Abstract
Background
The aim of this study was to examine the clinical characteristics of adrenal incidentalomas discovered by computed tomography (CT) and to investigate metabolic features of subclinical Cushing's syndrome (SCS) in patients with adrenal incidentalomas in a tertiary hospital in Korea.
Methods
This retrospective study examined the clinical aspects of 268 patients with adrenal incidentalomas discovered by CT at Soonchunhyang University Bucheon Hospital. Clinical data and endocrine function of the patients as well as histological findings were obtained from medical records, while anatomic characteristics were analyzed by reviewing imaging studies. Hormonal tests for pheochromocytoma, Cushing's syndrome, and aldosterone-secreting adenoma were performed.
Results
Most (n=218, 81.3%) cases were nonfunctioning tumors. Of the 50 patients with functioning tumors (18.7%), 19 (7.1%) were diagnosed with SCS, nine (3.4%) with overt Cushing's syndrome, 12 (4.5%) with primary aldosteronism, and 10 (3.7%) with pheochromocytoma. Malignant tumors (both primary and metastatic) were rare (n=2, 0.7%). Body mass index, fasting glucose, hemoglobin A1c, and total cholesterol were significantly higher in patients with SCS in comparison with those with nonfunctioning tumors. The prevalence of type 2 diabetes mellitus and hypertension were significantly higher in patients with SCS compared with those with nonfunctioning tumors.
Conclusion
Functioning tumors, especially those with subclinical cortisol excess, are commonly found in patients with adrenal incidentalomas, although malignancy is rare. In addition, patients with SCS in adrenal incidentalomas have adverse metabolic and cardiovascular profiles.
Keywords: Adrenal incidentalomas, Metabolic features, Subclinical Cushing's syndrome
INTRODUCTION
Adrenal incidentalomas are defined as clinically unapparent adrenal masses that are discovered during diagnostic testing or treatment for nonadrenal diseases. Adrenal masses are one of the most prevalent tumors in humans, and their reported prevalence is increasing with continued advances in imaging technology, making the management of adrenal incidentaloma important for modern medicine [1]. Based on clinical studies, the prevalence of adrenal incidentaloma is approximately 4% overall, with nearly 80% of these masses found to be benign [1,2,3,4,5]. A substantial proportion of these adrenal incidentalomas demonstrate subtle hormonal hypersecretion, mainly in the form of autonomous cortisol secretion, with a reported prevalence that varies from 1% to 47% according to the applied diagnostic criteria [6]. Subclinical Cushing's syndrome (SCS) is defined as a state of altered hypothalamic pituitary adrenal axis secretion in the absence of the classical signs or symptoms of overt cortisol excess. The diagnosis and treatment of SCS have recently become a topic of growing interest due to the high disease prevalence and are currently under debate [3,7]. Indeed, SCS is estimated to be present in 5% to 30% of patients with adrenal incidentalomas [8,9]. Some studies suggested that this condition is present in 1% to 10% of patients with diabetes or established osteoporosis [10,11,12,13]. The aim of the present study was to examine the clinical characteristics of adrenal incidentalomas discovered by computed tomography (CT) and to investigate metabolic features of SCS in patients with adrenal incidentalomas in a tertiary hospital in Korea.
METHODS
This retrospective study examined clinical aspects of patients with adrenal incidentalomas discovered by CT at Soonchunhyang University Bucheon Hospital from January 2002 to July 2012. Clinical data and endocrine function of the patient as well as histological findings were obtained from medical records, while the anatomic characteristics such as location and size of the masses were analyzed by reviewing imaging studies. Regarding functionality, hormone tests for pheochromocytoma, Cushing's syndrome, and aldosterone-secreting adenoma, which were the most frequently diagnosed conditions of the clinically significant functional adrenal masses, were performed. The following screening tests were performed in all patients: for pheochromocytoma, a 24-hour urinary vanillylmandelic acid (VMA), total metanephrine, 24-hour urinary catecholamine (epinephrine and norepinephrine), and plasma catecholamine (epinephrine and norepinephrine) were measured; for Cushing's syndrome, plasma adrenocorticotropic hormone (ACTH), early-morning cortisol, and a 24-hour urinary free cortisol were measured; for aldosterone-secreting adenoma, plasma aldosterone, renin activity, and potassium level were measured. Plasma ACTH (Cis-Bio international, Gif-sur-yvette, Cedex, France) and cortisol (DiaSorin Inc., Stillwater, MN, USA) were measured using radioimmunoassay kits. Urinary VMA and metanephrine were measured using high-performance liquid chromatography (Agilent Technologies, Santa Clara, CA, USA). If the 24-hour urinary free cortisol was higher than the reference range (75 to 270 µg/day), low early-morning plasma ACTH (<10 pg/mL) was observed, and the patient had any specific symptoms or signs of Cushing's syndrome, Cushing's syndrome was suspected. A low-dose dexamethasone suppression test (DST) also was performed to increase diagnostic specificity and to confirm Cushing's syndrome. If the 24-hour urinary free cortisol was higher than the reference range (75 to 270 µg/day), low early-morning plasma ACTH (<10 pg/mL) was observed, and the patient lacked specific symptoms or signs of Cushing's syndrome, a diagnosis of SCS was made [13,14,15]. Pheochromocytoma was determined using 24-hour urinary VMA, total metanephrine, 24-hour urinary catecholamine (epinephrine and norepinephrine), and plasma catecholamine (epinephrine and norepinephrine) levels. When plasma aldosterone was higher and plasma renin activity was lower than their respective reference values and the ratio of plasma aldosterone to renin activity was greater than 20, aldosterone-secreting adenoma was suspected. A saline-loading test or captopril challenge test was performed in most patients in order to confirm this diagnosis. Drugs affecting the renin-angiotensin-aldosterone system (ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, nonsteroidal anti-inflammatory drugs, β blockers) were discontinued for 14 days before measuring plasma renin and aldosterone levels.
Diabetes mellitus was diagnosed if the subject had a prior diagnosis or had been treated with oral hypoglycemic agents or insulin. In subjects with diabetes mellitus, diagnosis was established if fasting plasma glucose was ≥126 mg/dL or hemoglobin A1c (HbA1c) was ≥6.5%. Hypertension was diagnosed if the subject had a prior diagnosis or had been treated with antihypertensive agents. In subjects with hypertension, diagnosis was established if systolic blood pressure was ≥140 mm Hg or diastolic blood pressure was ≥90 mm Hg.
Statistical analysis
Statistical analyses were conducted using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). The comparison of the two groups was performed with Mann-Whitney U test or chi-square test. For all statistical analysis, a P<0.05 was considered to indicate statistical significance.
RESULTS
A total of 268 patients with adrenal incidentalomas were included in this study. Table 1 shows the clinical characteristics of the patients with adrenal incidentalomas. The mean age of the participants was 55.8 years (range, 15 to 90). The disease propensity was not different in men and women. The distribution of adrenal tumors was similar between right and left adrenal glands (42.6% and 44.9%, respectively), and bilateral tumors were found in 12.5% of patients. The largest adrenal tumor dimensions ranged from 0.37 to 12.00 cm, with a mean of 2.73±1.89 cm. The prevalences of type 2 diabetes mellitus and hypertension were 30.6% and 54.5%, respectively.
Table 1.
The Clinical Characteristics of 268 Patients with Adrenal Incidentalomas
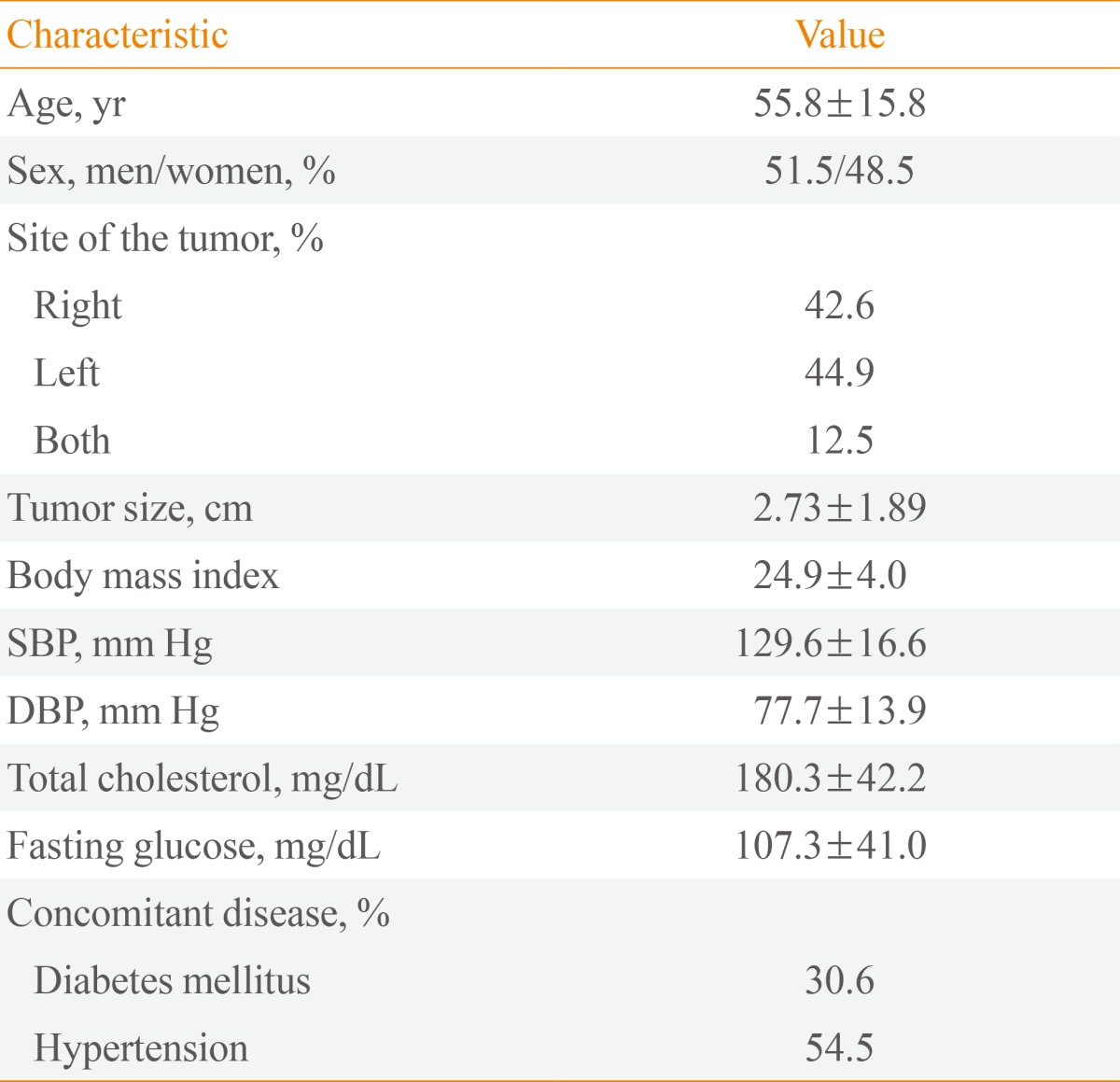
Values are expressed as mean±SD.
SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 2 shows the functional diagnosis of patients with adrenal incidentalomas. Most (n=218, 81.3%) cases were nonfunctioning tumors. Of the 50 patients with functioning tumors (18.7%), 19 (7.1%) were diagnosed with SCS, nine (3.4%) with overt Cushing's syndrome, 12 (4.5%) with primary aldosteronism, and 10 (3.7%) with pheochromocytoma. A saline-loading test or captopril challenge test was performed to confirm the diagnosis of primary aldosteronism in nine of 12 patients. Adrenal vein sampling was performed in the other three patients with primary aldosteronism. Adrenalectomy was performed at the hospital in 32 patients. Histological diagnoses included adenoma (n=16), pheochromocytoma (n=6), adrenal metastasis (n=1), adrenocortical carcinoma (n=1), and others (n=8). An overnight DST was performed in eight of nine patients with Cushing's syndrome and 10 of 19 patients with SCS. In three of 10 patients, 24-hour urinary free cortisol was higher than the reference range, but overnight DST cortisol was <2 µg/dL.
Table 2.
Functional Diagnosis of Patients with Adrenal Incidentalomas

Table 3 compares clinical and metabolic aspects of patients with SCS and those with nonfunctioning tumors. Body mass index, fasting glucose, HbA1c, and total cholesterol were significantly higher in patients with SCS in comparison with those with nonfunctioning tumors. The prevalences of type 2 diabetes mellitus and hypertension were significantly higher in patients with SCS compared to those with nonfunctioning tumors.
Table 3.
Clinical and Metabolic Aspects of Patients with Subclinical Cushing's Syndrome and Those with Nonfunctioning Tumors
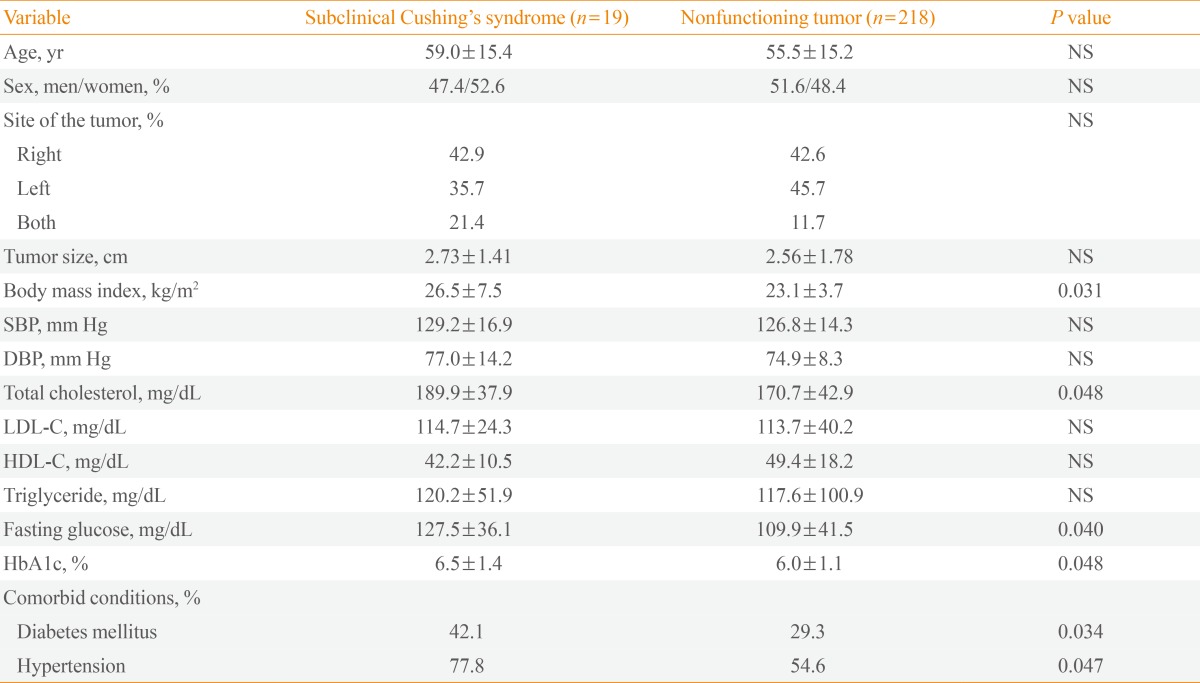
Values are expressed as mean±SD. Statistical significance was tested by Mann-Whitney U test for continuous variables and chi-square test for categorical variables.
NS, not significant; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; HbA1c, hemoglobin A1c.
DISCUSSION
In this retrospective study of 268 patients with adrenal incidentalomas discovered by CT, 18.7% of the tumors were found to be functioning and 81.3% were nonfunctioning. Adrenocortical carcinoma was found at a much lower prevalence (only one of 268 patients) than that reported in previous Korean studies (up to 6.0%) of adrenal incidentalomas [16,17,18].
Among the functioning tumors, 7.1% manifested with SCS, 3.4% with Cushing's syndrome, 4.5% with primary aldosteronism, and 3.7% with pheochromocytoma. The functional composition of the adrenal tumors was similar to that in Western studies [4,5,19]. In a previous Korean study, pheochromocytoma was seen at a much higher prevalence-up to 20%-of adrenal incidentalomas [16]. In our series, adrenal masses were found in similar proportions in men and women (51.5% vs. 48.5%). Many previous studies have reported that adrenal incidentalomas are found more frequently in females (female-to-male ratio, 1.3 to 1.5:1), although this could be due to a generally higher rate of abdominal imaging in females than in males [20,21,22,23]. In one previous Korean study, adrenal incidentalomas were found more frequently in females (44.8% vs. 55.2%) [18]. However, other studies reported that there were similar proportions of men and women (51.2% vs. 48.8%) [16] or a larger proportion of men than women (61.0% vs. 39.0%) [17] in Korean patients with adrenal incidentalomas. Taken together with our results, there seems to be no increased likelihood of tumor based on sex in Korean patients. In our study, 50 patients (18.7%) had functioning tumors. In previous Korean studies, the prevalences of functioning tumors reported varied from 13.8% to 41.0% [16,17,18]. In previous studies from different countries, the prevalences of functioning tumors varied from 16.0% to 29.0% and were similar to our study [4,5,19,24]. SCS is a condition of biochemical cortisol excess without the classical signs or symptoms of overt Cushing's syndrome. The diagnosis and treatment of SCS recently have become a topic of growing interest and are currently under debate [7,8,25]. In a previous Korean study, SCS was seen at a higher prevalence (9.0%) in adrenal incidentalomas than was observed in our study. In two previous Korean studies, patients with Cushingoid features were excluded, or the prevalence of Cushing's syndrome was not reported [17,18]. In a study from a different country, the prevalences of SCS and Cushing's syndrome were slightly higher compared to those in our study [24].
The diagnosis of SCS is a challenge for clinicians due to several reasons. First, particularly in patients with adrenal incidentalomas, cortisol secretion occurs on a continuum from completely normal to clearly increased levels and can be highly variable in the same individual [3,26]. Therefore, diagnosing SCS by arbitrary index cutoffs of cortisol secretion leads to unavoidable misclassifications in some patients. Second, because SCS is by definition not characterized by a specific clinical picture, a clinical "gold standard" for diagnosing SCS is lacking. In our study, fasting glucose, total cholesterol, and body mass index were significantly higher in patients with SCS than in those with nonfunctioning tumors. The prevalences of diabetes mellitus and hypertension were also significantly higher in patients with SCS compared to those with nonfunctioning tumors. Previous studies showed that patients with SCS related to adrenal incidentaloma have adverse metabolic and cardiovascular outcomes [12,27]. Diabetes mellitus, hypertension, and dyslipidemia have frequently been observed in patients with adrenal adenomas, the prevalence being even higher in those with SCS [17,24]. SCS might have negative effects on metabolism [11,28]; however, the higher prevalence may be due to the fact that patients with diabetes or hypertension have a higher rate of abdominal imaging compared to the healthy general population.
That prevalences of diabetes mellitus, dyslipidemia, and hypertension are higher in patients with SCS shows that it is not completely asymptomatic. The usefulness of screening for SCS in at-risk populations clearly depends on the possibility of SCS-associated increased morbidity and mortality, which is still unknown. Moreover, the economic costs of screening for SCS would probably be high. To date, no data are available on the cost-effectiveness of screening populations at risk. However, it seems rational to screen for SCS in patients with poorly controlled hypertension or type 2 diabetes despite adequate lifestyle modification and pharmacological treatment. Several studies have reported that adrenalectomy improved metabolic and cardiovascular outcomes in patients with adrenal incidentaloma and SCS [29,30,31,32]. Further studies are needed to confirm whether surgical treatment is indeed beneficial to patients with these diseases.
Our study has several limitations that must be taken into account. First, due to its cross-sectional design, we could not determine a causal relationship between SCS and metabolic derangements. Second, the patients were diagnosed with SCS by 24-hour urinary free cortisol levels and plasma ACTH, without overnight DST. Third, because our study population was a cohort of patients cared for in a single center, the results might have been affected by selection bias. Nonetheless, this single-center study also conferred a high degree of consistency regarding laboratory data for hormonal evaluation. Finally, the specificity and sensitivity for the criteria for SCS used in this study is not known, which could have lead to both under- and over-diagnosis of SCS.
In summary, functioning tumors, especially those with subclinical cortisol excess, are commonly found in patients with adrenal incidentalomas, although malignancy is rare. In addition, patients with SCS in adrenal incidentalomas have adverse metabolic and cardiovascular profiles. Further studies are needed to determine the accurate diagnostic criteria for SCS and to conclude whether surgical treatment can improve metabolic and cardiovascular outcomes in patients with adrenal incidentaloma and SCS.
ACKNOWLEDGMENTS
This work was supported by research grants from Soonchunhyang University.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–340. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 2.Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti GV, Angeli A, Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 3.Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically inapparent adrenal mass ("incidentaloma") Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 4.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–285. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 5.Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, Giovagnetti M, Opocher G, Angeli A. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 6.Tsagarakis S, Vassiliadi D, Thalassinos N. Endogenous subclinical hypercortisolism: diagnostic uncertainties and clinical implications. J Endocrinol Invest. 2006;29:471–482. doi: 10.1007/BF03344133. [DOI] [PubMed] [Google Scholar]
- 7.Nawar R, Aron D. Adrenal incidentalomas: a continuing management dilemma. Endocr Relat Cancer. 2005;12:585–598. doi: 10.1677/erc.1.00951. [DOI] [PubMed] [Google Scholar]
- 8.Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, Angeli A. Subclinical Cushing's syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am. 2005;34:423–439. doi: 10.1016/j.ecl.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Terzolo M, Reimondo G, Bovio S, Angeli A. Subclinical Cushing's syndrome. Pituitary. 2004;7:217–223. doi: 10.1007/s11102-005-4024-6. [DOI] [PubMed] [Google Scholar]
- 10.Garrapa GG, Pantanetti P, Arnaldi G, Mantero F, Faloia E. Body composition and metabolic features in women with adrenal incidentaloma or Cushing's syndrome. J Clin Endocrinol Metab. 2001;86:5301–5306. doi: 10.1210/jcem.86.11.8059. [DOI] [PubMed] [Google Scholar]
- 11.Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, Fazio S, Lombardi G. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–4878. doi: 10.1210/jc.2001-011766. [DOI] [PubMed] [Google Scholar]
- 12.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440–1448. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 13.Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM, Maffini MA, Scillitani A, Ambrosi B, Beck-Peccoz P, Chiodini I. The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol. 2009;160:87–92. doi: 10.1530/EJE-08-0485. [DOI] [PubMed] [Google Scholar]
- 14.Chiodini I, Guglielmi G, Battista C, Carnevale V, Torlontano M, Cammisa M, Trischitta V, Scillitani A. Spinal volumetric bone mineral density and vertebral fractures in female patients with adrenal incidentalomas: the effects of subclinical hypercortisolism and gonadal status. J Clin Endocrinol Metab. 2004;89:2237–2241. doi: 10.1210/jc.2003-031413. [DOI] [PubMed] [Google Scholar]
- 15.Chiodini I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96:1223–1236. doi: 10.1210/jc.2010-2722. [DOI] [PubMed] [Google Scholar]
- 16.Kim HY, Kim SG, Lee KW, Seo JA, Kim NH, Choi KM, Baik SH, Choi DS. Clinical study of adrenal incidentaloma in Korea. Korean J Intern Med. 2005;20:303–309. doi: 10.3904/kjim.2005.20.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho YY, Suh S, Joung JY, Jeong H, Je D, Yoo H, Park TK, Min YK, Kim KW, Kim JH. Clinical characteristics and follow-up of Korean patients with adrenal incidentalomas. Korean J Intern Med. 2013;28:557–564. doi: 10.3904/kjim.2013.28.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Bae KH, Choi YK, Jeong JY, Park KG, Kim JG, Lee IK. Clinical characteristics for 348 patients with adrenal incidentaloma. Endocrinol Metab (Seoul) 2013;28:20–25. doi: 10.3803/EnM.2013.28.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460–484. doi: 10.1210/edrv-16-4-460. [DOI] [PubMed] [Google Scholar]
- 20.Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110:1014–1021. [PubMed] [Google Scholar]
- 21.Barzon L, Fallo F, Sonino N, Boscaro M. Development of overt Cushing's syndrome in patients with adrenal incidentaloma. Eur J Endocrinol. 2002;146:61–66. doi: 10.1530/eje.0.1460061. [DOI] [PubMed] [Google Scholar]
- 22.Bulow B, Ahren B Swedish Research Council Study Group of Endocrine Abdominal Tumours. Adrenal incidentaloma: experience of a standardized diagnostic programme in the Swedish prospective study. J Intern Med. 2002;252:239–246. doi: 10.1046/j.1365-2796.2002.01028.x. [DOI] [PubMed] [Google Scholar]
- 23.Kasperlik-Zeluska AA, Rosłonowska E, Slowinska-Srzednicka J, Migdalska B, Jeske W, Makowska A, Snochowska H. Incidentally discovered adrenal mass (incidentaloma): investigation and management of 208 patients. Clin Endocrinol (Oxf) 1997;46:29–37. doi: 10.1046/j.1365-2265.1997.d01-1751.x. [DOI] [PubMed] [Google Scholar]
- 24.Comlekci A, Yener S, Ertilav S, Secil M, Akinci B, Demir T, Kebapcilar L, Bayraktar F, Yesil S, Eraslan S. Adrenal incidentaloma, clinical, metabolic, follow-up aspects: single centre experience. Endocrine. 2010;37:40–46. doi: 10.1007/s12020-009-9260-5. [DOI] [PubMed] [Google Scholar]
- 25.Reincke M. Subclinical Cushing's syndrome. Endocrinol Metab Clin North Am. 2000;29:43–56. doi: 10.1016/s0889-8529(05)70115-8. [DOI] [PubMed] [Google Scholar]
- 26.Terzolo M, Bovio S, Pia A, Conton PA, Reimondo G, Dall'Asta C, Bemporad D, Angeli A, Opocher G, Mannelli M, Ambrosi B, Mantero F. Midnight serum cortisol as a marker of increased cardiovascular risk in patients with a clinically inapparent adrenal adenoma. Eur J Endocrinol. 2005;153:307–315. doi: 10.1530/eje.1.01959. [DOI] [PubMed] [Google Scholar]
- 27.Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, Pagotto U, Pasquali R. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol. 2012;166:669–677. doi: 10.1530/EJE-11-1039. [DOI] [PubMed] [Google Scholar]
- 28.Terzolo M, Pia A, Ali A, Osella G, Reimondo G, Bovio S, Daffara F, Procopio M, Paccotti P, Borretta G, Angeli A. Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol Metab. 2002;87:998–1003. doi: 10.1210/jcem.87.3.8277. [DOI] [PubMed] [Google Scholar]
- 29.Chiodini I, Morelli V, Salcuni AS, Eller-Vainicher C, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Pellegrini F, Copetti M, Beck-Peccoz P, Arosio M, Ambrosi B, Trischitta V, Scillitani A. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J Clin Endocrinol Metab. 2010;95:2736–2745. doi: 10.1210/jc.2009-2387. [DOI] [PubMed] [Google Scholar]
- 30.Erbil Y, Ademoglu E, Ozbey N, Barbaros U, Yanik BT, Salmaslioglu A, Bozbora A, Ozarmagan S. Evaluation of the cardiovascular risk in patients with subclinical Cushing syndrome before and after surgery. World J Surg. 2006;30:1665–1671. doi: 10.1007/s00268-005-0681-x. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell IC, Auchus RJ, Juneja K, Chang AY, Holt SA, Snyder WH, 3rd, Nwariaku FE. "Subclinical Cushing's syndrome" is not subclinical: improvement after adrenalectomy in 9 patients. Surgery. 2007;142:900–905. doi: 10.1016/j.surg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Mondi I, Tropea S, Nitti D, Favia G. Adrenalectomy may improve cardiovascular and metabolic impairment and ameliorate quality of life in patients with adrenal incidentalomas and subclinical Cushing's syndrome. Surgery. 2012;152:991–997. doi: 10.1016/j.surg.2012.08.054. [DOI] [PubMed] [Google Scholar]


