Abstract
Background
Increased serum ferritin and decreased vitamin D levels associated with nonalcoholic fatty liver disease (NAFLD). However, their association with the severity of NAFLD has not been fully evaluated. The aim of this study was to compare the association of serum ferritin and 25(OH)D3 levels with the severity of ultrasonographically detected NAFLD (US-NAFLD) and hepatic steatosis defined by fatty liver index (FLI) in Korean adults.
Methods
A cross-sectional analysis of clinical and anthropometric data, including serum ferritin and 25(OH)D3, from men (n=295) and women (n=263) who underwent a routine health check-up in 2012.
Results
In men, with an increase in the quartile of serum ferritin level, the incidences of subjects with metabolic syndrome (P=0.002), US-NAFLD (P=0.041), and FLI ≥60 (P=0.010) were significantly elevated. In women, the incidence of subjects with US-NAFLD was also significantly elevated with increases in the serum ferritin quartile (P=0.012). Regarding 25(OH)D3, no statistical differences were observed among the different quartiles in either gender. Serum ferritin level significantly increased as the severity of US-NAFLD increased (P<0.001); however, no significant differences in 25(OH)D3 level were observed in men. No significant differences in either serum ferritin or 25(OH)D3 level were observed among women with different levels of severity of US-NAFLD.
Conclusion
Increased serum ferritin level showed a closer association with severity of NAFLD compared with level of serum vitamin D, suggesting that serum ferritin level may be a better marker than vitamin D level for predicting the severity of US-NAFLD and hepatic steatosis in a clinical setting.
Keywords: Ferritin, Vitamin D, Nonalcoholic fatty liver disease, Metabolic syndrome
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) refers to fat accumulation in the liver exceeding 5% to 10% by weight in the absence of excessive alcohol consumption and is now considered to be the hepatic component of metabolic syndrome (MetS) [1,2,3], a constellation of risk factors for cardiovascular diseases, including insulin resistance, abdominal obesity, dyslipidemia, glucose intolerance, and hypertension [4,5]. The prevalence of NAFLD is increasing and has been estimated to range from 10% to 24% worldwide [6], including Asian populations [7]. NAFLD reflects insulin resistance, and patients with NAFLD are also likely to have the component disorders of MetS [8]. Although abdominal ultrasonography (US) has been found to be not sufficiently sensitive to detect liver inflammation and fibrosis, it has been shown to reveal a good correlation with histological findings of fatty infiltration and has been used for the diagnosis of different degrees of steatosis [9,10,11]. Moreover, a recent study showed that ultrasonographically-detected NAFLD (US-NAFLD) is an independent predictor for insulin resistance in nonobese, nondiabetic middle-aged Asian adults [12]. There are some markers for the identification of liver steatosis that are used to determine the severity of fatty liver [3], such as NAFLD liver fat score [13], and fatty liver index (FLI) [14].
There have been several cross-sectional studies that showed that an elevated serum ferritin levels associated with insulin resistance and NAFLD [15,16]. One recent large-scale study demonstrated that increased serum ferritin level was an independent risk factor of incident fatty liver detected by US, even in nonobese, healthy Korean men [17]. In association with MetS, one large, prospective study in middle-aged Korean men reported that elevated serum ferritin level showed an independent association with future development of MetS during a 5-year follow-up period [18].
Decreased vitamin D level has also been demonstrated to have an association with insulin resistance and cardiovascular risk factors, including MetS [19,20,21,22]. One large, cross-sectional study of healthy Korean men reported that participants with higher serum vitamin D level assessed by serum 25-hydroxyvitamin D3 showed a significantly reduced risk for NAFLD compared with patients with low vitamin D, independent of obesity and MetS [23].
Although several studies have examined the associations of serum ferritin and vitamin D with NAFLD, few studies have evaluated whether these markers predict the severity of NAFLD. In addition, to the best of our knowledge, there has been little data reported on the associations of serum ferritin and vitamin D with NAFLD in women. Therefore, we evaluated the associations of these two recently identified metabolic markers with the severity of NAFLD as defined by US and FLI in both genders.
METHODS
Study subjects
We used data from Korean adults who participated in a comprehensive health examination at Pusan National University Yangsan Hospital in Yangsan, Korea in 2012 (n=3,380) and evaluated subjects who had data that included both serum ferritin and vitamin D (n=695) and performed a medical chart review. There were no subjects who had a history of excessive alcohol intake (>20 g/day) or medication use that could affect the development of NAFLD. Of the 695 subjects, we excluded adults with liver or hepatobiliary disease (n=47), positive serologic markers for hepatitis B or C virus (n=33), low hemoglobin level (<10 g/dL; n=8), elevated white blood cell count (>10,000/mm3; n=7), elevated high sensitivity C-reactive protein (hs-CRP) level (>10.0 mg/L; n=5), or highly elevated serum ferritin level (>300 ng/mL; n=27). Finally, 558 adults (295 men, 263 women) were enrolled in the analysis. Informed consent for use of the health screening data in the research was obtained from the subjects. This study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital.
Anthropometric and biochemical data
Measurement of height and weight was performed while subjects wore light clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared and was used as an estimate of overall adiposity. Waist circumference, an estimate of central obesity, was measured using a soft tape midway between the lowest rib and the iliac crest on standing subjects. Blood pressure was measured on the right arm with subjects in a sitting position after a 5-minute rest period. Blood specimens were sampled from the antecubital vein after an overnight fast. Fasting glucose was measured using the glucose oxidase method (Synchron LX-20, Beckman Coulter Inc., Fullerton, CA, USA). Concentrations of standard liver enzyme, total cholesterol, high density lipoprotein cholesterol (HDL-C), and serum triglyceride (TG), and gamma-glutamyltransferase (GGT) were measured using an autoanalyzer and an enzymatic colorimetric method (Hitachi 7600, Hitachi Ltd., Tokyo, Japan). Glycated hemoglobin was measured using high performance liquid chromatography on a Varian II Turbo (Bio-Rad Laboratories, Hercules, CA, USA). Concentrations of serum ferritin were measured using an immunoturbidimetric assay (Cobas 6000, Roche Diagnostics, Mannheim, Germany). To assess vitamin D status, serum 25-hydroxy vitamin D3 [25(OH)D3] was measured using a chemiluminescent immunoassay (CLIA, Liaison, DiaSorin, Italy). The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated using the following formula:
HOMA-IR=[fasting serum insulin (mU/L)×fasting plasma glucose (mmol/L)]/22.5
Ultrasound is currently the most commonly used tool for screening asymptomatic patients with elevated liver enzymes and suspected NAFLD [3]. US findings of fatty liver include hepatomegaly, diffuse increases in echogenicity of liver parenchyma, and vascular blunting. Nonsteatotic hepatic parenchyma exhibits an echotexture similar to that of renal parenchyma but becomes "brighter" when infiltrated with fat [10]. Abdominal US was performed by one experienced radiologist who was blinded to the subjects' clinical statuses, using a 3.5-MHz transducer (Acuson Sequoia 512 scanner, Siemens Medical Solutions, Mountain View, CA, USA). Images were captured in a standard fashion with the patient in the supine position with the right arm raised above the head [24]. A US-NAFLD was defined as the presence of a diffuse increase in fine echoes in liver parenchyma compared to kidney or spleen parenchyma [25]. The severity of US-NAFLD was defined as follows: mild, slightly diffuse increase in fine echoes in the hepatic parenchyma with normal visualization of the diaphragm and intrahepatic vessel borders; moderate, moderate diffuse increase in fine echoes with slightly impaired visualization of the diaphragm and intrahepatic vessels; severe, marked increase in fine echoes with poor or no visualization of the diaphragm, intrahepatic vessels, and posterior portion of the right lobe of the liver [26].
NAFLD was also determined by the FLI using metabolic parameters, such as TG, BMI, GGT, and waist circumference. The calculation formula of FLI was as follows [14,27] and an FLI ≥60 was considered to indicate hepatic steatosis:
FLI=[e 0.953×loge (TG)+0.139×BMI+0.718×loge (GGT) +0.053×waist circumference-15.745)]/[1+e 0.953×loge (TG)+0.139×BMI+0.718×loge (GGT)+0.053×waist circumference-15.745]×100
MetS was defined according to the modified, revised National Cholesterol Education Program Adult Treatment III as the presence of three or more of the following criteria [28,29]: (1) abdominal obesity defined as waist circumference >90 cm; (2) impaired fasting glucose as defined by FBG ≥100 mg/dL; (3) high TG as defined by TG ≥150 mg/dL (for conversion to mmol/L, multiply by 0.01129); (4) low HDL-C as defined by HDL-C <40 mg/dL in men and <50 mg/dL in women (for conversion to mmol/L, multiply by 0.02586); and (5) blood pressure ≥130/85 mm Hg.
Statistical analyses
All data are given as mean±SD for continuous variables. Median values are also indicated in the case of TG, GGT, and hs-CRP, which have skewed distributions. To evaluate the associations of ferritin and 25(OH)D3 with other variables, we performed Pearson partial correlation coefficients analyses, adjusting for age. Components of MetS and other clinical characteristics were compared between subjects with and without MetS using independent-sample Student t test and Mann-Whitney U test in variables with a skewed distribution. All subjects were divided into quartiles based on the distribution of serum ferritin and 25(OH)D3. The prevalence of MetS, NAFLD, and FLI >60 in each quartile was indicated as a percent and compared using the chi-square test. Finally, the mean ferritin and 25(OH)D3 levels were compared with regard to the severity of US-NAFLD using one-way analysis of variance analysis followed by post hoc testing using the S-N-K test. Statistical analyses were performed using an SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). A probability value less than 0.05 was considered significant.
RESULTS
Mean ferritin level was 149.5±60.4 ng/mL in men and 42.1±35.1 ng/mL in women. Mean 25(OH)D3 level was 18.5±6.4 ng/mL in men and 15.2±5.5 ng/mL in women. MetS was diagnosed in 49 men (16.6%) and 21 women (8.0%). Regarding hepatic steatosis, US-NAFLD was diagnosed in 132 men (44.7%) and 40 women (15.2%). FLI ≥60 was diagnosed in 63 men (21.4%) and 11 in women (4.2%) (Table 1).
Table 1.
Clinical Characteristics of Subjects (n=558)
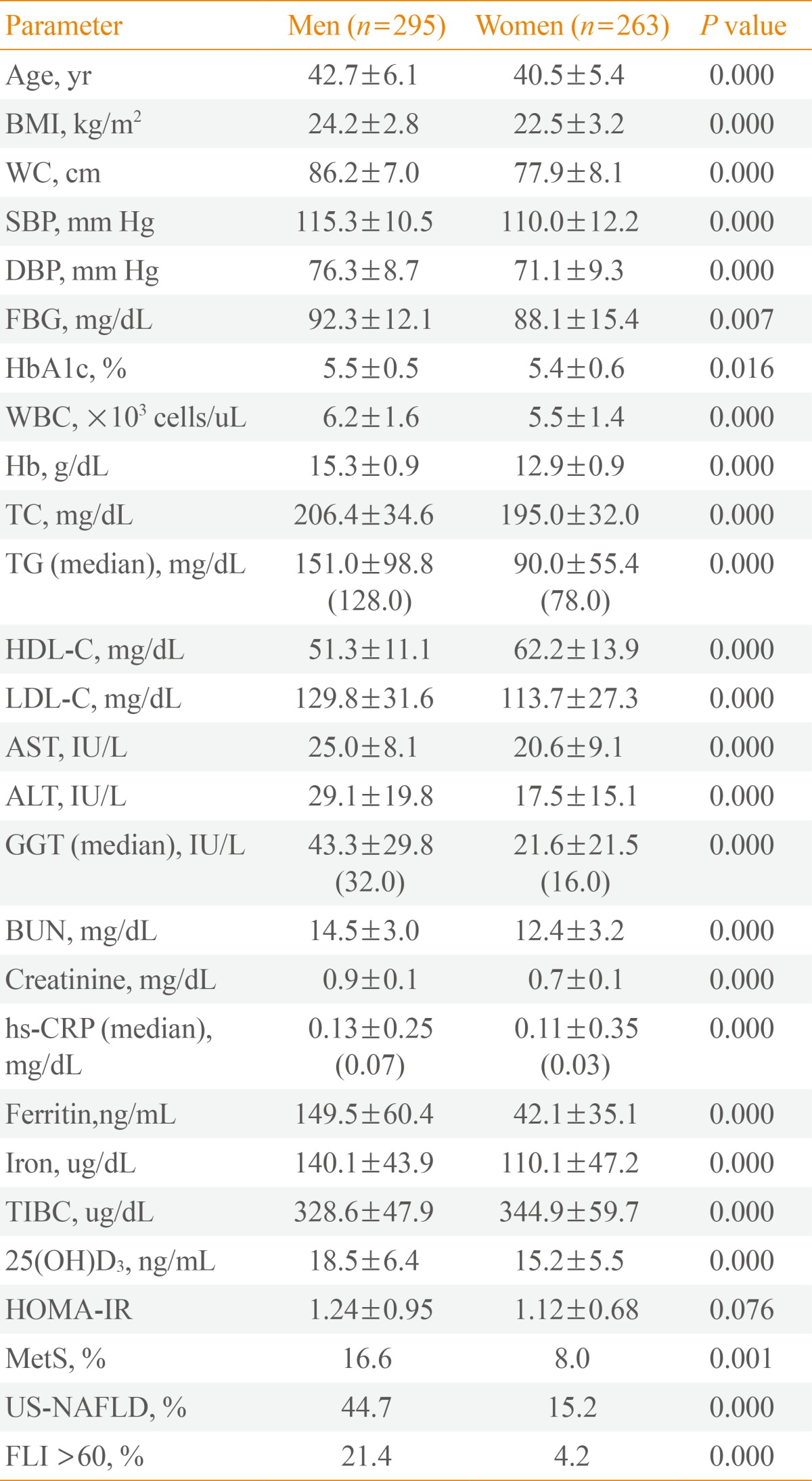
Values are expressed as mean±SD or unless otherwise indicated.
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; WBC, white blood cell; Hb, hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; BUN, blood urea nitrogen; hs-CRP, high sensitivity C-reactive protein; TIBC, total iron binding capacity; HOMA-IR, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome; US-NAFLD, ultrasonographically-detected nonalcoholic fatty liver disease; FLI, fatty liver index.
In a partial correlation analysis adjusted for age, ferritin level showed significant positive correlations with waist circumference (r=0.179, P=0.002), BMI (r=0.166, P=0.005), aspartate aminotransferase (AST; r=0.284, P<0.001), alanine aminotransferase (ALT; r=0.314, P<0.001), log (GGT) (r=0.187, P=0.001], log (hs-CRP) (r=0.117, P=0.046), and FLI (r=0.148, P=0.011] in men and showed significant positive correlations with waist circumference (r=0.131, P=0.034), ALT (r=0.152, P=0.014), log (GGT) (r=0.192, P=0.002), and log (hs-CRP) (r=0.171, P=0.006) in women. In contrast, 25(OH)D3 showed significant negative correlations with log (TG) (r=-0.238, P<0.001) and FLI (r=-0.130, P=0.026) in men, while there was no significant correlation between 25(OH)D3 and any parameters in women (Table 2).
Table 2.
Partial Age-Adjusted Correlation Coefficients of Ferritin, 25(OH)D3, and Metabolic Risk Factors
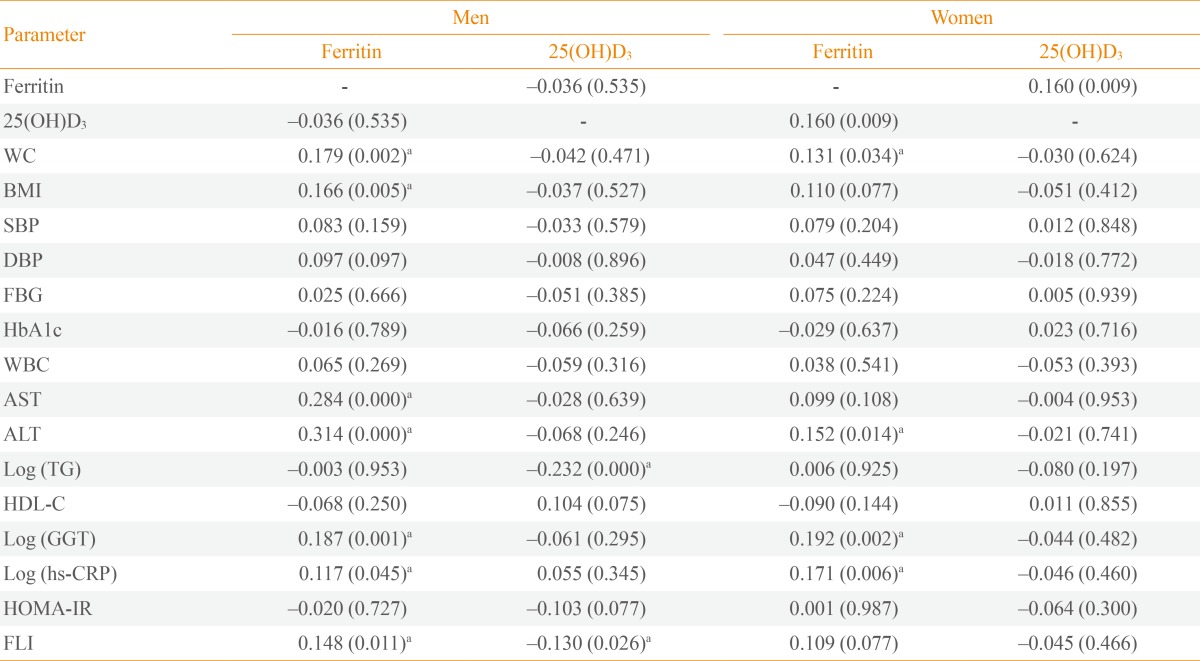
Values are expressed as coefficient r (P value).
WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TG, triglycerides; HDL-C, high density lipoprotein cholesterol; GGT, gamma-glutamyltransferase; hs-CRP, high sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; FLI, fatty liver index.
aP<0.05.
Serum ferritin level was significantly increased in men with obesity (163.9±58.6 ng/dL vs. 143.5±60.2 ng/dL, P=0.008), MetS (171.4±64.2 ng/dL vs. 145.1±58.7 ng/dL, P=0.010), US-NAFLD (157.5±63.1 ng/dL vs. 142.9±57.4 ng/dL, P=0.042), and FLI ≥60 (165.1±64.3 ng/dL vs. 145.2 ± 58.7 ng/dL, P=0.029). Serum 25(OH)D3 level was significantly decreased only in men with hyper-TG (17.4±6.3 ng/dL vs. 19.2±6.4 ng/dL, P=0.021). In women, ferritin level was significantly increased in subjects with obesity (50.5±44.3 ng/dL vs. 37.7±28.4 ng/dL, P=0.015) and US-NAFLD (54.3±41.6 ng/dL vs. 39.9±33.5 ng/dL, P=0.045), and 25(OH)D3 level was significantly decreased only in subjects with MetS (13.3±4.2 ng/dL vs. 15.4±5.5 ng/dL, P=0.046) (Table 3).
Table 3.
Comparison of Mean Ferritin and 25(OH)D3 Levels between Subjects with and without Each Metabolic Component Disorder
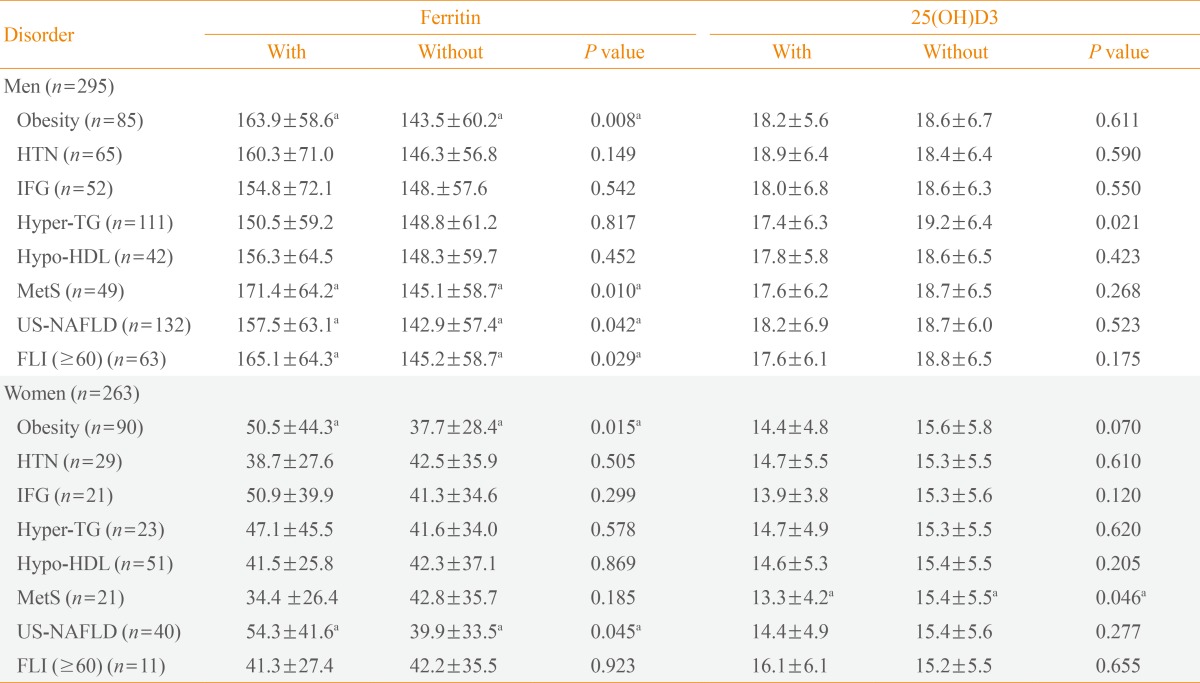
Values are expressed as mean±SD.
HTN, hypertension; IFG: impaired fasting glucose; Hyper-TG, hyper-triglyceridemia; Hypo-HDL, hypo-high density lipoprotein cholesterol; MetS, metabolic syndrome; US-NAFLD, ultrasonographically-detected nonalcoholic fatty liver disease; FLI, fatty liver index.
aP<0.05.
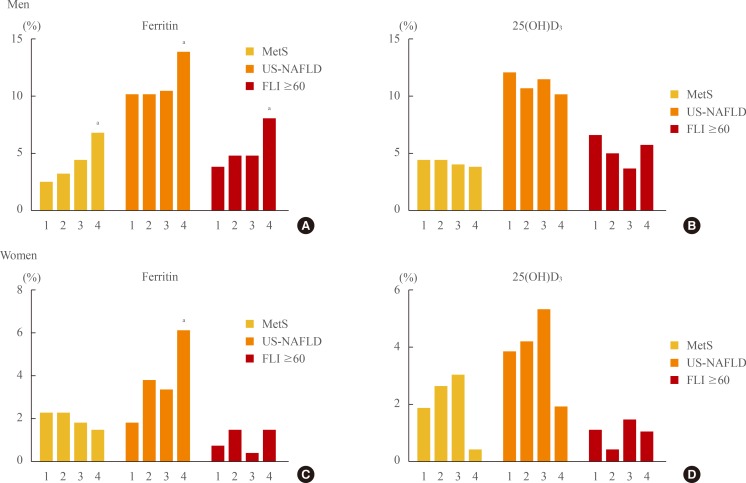
With an increase in the quartile of serum ferritin level, the incidences of subjects with MetS (2.4%, 3.1%, 4.4%, 6.8%; P=0.002), US-NAFLD (10.2%, 10.2%, 10.5%, 14.9%; P=0.041), and FLI ≥60 (3.7%, 4.7%, 4.7%, 8.1%; P=0.010) were significantly increased in men. In women, only the incidence of subjects with US-NAFLD increased significantly with the increase of the quartile of serum ferritin level (1.9%, 3.8%, 3.4%, 6.1%; P=0.012). Regarding 25(OH)D3, no statistical differences were observed among quartiles in either gender (Fig. 1).
Fig. 1.
Incidence of metabolic syndrome (MetS), ultrasonographically-detected nonalcoholic fatty liver disease (US-NAFLD), and fatty liver index (FLI) ≥60 by quartile rankings of serum ferritin and 25(OH)D3 levels. 1, first quartile; 2, second quartile; 3, third quartile; 4, fourth quartile. (A) With an increase in the quartile of ferritin level, the incidences of subjects with MetS, US-NAFLD, and FLI ≥ 60 were significantly increased in men. (B) With an increase in the quartile of vitamin D level, no statistical differences were observed among quartiles in men. (C) With an increase in the quartile of ferritin level, only the incidence of subjects with US-NAFLD increased significantly in women. (D) With an increase in the quartile of vitamin D level, no statistical differences were observed among quartiles in women.
aP<0.05 compared with the first quartile using one-way analysis of variance analysis followed by post hoc testing with the S-N-K test.
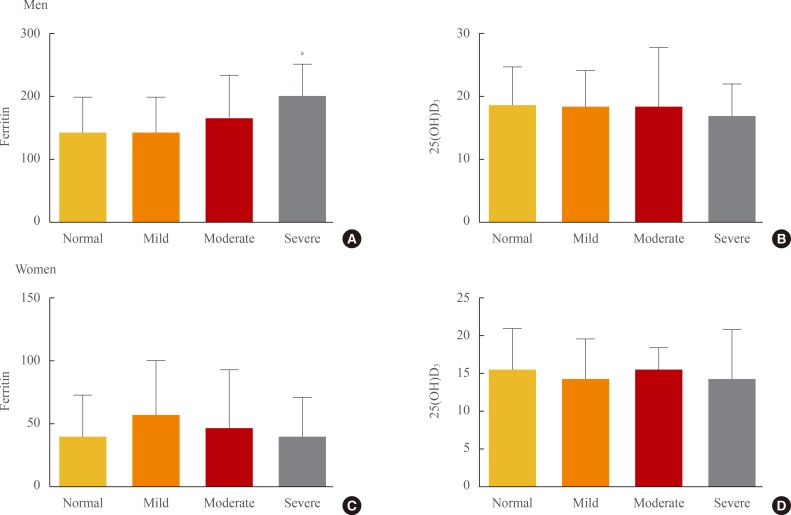
Serum ferritin level was significantly increased as the severity of US-NAFLD increased from normal to severe (normal, 142.9±57.4; mild, 142.2±57.9; moderate, 166.3±68.4; severe, 200.4±49.0 ng/mL; P<0.001). However, no significant differences were observed in 25(OH)D3 level (normal, 18.7±6.0; mild, 18.4±5.8; moderate, 18.5±9.3; severe, 17.0±5.0 ng/mL) in men. No significant differences in either serum ferritin or 25(OH)D3 level were observed among women with different severities of US-NAFLD (Fig. 2).
Fig. 2.
Serum ferritin and 25(OH)D3 levels according to the severity of ultrasonographically-detected nonalcoholic fatty liver disease (US-NAFLD). (A) Ferritin level was significantly increased as the severity of US-NAFLD increased from normal to severe in men. (B) No significant differences were observed in vitamin D level among men with different severities of US-NAFLD. (C) No significant differences in ferritin level were observed among women with different severities of US-NAFLD. (D) No significant differences in vitamin D level were observed among women with different severities of US-NAFLD.
aP<0.001 compared with the first quartile using one-way analysis of variance analysis followed by post hoc testing with the S-N-K test.
DISCUSSION
In this study, serum ferritin level showed a significant increase with severity of US-NAFLD, and increase in the quartile of serum ferritin level was significantly associated with the incidence of US-NAFLD in men. On the other hand, no significant differences in 25(OH)D3 level were observed among the degrees of severity in US-NAFLD and the incidence of US-NAFLD also showed no statistical differences among the different quartiles of 25(OH)D3 level, suggesting that serum ferritin level may have a closer association in men with the severity of US-NAFLD compared with serum vitamin D. Serum ferritin level showed significant positive correlations with waist circumference, BMI, AST, ALT, log (GGT), and log (hs-CRP). Ferritin level was significantly increased in obese subjects and those with MetS. However, 25(OH)D3 level in men showed a significant negative correlation only with log (TG) and was significantly decreased only in the hyper-TG group. In hepatic steatosis, these two markers showed a significant association with FLI, positive for ferritin and negative for 25(OH)D3. Serum ferritin level was significantly increased in subjects with US-NAFLD and FLI ≥60; however, there were no significant differences in 25(OH)D3 level between subjects with and without US-NAFLD or FLI ≥60. These data also may suggest that ferritin level more closely reflects hepatic steatosis compared with vitamin D level. Regarding the association of serum ferritin with hepatic steatosis, Kowdley et al. [30] reported that a serum ferritin level >1.5×the upper limit of normal is associated with worsened histologic activity and is an independent predictor of advanced hepatic steatosis among patients with biopsy-proven NAFLD. Although NAFLD was defined using US and hepatic steatosis was defined by FLI, our results are consistent with those of that previous study [30].
Most cross-sectional studies regarding the association of decreased serum vitamin D level with NAFLD reported positive results [19,20,21,22,23]. However, Katz et al. [31] reported that vitamin D level was not associated with suspected NAFLD assessed only by elevated ALT level after adjustment for obesity using the National Health and Nutrition Examination Survey database of 1,630 American adolescents. Furthermore, our study also showed no association of vitamin D level with NAFLD. The current study, using US and FLI instead of ALT levels to define hepatic steatosis, showed some findings that were consistent with those of Katz et al. [31], demonstrating no significant relationship between vitamin D level and hepatic steatosis detected by US-NAFLD and FLI and no significant differences among the different levels of severity of US-NAFLD.
For women, there were no significant differences in either serum ferritin or 25(OH)D3 level among the different levels of severity of US-NAFLD, but the incidence of US-NAFLD significantly increased with an increase in quartile rankings of ferritin level, findings that were not observed with vitamin D level. Serum ferritin level showed a significant positive correlation with waist circumference, ALT, log (GGT), and log (hs-CRP) and showed a significant increase among subjects with obesity and US-NAFLD. In the case of vitamin D in women, 25(OH)D3 levels showed no significant correlation with any metabolic parameters and showed a significant decrease only in the MetS group. Few studies have reported on serum ferritin level as a metabolic cardiovascular risk factor in women. In one study by Williams et al. [32], serum ferritin was significantly associated with CRP, waist circumference, BMI, and TG in 443 women in New Zealand. In this study, waist circum ference was a significant predictor of serum ferritin in men, and CRP, a useful marker of systemic inflammation, was the most significant predictor of serum ferritin in women [32]. Our study also demonstrated that serum ferritin showed significant association with waist circumference, log (GGT), and log (hs-CRP) in both genders, suggesting that serum ferritin may have a close relationship with central obesity and low grade systemic inflammation in both men and women. GGT has also been recognized as a reliable marker of cardiovascular disease, including MetS [33,34] and NAFLD [35,36], and serum ferritin showed significant association with GGT in both genders in our study.
Our study has some limitations. First, it was conducted using a cross-sectional design and thus did not identify a causal relationship between clinical markers and hepatic steatosis, and the total number of enrolled subjects in both genders was relatively small. This limitation may have affected our results that showed no statistical correlation between HOMA-IR and ferritin or 25(OH)D3. Second, we could not obtain social data such as history of exercise, diet, and smoking, which may affect the incidence of hepatic steatosis. Third, the definition of hepatic steatosis and severity were not histologically confirmed but were made based on US and FLI calculated using related metabolic parameters. Fourth, we did not consider the seasonal variations in serum vitamin D or menopausal status and estrogen level in women, which could have effected the low mean 25(OH)D3 levels in both genders compared with those in previous reports.
In conclusion, findings of the current study indicate that serum ferritin has a closer association with severity of US-NAFLD than does serum vitamin D, reflected by serum 25(OH)D3 levels in men and women. Therefore, serum ferritin level may be a better marker than serum vitamin D level for predicting the severity of US-NAFLD and hepatic steatosis in a clinical setting.
ACKNOWLEDGMENTS
This study was supported by a Clinical Research Grant (2013) of Pusan National University Yangsan Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22:293–303. doi: 10.1111/j.1440-1746.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- 2.Souza MR, Diniz Mde F, Medeiros-Filho JE, Araujo MS. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol. 2012;49:89–96. doi: 10.1590/s0004-28032012000100015. [DOI] [PubMed] [Google Scholar]
- 3.Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. doi: 10.1155/2012/145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 7.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome. World J Gastroenterol. 2013;19:3375–3384. doi: 10.3748/wjg.v19.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25–29. doi: 10.1002/(SICI)1097-0096(199601)24:1<25::AID-JCU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Kim HC, Choi SH, Shin HW, Cheong JY, Lee KW, Lee HC, Huh KB, Kim DJ. Severity of ultrasonographic liver steatosis and metabolic syndrome in Korean men and women. World J Gastroenterol. 2005;11:5314–5321. doi: 10.3748/wjg.v11.i34.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinn DH, Gwak GY, Park HN, Kim JE, Min YW, Kim KM, Kim YJ, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107:561–567. doi: 10.1038/ajg.2011.400. [DOI] [PubMed] [Google Scholar]
- 13.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstrale M, Groop L, Orho-Melander M, Yki-Jarvinen H. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004;28:167–172. doi: 10.1038/sj.ijo.0802519. [DOI] [PubMed] [Google Scholar]
- 16.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–707. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Kim CW, Chang Y, Sung E, Shin H, Ryu S. Serum ferritin levels predict incident non-alcoholic fatty liver disease in healthy Korean men. Metabolism. 2012;61:1182–1188. doi: 10.1016/j.metabol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Park SK, Ryoo JH, Kim MG, Shin JY. Association of serum ferritin and the development of metabolic syndrome in middle-aged Korean men: a 5-year follow-up study. Diabetes Care. 2012;35:2521–2526. doi: 10.2337/dc12-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab) J Clin Endocrinol Metab. 2012;97:1953–1961. doi: 10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 20.Lim S, Shin H, Kim MJ, Ahn HY, Kang SM, Yoon JW, Choi SH, Kim KW, Song JH, Choi SI, Chun EJ, Shin CS, Park KS, Jang HC. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. J Clin Endocrinol Metab. 2012;97:169–178. doi: 10.1210/jc.2011-1580. [DOI] [PubMed] [Google Scholar]
- 21.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, Kang MI, Park SW, Kim SW, Oh KW. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J. 2013;60:743–752. doi: 10.1507/endocrj.ej12-0387. [DOI] [PubMed] [Google Scholar]
- 24.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53:686–692. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 25.Mathiesen UL, Franzen LE, Aselius H, Resjo M, Jacobsson L, Foberg U, Fryden A, Bodemar G. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34:516–522. doi: 10.1016/s1590-8658(02)80111-6. [DOI] [PubMed] [Google Scholar]
- 26.Mittelstaedt CA. General ultrasound. New York: Churchill Livingstone; 1992. [Google Scholar]
- 27.Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;10:98. doi: 10.1186/1471-230X-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 30.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz K, Brar PC, Parekh N, Liu YH, Weitzman M. Suspected nonalcoholic Fatty liver disease is not associated with vitamin D status in adolescents after adjustment for obesity. J Obes. 2010;2010:496829. doi: 10.1155/2010/496829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams MJ, Poulton R, Williams S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis. 2002;165:179–184. doi: 10.1016/s0021-9150(02)00233-2. [DOI] [PubMed] [Google Scholar]
- 33.Kang YH, Min HK, Son SM, Kim IJ, Kim YK. The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract. 2007;77:306–313. doi: 10.1016/j.diabres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Liu CF, Zhou WN, Fang NY. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract. 2012;66:692–698. doi: 10.1111/j.1742-1241.2012.02959.x. [DOI] [PubMed] [Google Scholar]
- 35.Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G, Hatemi I, Olgac V, Sonsuz A, Ozbay G, Yurdakul I, Senturk H. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology. 2008;55:1433–1438. [PubMed] [Google Scholar]
- 36.Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramírez JC, Castro MG. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24:805–810. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]