi: 10.1093/jac/dkg262. [DOI] [PubMed] [Google Scholar]
- 6.Rodvold KA. Plasma and intrapulmonary concentrations of oritavancin and vancomycin in normal healthy adults. Abstracts of the 14th European congress of clinical microbiology and infectious diseases; 14th European congress of clinical microbiology and infectious diseases; Prague, Czech Republic. Basel, Switzerland: ESCMID; 2004. p. 44. Abstract O-254. [Google Scholar]
- 7.Mimoz O, Rolland D, Adoun M, Marchand S, Breilh D, Brumpt I, Debaene B, Couet W. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med. 2006;32:775–779. doi: 10.1007/s00134-006-0136-3. [DOI] [PubMed] [Google Scholar]
- 8.Gotfried MH, Shaw J, Benton BM, Krause KM, Goldberg MR, Kitt MM, Barriere SL. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother. 2008;52:92–97. doi: 10.1128/AAC.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte JE, Jr, Golden JA, Kelley MG, Zurlinden E. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int J Antimicrob Agents. 2005;25:523–529. doi: 10.1016/j.ijantimicag.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Lamer C, de Beco V, Soler P, Calvat S, Fagon JY, Dombret MC, Farinotti R, Chastre J, Gibert C. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob Agents Chemother. 1993;37:281–286. doi: 10.1128/aac.37.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodise TP, Drusano GL, Butterfield JM, Scoville J, Gotfried M, Rodvold KA. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob Agents Chemother. 2011;55:5507–5511. doi: 10.1128/AAC.00712-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nau R, Sörgel F, Prange HW. Lipophilicity at pH 7.4 and molecular size govern the entry of the free serum fraction of drugs into the cerebrospinal fluid in humans with uninflamed meninges. J Neurol Sci. 1994;122:61–65. doi: 10.1016/0022-510x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 13.Fernández A, Cabellos C, Tubau F, Maiques JM, Doménech A, Ribes S, Liñares J, Viladrich PF, Gudiol F. Experimental study of teicoplanin, alone and in combination, in the therapy of cephalosporin-resistant pneumococcal meningitis. J Antimicrob Chemother. 2004;55:78–83. doi: 10.1093/jac/dkh496. [DOI] [PubMed] [Google Scholar]
- 14.Barcia-Macay M, Seral C, Mingeot-Leclercq M, Tulkens PM, Van Bambeke F. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers HF, Kennedy S. Effects of dosage, peak and trough concentrations in serum, protein binding and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob Agents Chemother. 1990;34:510–514. doi: 10.1128/aac.34.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laue H, Valensise T, Seguin A, Hawser S, Lociuro S, Islam K. Effect of human plasma on the antimicrobial activity of iclaprim in vitro. J Antimicrob Chemother. 2007;60:1388–1390. doi: 10.1093/jac/dkm392. [DOI] [PubMed] [Google Scholar]
- 17.Koch-Weser J, Sellers EM. Binding of drugs to serum albumin (first of two parts) New Engl J Med. 1976;294:311–316. doi: 10.1056/NEJM197602052940605. [DOI] [PubMed] [Google Scholar]
- 18.Craig WA, Suh B. Protein Binding and the antimicrobial effects: methods for the determination of protein binding. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, MD: Williams and Wilkins; 1996. pp. 367–402. [Google Scholar]
- 19.Wise R. Protein binding of beta-lactams: the effects on activity and pharmacology particularly tissue penetration. I. J Antimicrob Chemother. 1983;12:1–18. doi: 10.1093/jac/12.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Silverman JA, Mortin LI, VanPraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 21.Herkner H, Műller MR, Keischitz N, Mayer BX, Frossard M, Joukhadar C, Klein N, Lackner E, Müller M. Closed-chest microdialysis to measure antibiotic penetration into human lung tissue. Am J Respir Crit Care Med. 2002;165:273–276. doi: 10.1164/ajrccm.165.2.2106082. [DOI] [PubMed] [Google Scholar]
- 22.Hutschala D, Skhirtladze K, Zuckermann A, Wisser W, Jaksch P, Mayer-Helm BX, Burgmann H, Wolner E, Müller M, Tschernko EM. In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob Agents Chemother. 2005;49:5107–5111. doi: 10.1128/AAC.49.12.5107-5111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomaselli F, Dittrich P, Maier A, Woltsche M, Matzi V, Pinter J, Nuhsbaumer S, Pinter H, Smolle J, Smolle-Jüttner FM. Penetration of piperacillin and tazobactam into pneumonic human lung tissue measured by in vivo microdialysis. Br J Clin Pharmacol. 2003;55:620–624. doi: 10.1046/j.1365-2125.2003.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeitlinger MA, Traunmüller F, Abrahim A, Müller MR, Erdogan Z, Müller M, Joukhadar C. A pilot study testing whether concentrations of levofloxacin in interstitial space fluid of soft tissues may serve as a surrogate for predicting its pharmacokinetics in lung. Int J Antimicrob Agents. 2007;29:44–50. doi: 10.1016/j.ijantimicag.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moise PA, Forrest A, Bhavnani SM, Birmingham MC, Schentag JJ. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm. 2000;57(Suppl 2):S4–S9. doi: 10.1093/ajhp/57.suppl_2.S4. [DOI] [PubMed] [Google Scholar]
- 27.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]