Abstract
Human infection caused by Shewanella algae is rare, which usually occurred after direct contact with seawater or ingestion of raw seafood in the immunocompromised host. There have been anecdotal reports about Shewanella infections in human, but their pathogenic role and microbiologic data are limited. Here, we report a fatal case of spontaneous bacterial peritonitis with bacteremia due to S. algae in a 57-year-old male with liver cirrhosis who had no history of exposure to seawater or raw seafood. Polymicrobial infection with Streptococcus mitis and Escherichia coli was combined and the patient died in spite of early appropriate antimicrobial therapy and early goal-directed therapy for sepsis.
Keywords: Bacteremia, Liver Cirrhosis, Peritonitis, Polymicrobial infection, Shewanella algae
Introduction
Shewanella species are Gram-negative, non-fermentative, motile bacilli mainly found in seawater and other underwater environments (fresh water, stagnant water, lakes, rivers, sewage), as well as soil, fish, meat, poultry and dairy products [1, 2]. This species was initially known as Achromobacter putrefaciens, followed by Pseudomonas putrefaciens, and was reclassified into the novel genus Shewanella in 1985, which contained approximately 30 Shewanella spp. [1]. Among these, only S. algae and S. putrefaciens are known to cause human infection. Human infection caused by Shewanella spp. is rare and occurs in mainly immunocompromised hosts who have a history of contact with seawater or ingestion of raw seafood. Infection is also associated with ulcerations on the lower extremities [1, 2, 3].
Recently, we experienced a case of spontaneous bacterial peritonitis (SBP) with bacteremia caused by Shewanella spp. in a patient with liver cirrhosis (LC) who had no history of exposure to seawater or raw seafood. Through biochemical studies and 16S rRNA polymerase chain reaction (PCR) with sequencing analysis, we confirmed the clinical isolates as S. algae. Polymicrobial infection by Streptococcus mitis and Escherichia coli was combined, which resulted in a fatal clinical course.
Case Report
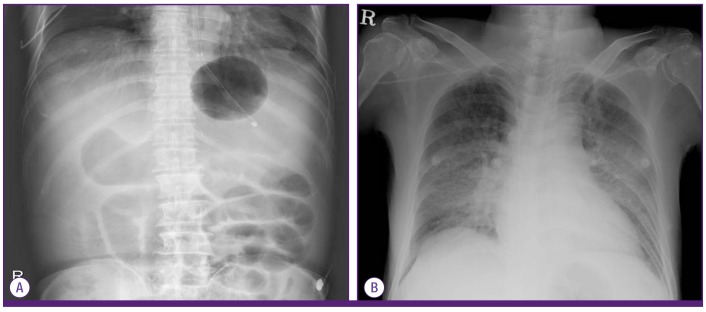
A 57-year-old male was transferred to the emergency department after endoscopic hemostasis for 1,000 mL of massive hematemesis due to esophageal and cardiac variceal bleeding. The patient had been diagnosed with alcoholic LC with varices 1 year ago. The patient was taking propranolol for varices and metformin for diabetes. The patient had no history of travel, no contact with seawater or fresh water, and did not consume raw fish within the last 6 months. On physical examination, the blood pressure was 100/40 mmHg, heart rate was 176 beats/min, respiration rate was 28/min and body temperature was 35℃. The mental state was alert but confused and icteric sclerae were observed. The heart sound was regular without murmur, and the breathing sound was clear. The abdomen was distended with decreased bowel sounds. Complete blood count showed white blood cells (WBC) counts of 12,960/mm3 (neutrophils, 78%), hemoglobin levels of 5.7 g/dL, and platelet counts of 46,000/mm3. A coagulation test showed a prothrombin time (PT) of 22.8 seconds (33.8%, INR 2.04) and an activated partial thromboplastin time of 58.7 seconds. Blood chemistry showed the following: aspartate aminotransferase/alanine aminotransferase 522/103 IU/L, total bilirubin/direct bilirubin 3.21/1.18 mg/dL, total protein/albumin 4.3/2.29 g/dL, creatine phosphokinase/lactate dehydrogenase 281/2,358 IU/L, blood urea nitrogen/creatinine 14.0/0.96 mg/dL, sodium/potassium 152/3.9 mmol/L. Serum osmolarity was 349 mOsm/kg, lactic acid was 119.5 mg/dL (1-13 mg/dL) and highly sensitive C-reactive protein (hsCRP) was 29.34 mg/L. Arterial blood gas analysis under 4 L of oxygen via nasal cannula revealed a pH of 7.19, PaCO2 of 15.6 mmHg, PaO2 of 109.5 mmHg, HCO3- of 5.9 mmol/L, and oxygen saturation of 97.3%, which represented metabolic acidosis with an anion gap of 35.1. Chest X-ray was normal but, abdominal X-ray showed moderate ascites and paralytic ileus (Fig. 1A). Because of persistent hemodynamic instability, we could not perform endoscopic hemostasis. Instead, we inserted a Sengstaken Blakemore (SB) tube. On the second hospital day (HD), the patient had a fever up to 40℃ and showed decreased mentality. To exclude SBP, we performed ascites tapping. The ascites fluid analysis revealed WBC counts of 5,800/mm3 (neutrophils, 93%), albumin levels of 0.43 g/dL and the serum-ascites albumin gradient of 2.24, which agreed with SBP. We initiated cefotaxime (6 g/day) and metronidazole (1,500 mg/day) and continued early goal-directed therapy (EGDT) for sepsis. On the third HD, the patient was intubated and underwent mechanical ventilation. Based on the follow-up chest X-ray (Fig. 1B) and clinical course, we assessed the patient having the respiratory failure which was combined with pulmonary edema due to massive hydration and transfusion for gastrointestinal bleeding and aspiration related to hematemesis. On the third HD, the blood culture revealed growth of Gram-positive cocci and Gram-negative bacilli. We added gentamicin (160 mg/day) because we could not exclude the possibility of infective endocarditis. Using the Microscan system (Siemens, Inc., Renton, WA, USA), Shewanella spp. and S. mitis were isolated from both peripheral and central blood (4 of 4 bottles) and Shewanella spp. and E. coli were isolated from ascites. S. mitis was susceptible to penicillin and E. coli was susceptible to all of the antibiotics including ampicillin, cefotaxime, levofloxacin and gentamicin. The Shewanella isolate was sub-cultured on MacConkey agar at 37℃, and growth was found after 16 hours of incubation. Based on the API 20NE kit (bioMérieux Inc., Marcy-l'Etoile, France), the organism was identified as S. putrefaciens with 99.9% certainty. We performed additional biochemical tests, as well as 16S rRNA PCR with sequencing analysis because it is known that an automatic bacterial culture system and biochemical identification system, such as ID32E, ID32GN, or API 20E could not distinguish S. algae from S. putrefaciens [2]. The isolate was incubated on sheep blood agar and showed growth at 42℃ (but not 4℃) and on nutrient agar containing 6.5% NaCl, which was compatible with S. algae. 16S rRNA gene sequencing analysis was performed with the following primers [4]: HDA1 forward primer (5'-ACTCCTACGGGAGGCAGCAGT-3') and HDA2 reverse primer (5'-GTATTACCGCGGCTGCTGGCA-3'). The isolate gene sequence showed 100% concordance with the S. algae strain QC39 (GenBank accession number: JN384129). S. mitis was susceptible to penicillin, and S. algae was susceptible to piperacillin, cefotaxime, ceftazidime, cefepime, carbapenems, aminoglycosides and quinolones. A transthoracic echocardiogram showed no evidence of vegetation. Because the patient had persistent fever, leukocytosis, and elevated hsCRP with progressing pneumonia, we changed the antibiotics to piperacillin/tazobactam (12/1.5 g/day) and amikacin (500 mg/day) on the 9th HD, which could reinforce the antimicrobial activity for nosocomial pathogens such as Pseudomonas. After then, fever subsided and clinical condition was improved with normalization of WBC and hsCRP levels. Follow-up culture of ascites on the 8th HD and blood on the 13th HD, showed no bacterial pathogens. Amikacin was discontinued on the 15th HD. The SB tube was removed on the 16th HD. However, hepatic failure was progressing with total bilirubin up to 30.84 md/dL and prolonged PT to 34.3 seconds (21.8%). Urine volume decreased below 500 mL/day despite diuretics use. Metabolic acidosis due to renal failure worsened and the mental state remained confused. Hepatorenal syndrome was suspected and planned dialysis was proposed, but his family refused further treatment. He died on the 19th HD.
Figure 1.
(A) Abdomen X-ray at admission showed moderate ascites and mild paralytic ileus. Sengstaken Blakemore tube was inserted in stomach. (B) Follow up chest X-ray at the second hospital day showed bilateral perihilar ill-defined hazziness.
Discussion
A patient with alcoholic LC suffered SBP with bacteremia caused by S. algae, though he had no history of previous exposure to seawater and raw seafood, nor ulcerations on the extremities. The patient showed a fatal clinical course accompanied with co-infection by S. mitis and E. coli. As S. algae is known to be more virulent than S. putrefaciens and these two species seem to exhibit different pathogenicities in humans, correct identification is important [1]. However, automatic bacterial identification systems fail to differentiate between S. algae and S. putrefaciens because the S. algae is not included in the databases of these systems [3]. We performed species identification using the API 20NE kit, which resulted in S. putrefaciens with 99.9% certainty. However, we could ultimately confirm the organism as S. algae by 16S rRNA PCR and gene sequence analysis.
Shewanella spp., initially known as Achromobacter putrefaciens, was reclassified under the genus Pseudomonas with the name of Pseudomonas putrefaciens in 1941. During the next three decades, P. putrefaciens was classified as Pseudomonas group IV by Shewan et al. In 1985, further phylogenetic studies resulted in a reclassification of these organisms into the family Vibrionaceae, and the description of a novel genus, Shewanella, named after James Shewan in honor of his work in marine microbiology. In the early 1990s, it was reclassified as a new species, S. alga and S. putrefaciens, although DNA homology between the two species was less than 10%. In 1997, the name of this new species was corrected to S. algae. Recent results of 16S rRNA gene sequence analyses led to a proposal for a new family, Shewanellaceae [1].
Shewanella spp. are found throughout the environment and human infection is rare but reports are increasing [1, 5]. Shewanella spp. commonly involve skin, soft tissues and ears. There are several reports regarding hepatobiliary infection, spondylodiscitis, infective endocarditis and severe systemic infection such as sepsis [2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. In Korea, three cases of systemic infection caused by S. algae and one case of endophthalmitis (which developed after trauma) have been reported [3, 6, 7, 8]. Among the three cases of S. algae systemic infection, two were skin and soft tissue infections (SSTI) with bacteremia. One patient was diagnosed with end-stage renal disease receiving hemodialysis and another was alcoholic LC. The other case was a spinal epidural abscess in a patient who had undergone distal pancreatectomy with cholecystectomy for an intraductal papillary mucinous tumor. To our knowledge, this is the first case of SBP with Shewanella bacteremia in Korea, particularly accompanied by polymicrobial infection.
Risk factors for Shewanella human infection include hepatobiliary disease, malignancies, severe heart failure, renal failure, peripheral vascular diseases and chronic ulcerations on the lower extremities, poor hygiene, and low socioeconomic status [3, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. Exposure to seawater or ingestion of raw seafood might be a predisposing factor of Shewanella infection. Lee et al., previously reviewed 16 cases of Shewanella human infection [3]. We also reviewed global studies and found a total of 21 cases with bacteremia by Shewanella spp.. Among these, in only 10 cases, we could identify the history of exposure to seawater. Six out of 10 (60%) were previously exposed to seawater or raw seafood, and those cases were presented as bacteremia with SSTI. Four cases, which had no history of exposure to seawater, were presented as primary bacteremia (n=2), SSTI (n=1) and SBP (this case). In particular, hepatobiliary disease has been proposed as a risk factor for poor outcome [5, 9, 10, 11]. Fifteen out of 21 (71.4%) developed in patients with hepatobiliary disease [5, 8, 9, 10, 11, 12, 13, 14, 15]. The mortality rate of Shewanella bacteremia was higher in patients with hepatobiliary disease, at 53.3% (8/15) compared to 16.7% (1/6) in cases without hepatobiliary diseases.
Shewenella spp. are usually susceptible to common antibiotics, including quinolones, carbapenems, aminoglycosides and erythromycin. Susceptibility to ampicillin and cephalosporins is variable, with more isolates being susceptible to third- and fourth- than first- and second-generation cephalosporins, but resistant to penicillin [1]. There is a report that 68% of S. putrefaciens are resistant to imipenem [12]; They recommended a combination of amikacin or gentamicin as an empirical therapy. In addition, the rapid emergence of resistance during imipenem treatment which was initially susceptible, was reported [8]. However, in the majority of community-onset Shewanella infections, resistance is not an issue because the clinical isolates are susceptible to commonly used empirical antibiotics [9, 12]. Further studies of susceptibility patterns according to the geographical region and antibiotics commonly selected for empirical therapy are required.
In our case, culture results for blood and ascites on admission showed polymicrobial infection with S. mitis, E. coli and S. algae. Among these isolates, S. algae was identified from all four blood bottles (two aerobic and two anaerobic) and from the ascites, with clinical manifestations compatible with SBP. Therefore, S. algae was considered to be the major pathogen of peritonitis and bacteremia. However, S. mitis and E. coli are also well known significant human pathogens that can cause bacteremia and SBP. We thought these organisms also played as significant pathogens and affected the outcome of this patient. In addition, there were several reasons for the poor outcome despite early administration of susceptible antibiotics and recovery from bacteremia. First, the patient suffered from alcoholic LC with Child-Pugh class C, which is known to be a predictive factor for death. Second, this disease was accompanied by polymicrobial infection, which could be associated with a poorer outcome than monomicrobial infections [20].
In conclusion, Shewanella infection can occur without exposure to seawater nor leg ulcers in the immunocompromised patients and causes not only SSTI but also severe systemic infections, especially in patients with hepatobiliary diseases. Here, we report a fatal case of SBP with severe sepsis caused by S. algae, accompanied by S. mitis and E. coli coinfection. The patient died from hepatorenal syndrome and renal failure, presenting with progressive metabolic acidosis, despite early and appropriate antimicrobial therapy in combination. Although it is an uncommon cause of disease in humans, we should be aware of the pathogenicity of this emerging bacterium.
References
- 1.Holt HM, Gahrn-Hansen B, Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect. 2005;11:347–352. doi: 10.1111/j.1469-0691.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 2.Gressier M, Mbayo D, Deramond H, Grados F, Eb F, Canarelli B. First case of human spondylodiscitis due to Shewanella algae. Int J Infect Dis. 2010;14:e261–e264. doi: 10.1016/j.ijid.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Lee ST, Lee SJ, Yun MJ, Oh HJ, Gang NR, Koo MS, Choi JP. A case of Shewanella algae bacteremia accompanying cellulitis in both legs of a patient on hemodialysis: case report and literature review. Infect Chemother. 2012;44:193–196. [Google Scholar]
- 4.Todorova SG, Costello AM. Design of Shewanella-specific 16S rRNA primers and application to analysis of Shewanella in a minerotrophic wetland. Environ Microbiol. 2006;8:426–432. doi: 10.1111/j.1462-2920.2005.00908.x. [DOI] [PubMed] [Google Scholar]
- 5.Goyal R, Kaur N, Thakur R. Human soft tissue infection by the emerging pathogen Shewanella algae. J Infect Dev Ctries. 2011;5:310–312. doi: 10.3855/jidc.1436. [DOI] [PubMed] [Google Scholar]
- 6.Myung DS, Jung YS, Kang SJ, Song YA, Park KH, Jung SI, Kim SH, Shin JH. Primary Shewanella algae bacteremia mimicking Vibrio septicemia. J Korean Med Sci. 2009;24:1192–1194. doi: 10.3346/jkms.2009.24.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh SY, Lee SJ, Park JM. A case of endophthalmitis caused by Shewanella algae after trauma. J Korean Ophthalmol Soc. 2013;54:365–369. [Google Scholar]
- 8.Kim DM, Kang CI, Lee CS, Kim HB, Kim EC, Kim NJ, Oh MD, Choe KW. Treatment failure due to emergence of resistance to carbapenem during therapy for Shewanella algae bacteremia. J Clin Microbiol. 2006;44:1172–1174. doi: 10.1128/JCM.44.3.1172-1174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otsuka T, Noda T, Noguchi A, Nakamura H, Ibaraki K, Yamaoka K. Shewanella infection in decompensated liver disease: a septic case. J Gastroenterol. 2007;42:87–90. doi: 10.1007/s00535-006-1957-0. [DOI] [PubMed] [Google Scholar]
- 10.Tsai MS, You HL, Tang YF, Liu JW. Shewanella soft tissue infection: case report and literature review. Int J Infect Dis. 2008;12:e119–e124. doi: 10.1016/j.ijid.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt U, Kapila R, Kaminski Z, Louria D. Pseudomonas putrefaciens as a cause of septicemia in humans. J Clin Microbiol. 1979;10:385–387. doi: 10.1128/jcm.10.3.385-387.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brink AJ, van Straten A, van Rensburg AJ. Shewanella (Pseudomonas) putrefaciens bacteremia. Clin Infect Dis. 1995;20:1327–1332. doi: 10.1093/clinids/20.5.1327. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Cooper RA, Welty-Wolf KE, Harrell LJ, Zwadyk P, Klotman ME. Pseudomonas putrefaciens bacteremia. Rev Infect Dis. 1989;11:97–104. [PubMed] [Google Scholar]
- 14.Chen YS, Liu YC, Yen MY, Wang JH, Wang JH, Wann SR, Cheng DL. Skin and soft-tissue manifestations of Shewanella putrefaciens infection. Clin Infect Dis. 1997;25:225–229. doi: 10.1086/514537. [DOI] [PubMed] [Google Scholar]
- 15.Vandepitte J, Debois J. Pseudomonas putrefaciens as a cause of bacteremia in humans. J Clin Microbiol. 1978;7:70–72. doi: 10.1128/jcm.7.1.70-72.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domínguez H, Vogel BF, Gram L, Hoffmann S, Schaebel S. Shewanella algae bacteremia in two patients with lower leg ulcers. Clin Infect Dis. 1996;22:1036–1039. doi: 10.1093/clinids/22.6.1036. [DOI] [PubMed] [Google Scholar]
- 17.Iwata M, Tateda K, Matsumoto T, Furuya N, Mizuiri S, Yamaguchi K. Primary Shewanella alga septicemia in a patient on hemodialysis. J Clin Microbiol. 1999;37:2104–2105. doi: 10.1128/jcm.37.6.2104-2105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saidel-Odes L, Borer A, Riesenberg K, Schlaeffer F. Shewanella spp. infection following treatment for upper gastrointestinal bleeding. Scand J Infect Dis. 2007;39:360–361. doi: 10.1080/00365540600978948. [DOI] [PubMed] [Google Scholar]
- 19.Pagani L, Lang A, Vedovelli C, Moling O, Rimenti G, Pristerà R, Mian P. Soft tissue infection and bacteremia caused by Shewanella putrefaciens. J Clin Microbiol. 2003;41:2240–2241. doi: 10.1128/JCM.41.5.2240-2241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiani D, Quinn EL, Burch KH, Madhavan T, Saravolatz LD, Neblett TR. The increasing importance of polymicrobial bacteremia. JAMA. 1979;242:1044–1047. [PubMed] [Google Scholar]