Abstract
This paper reviews the current literature on individual differences in susceptibility to the effects of background sound on visual-verbal task performance. A large body of evidence suggests that individual differences in working memory capacity (WMC) underpin individual differences in susceptibility to auditory distraction in most tasks and contexts. Specifically, high WMC is associated with a more steadfast locus of attention (thus overruling the call for attention that background noise may evoke) and a more constrained auditory-sensory gating (i.e., less processing of the background sound). The relation between WMC and distractibility is a general framework that may also explain distractibility differences between populations that differ along variables that covary with WMC (such as age, developmental disorders, and personality traits). A neurocognitive task-engagement/distraction trade-off (TEDTOFF) model that summarizes current knowledge is outlined and directions for future research are proposed.
Keywords: distraction, individual differences, noise, selective attention, sound, working memory capacity
The scientific investigation of individual differences and identifying the general principles and underlying mechanisms that explain those differences is not only a valuable but a necessary endeavor in the pursuit of understanding human cognition. This paper reviews recent studies that have used this approach in an attempt to understand how the human mind creates selective attention.
Try to read this paper in a noisy environment. Are you distracted? Individual differences in distractibility vary quite a lot, extending from slight facilitation from a noisy background to severe disruption (Ellermeier & Zimmer, 1997; Sörqvist, 2010b). What is the basis of these individual differences? In a previous review of the current literature, Sörqvist (2010c) attempted to answer this question. The review resulted in three general conclusions. First, individual differences in working memory capacity (WMC) underpin individual differences in susceptibility to auditory distraction across a wide range of tasks and contexts. WMC is typically measured by so-called complex-span tasks that combine mnemonic short-term memory processes with distracter activities (Conway et al., 2005). Complex-span tasks show tremendous predictive power and are basically able to predict individual differences on any task that requires some cognitive control, particularly if there is a need to overcome distraction (Engle, 2002). Based on this finding, it has been argued that WMC actually reflects individual differences in the ability to control attention and avoid distraction (Conway, Cowan, & Bunting, 2001; Kane, Bleckley, Conway, & Engle, 2001). Auditory distraction is indeed no exception. By using complex-span tasks as a measure of WMC and correlating this variable with person-specific measures of distractibility, it has, for instance, been shown that high-WMC individuals are less susceptible to the effects of aircraft noise (Sörqvist, 2010a) and background speech (Beaman, 2004; Sörqvist, Halin, & Hygge, 2010; Sörqvist, Ljungberg, & Ljung, 2010) on memory and comprehension of written materials.
The second conclusion that emerged from a prior review (Sörqvist, 2010c) is that individual differences in WMC seem to be able to explain differences between age groups and other populations. Older adults (Bell, Buchner, & Mund, 2008; Boman, Enmarker, & Hygge, 2005) and children (Elliott, 2002) are typically more susceptible to auditory distraction than young adults. As the capacity of working memory increases throughout adulthood (Gathercole, Pickering, Ambridge, & Wearing, 2004) and then declines at older ages (Gazzaley, Cooney, Rissman, & D'Esposito, 2005), it seems like age differences in distractibility reflect life-span changes in WMC. Likewise, children with attention-deficit/hyperactivity disorder (ADHD; Gumenyuk et al., 2005; Zentall & Shaw, 1980) and children with low intelligence (Johansson, 1983) are more susceptible to auditory distraction than their counterparts. This fits well with the finding that these populations typically demonstrate low WMC (Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005; Shelton, Elliott, Matthew, Hill, & Gouvier, 2010) and with the notion that low WMC makes individuals more distractible.
The third conclusion that was reached in a previous review (Sörqvist, 2010c) is that WMC—although apparently a very reliable predictor of individual differences in susceptibility to distraction in general—is unrelated to a specific form of distraction, with the key signature being the so-called changing-state effect (Beaman, 2004; Sörqvist, 2010b). The changing-state effect is the observation that serial recall of a visually presented sequence of items (e.g., digits) is more impaired by a concurrently presented sound stream that changes across time (e.g., “k l m v r q c”) compared with the repetitive presentation of the same sound element (e.g., “c c c c c c c”). Whilst WMC is unrelated to the changing-state effect, individual differences in perceptual abilities—specifically the ability to detect subtle changes in tone streams—appear to underpin the magnitude of this effect (Macken, Phelps, & Jones, 2009). In contrast, high WMC attenuates the deviation effect—the observation that serial recall is more impaired by a sound stream that contains a single deviating element (e.g., “c c c m c c c”) compared with a steady-state sound stream (e.g., “c c c c c c c”). Together, this was regarded as evidence in favor of a duplex-mechanism account of auditory distraction (Hughes, Vachon, & Jones, 2007) wherein sound can impair cognitive performance by two functionally different mechanisms: either by capturing attention (which underpins the deviation effect) or by an involuntary (uncontrollable) analysis of order information that inevitably interferes with the deliberate act of serially rehearing to-be-recalled material (which underpins the changing-state effect). A distinguishing feature of the duplex-mechanism account is, hence, that distraction is a function of the characteristics of the sound as well as a function of the processes that the task entails. For example, whilst both a changing state sound sequence (e.g., “k l m v r q c”) and a deviant sound sequence (e.g., “m m m v m m m”) are more disruptive to serial short-term memory (wherein the task is to recall a sequence of visually presented items in their order of presentation) than a steady-state sequence (e.g., “m m m m m m m”), only a deviant sound sequence disrupts short-term memory tasks that do not entail serial order memory (e.g., when the task is to identify the missing weekday from a set of six weekdays). This is because the deviating sound is disruptive owing to it capturing attention, whereas the perceptual processing of a changing state sequence is only disruptive inasmuch as it comes into conflict with the processes that are required by the task.
Here, the original review is expanded upon in an attempt to test whether the same general patterns still hold against more recent evidence. Furthermore, studies that have tried to identify the mechanisms and the neural basis of the relation between WMC and auditory distraction are discussed. Finally, the current knowledge is integrated into a model that attempts to describe the role of WMC in auditory distraction and we discuss the model's generalizability and future directions.
Individual differences in distractibility
Working memory capacity
Studies reported under this heading have all measured individual differences in WMC directly using complex-span tasks. A couple of studies have replicated the finding that high-WMC individuals are less distracted by auditory deviants (Hughes, Hurlstone, Marsh, Vachon, & Jones, 2013; Sörqvist, Nöstl, & Halin, 2012b). For instance, in a study by Sörqvist et al. (2012b), the standard cross-modal oddball paradigm was used, wherein the participants were requested to categorize visually presented target arrows as either pointing to the left or to the right. Each target was preceded by a task-irrelevant background sound. The sound was identical on most trials (standard tones), but occasionally it was replaced by a different sound (a noise burst). The deviant sound captured attention, as response time to the visual target was increased. The magnitude of this deviation effect was larger at the beginning of the experiment compared with at the end. However, the habituation process was only evident in high-WMC individuals. Whilst the deviation effect was of approximately the same size in high- and low-WMC individuals at the beginning of the experiment, the deviation effect was completely abolished in high-WMC individuals at the end of the experiment but remained at the same magnitude throughout the experiment in low-WMC individuals. Thus, individual differences in WMC are related to individual differences in susceptibility to attentional capture from background sound.
Evidence in favor of the assumption that WMC is indeed unrelated to the changing-state effect (i.e., the larger disruption from an acoustically changing, as compared with an acoustically nonchanging sound stream to serial short-term memory) has also grown (Elliott & Briganti, 2012; Hughes et al., 2013). It does not seem to matter whether the changing-state sound sequence consists of speech-tokens (such as words or spoken letters; Elliott & Briganti, 2012; Hughes et al., 2013) or of tones (Sörqvist, 2010b): Individual differences in WMC are unrelated to this type of distraction. A recent meta-analysis, which spanned across all experiments that have looked at the relation between WMC and the changing-state effect to date, found relatively strong evidence in favor of this conclusion (Sörqvist, Marsh, & Nöstl, 2013). This meta-analysis used Bayesian statistics that, contrary to conventional statistical techniques, can provide quantitative evidence in support of the null hypothesis (i.e., the absence of a relation) by calculating the actual probability of a hypothesis given the data, namely as p(H0|D), instead of calculating the probability of the observed data, as p(D|H0), under the assumption that H0 is true. Taken together, high WMC appears to protect against the involuntary capture of attention by sound (e.g., distraction that is caused by a deviant sound embedded in an otherwise nonchanging sound stream), but not against distraction that is underpinned by the mechanism responsible for the changing-state effect.
Whether WMC is also related to the magnitude of semantic auditory distraction (often called “informational masking” in the auditory-perception literature)—that is, when the semanticity of background speech is responsible for the distraction rather than the acoustic properties of the speech sound—remains to be properly explored. The few pioneering studies suggest that it is (Beaman, 2004; Sörqvist & Rönnberg, 2012; Zekveld et al., 2011). For example, we found that episodic long-term memory for spoken information is impaired when the target speech is masked by a task-irrelevant speech stream in comparison with when the to-be-attended speech stream is masked by a spectrally rotated version (acoustically very similar but meaningless) of the task-irrelevant speech stream (Sörqvist & Rönnberg, 2012). This disruption was smaller in magnitude to high-WMC individuals. In a related study, Zekveld, Rudner, Johnsrude, and Rönnberg (2013) used a sentence-in-noise speech perception task where each sentence was preceded by a visually presented text cue that was either related or unrelated to the semantic contents of the sentence. The text cue made it easier to identify the noise-masked sentence. The cue benefit was related to WMC only when the auditory masker was interfering speech compared with modulated or stationary noise backgrounds. Also, better WMC was associated with enhanced delayed recognition of sentences preceded by a cue word. This suggests that WMC is not only related to the magnitude of disruption from informational masking, but it is also related to the ability to capitalize on semantically related information, keeping cues in mind while disambiguating sentences, and encoding speech content into episodic long-term memory.
Age differences
It is quite clear that children are more susceptible to the effects of noise on task performance than are young adults (for a review see Klatte, Bergström, & Lachmann, 2013). This general conclusion has received further support in recent years (e.g., Elliott & Briganti, 2012; Muenssinger et al., 2013; but see Ruhnau, Wetzel, Widmann, & Schröger, 2010). Just as low-WMC individuals habituate to auditory distraction at a slower pace than high-WMC individuals (Sörqvist et al., 2012b), children appear to habituate at a slower pace than young adults (Muenssinger et al., 2013). Similar to Sörqvist et al. (2012b), Muenssinger et al. (2013) used a cross-modal paradigm, but the participants focused their attention on a silent movie while passively listening to tone sequences. The tone sequences consisted of five standard tones, followed by one deviant tone, followed by two standard tones. Both children's and adults' attention was captured by the presentation of the first standard tone in the sequence, as shown in event-related potential recordings. The expected pattern of habituation was found in adults (a decreased response to the following standards, and a dishabituation when the deviant tone was presented), but there was no evidence of habituation in children.
Age differences in susceptibility to distraction in the context of serial short-term memory have also been further explored. Children are more susceptible to the effects of background speech on serial short-term memory when compared with a silent condition (Elliott & Briganti, 2012). However, children seem to be no more susceptible than adults to the changing-state effect (Elliott, Hughes, Macken, Briganti, & Kytola, 2012). Taken together, the differences in distractibility between children and young adults are generally very similar to the differences that have been found between adults with low and high WMC respectively (a greater susceptibility to distraction from attention capture in children and low-WMC adults, but no relation between age and susceptibility to the changing-state effect, just as with individual differences in WMC). This is not surprising considering that children generally have a lower WMC than adults, and the finding is well in line with the view advocated here: The relation between WMC and distractibility is responsible for systematic differences in distractibility across individuals, as found in studies wherein WMC has not been measured directly, but indirectly with variables that covary with WMC.
Older adults are more susceptible to distraction than are younger adults, arguably because they have poorer inhibitory ability (Lustig, Hasher, & Zacks, 2008). In the context of auditory distraction, a typical finding when older adults are compared with younger is that older adults are more susceptible to semantic distraction (e.g., Bell et al., 2008) and to auditory attentional capture (Andrés, Parmentier, & Escera, 2006; Parmentier & Andrés, 2010) but not to the changing-state effect (Beaman, 2005). However, recent studies have often failed to confirm the general assumption that older adults are more susceptible to auditory distraction than younger adults. In a recent study by P. A. Smith (2010), participants were asked to make speeded grammaticality judgments of read sentences in the presence of background cafeteria noise, narrative background speech, or in silence. While older participants showed no difference between the three sound conditions, younger adults made faster responses in the narrative background speech condition compared with the other sound conditions. Furthermore, Van Gerven and Murphy (2010) requested younger and older adults to undertake a visual counting task in silence or while being exposed to background speech (random numbers intermixed either with emotionally neutral words, positive words or negative words, depending on the condition). The task-irrelevant speech increased counting time and accuracy, but to the same extent in younger and older participants. There was no effect of the emotional valence manipulation. In a separate study, Guerreiro and Van Gerven (2011) asked the participants to conduct an n-back task that required memory of visually presented digits while they listened to to-be-ignored spoken digits. Again, there was no difference in auditory distraction susceptibility between younger and older adults. Comparable results were also reported in a subsequent study from the same researchers (Guerreiro, Murphy, & Van Gerven, 2013).
In all, a new body of evidence points towards the possibility that older adults are actually no more susceptible to auditory distraction than younger adults (cf. Guerreiro & Van Gerven, 2011). However, in neither of these studies was the sound specifically designed to elicit attention capture by embedding a single deviant sound in a steady-state speech stream as in the studies by Andrés and colleagues (Andrés et al., 2006; Parmentier & Andrés, 2010). Moreover, the role of the semantic relation between the task-irrelevant background speech and the to-be-attended information is unclear. One study has found that meaningful background speech that is semantically related to to-be-remembered material is more distracting to memory than semantically unrelated background speech, and that this effect is larger in older adults (Bell et al., 2008). Thus, when controlling for the semantic relation between target and distracter, older adults appear to be more distracted than younger adults. In the studies by Guerreiro and colleagues (Guerreiro et al., 2013; Guerreiro & Van Gerven, 2011), the distracter was semantically related to the to-be-attended information, but there was no control condition with a background speech that was semantically unrelated to the to-be-attended information. Taken together, the results suggest that older adults are more susceptible to auditory distraction, at least when the sound calls for attention. From one standpoint, it might be the nature of the cognitive processes that the task involves (e.g., semantic or rehearsal processes), the nature of the sound, and the relation between the sound and the task, that modulates when, and when not, older adults are more distractible than younger adults. Another possibility is that the complexity/difficulty of the task, and the participants' capability to deal with this difficulty, is what distinguishes younger from older adults. In the context of more complex/difficult tasks, like reading prose for later recall, older adults are more susceptible to distraction, perhaps because younger adults can engage more fully in the task (as they have more cognitive capacity available), whereas with easier tasks both younger and older adults reach a high state of task engagement. We return to this issue later.
Disorders
Positive effects of noise are rare, but there are notable exceptions documented in the literature. A few studies have found that masking broadband noise can enhance memory of spoken words in children with ADHD (Söderlund, Sikström, Loftesnes, & Sonuga-Barke, 2010) and help them keep focus on a visual task (Lederer et al., 2013), presumably by helping these individuals reach a more optimal level of domain release (cf. Sikström & Söderlund, 2007), but opposing results have also been reported (e.g., Pelletier, Hodgetts, Lafleur, Vincent, & Tremblay, 2013; Jin, Liu, Li, & Lan, 2013). Pelletier et al. (2013) requested adult participants with ADHD and controls to undertake serial recall of visually presented sequences of items, either in silence or against a background of sound. They found that the ADHD participants were more distracted by the irrelevant sound. Thus it is still unclear whether, and under what conditions, sound may be beneficial or particularly detrimental to persons with ADHD. One possibility is that, although low WMC is a disadvantage in most cases, it may actually facilitate positive effects of background noise in very specific circumstances. Generally, however, individuals with ADHD appear to be more impaired by noise than their counterparts and the beneficial effects of noise that have been documented should be treated with caution. Sikström and Söderlund's (2007) model may be valid, in the sense that background stimulation releases dopamine that facilitates memory processes, but the literature still indicates that people with ADHD are generally more susceptible to distraction.
Overall, disorders are typically associated with a greater susceptibility to auditory distraction, including auditory processing disorders (Elliott, Bhagat, & Lynn, 2007) as well as disorders that are not directly related to auditory processing such as schizophrenia (Cao et al., 2013; Smucny, Rojas, Eichman, & Tregellas, 2013), depression (Lepistö et al., 2004), and anxiety (Eysenck & Derakshan, 2011). Notably, both schizophrenia (Lee & Park, 2005) and depression and anxiety (Christopher & MacDonald, 2005) are associated with working memory deficits. Taken together, these observations support the hypothesis that the covariation of WMC and mental disorders is the underpinning mechanism that makes people with disorders unusually distractible.
Hearing impairment
The relation between hearing impairment and auditory distraction should also be mentioned, as these individual differences have a natural place in this review even though neither the risk of acquiring a hearing impairment, or the severity of it, covaries with WMC (e.g., Lyxell & Rönnberg, 1989). However, individual differences in WMC influence how handicapping a hearing impairment becomes as WMC supports listening in noisy environments (Rönnberg et al., 2013; Rönnberg, Rudner, Foo, & Lunner, 2008). Particularly in “mismatching” conditions (i.e., when the perceived signal does not match the corresponding syllabic representations in long-term memory) explicit WMC needs to be used to support attention, inferential processing, and lexical access. Manipulations of mismatch involve using nonhabitual signal processing in hearing aids to increase mismatch (Foo, Rudner, Rönnberg, & Lunner, 2007) and the opposite reduction of mismatch via acclimatization to a signal processing algorithm (Ng, Rudner, Lunner, Pedersen, & Rönnberg, 2013; Rudner, Foo, Rönnberg, & Lunner, 2009). Interestingly, the ability to cope with the nonhabitual signal processing in hearing aids is only WMC-dependent in the beginning. As the signal processing algorithm becomes more familiar (and thus habitual), the correlation between WMC and speech comprehension decreases in magnitude (Ng, Classon, et al., 2013). This may have some commonality with the relation seen between WMC and habituation to distraction (Sörqvist et al., 2012b). Together, individual differences in WMC appear to underpin people's ability to adapt to a changing environment.
With regard to cross-modal auditory distraction, wherein hearing-impaired persons have a visual task and the sound is to be ignored, one straightforward assumption is that hearing impairment should shield against distraction for the simple reason that the hearing impaired cannot hear the sound as well as normal-hearing listeners. However, on testing this hypothesis, the opposite result has been revealed. Hearing-impaired participants are more distracted by background noise than their normal-hearing counterparts, at least when they use their hearing aids (Jahncke & Halin, 2012). Hearing-impaired individuals also rate visual tasks as more effortful in noisy environments in comparison with normal-hearing individuals (Hua, Karlsson, Widén, Möller, & Lyxell, 2013).
Personality and preferences
Interest in the effects of music on cognitive performance has been great in recent years (e.g., Schlittmeier, Hellbrück, & Klatte, 2008; Smith, Waters, & Jones, 2010). For instance, it has been shown that liked music is just as distracting to serial short-term memory as disliked music (Perham & Vizard, 2011), at least as long as the two sound sources are acoustically similar. However, some findings indicate that disliked music can actually be better for performance (Perham & Sykora, 2012). In the context of individual differences, Doyle and Furnham (2012) asked participants to undertake a reading comprehension task either in silence or against background music, and found that creative persons (operationalized as high scores on three creativity measures: the Biographical Inventory of Creative Behavior, the Guilford Alternate Uses test, and the Runco Ideational Behavior Scale) were less impaired by the background music. The evidence for this finding was weak, but it is particularly interesting here because high WMC, whilst strongly linked to analytic thinking, may actually be harmful to creativity (Wiley & Jarosz, 2012). Extrapolating, one possibility is therefore that the creative participants in Doyle and Furnham's study had relatively low WMC and they, like persons with ADHD who also tend to experience working memory deficits, may actually benefit from noisy environments under specific conditions.
Studies of individual differences in distractibility amongst extraverts and introverts are more in line with the typical pattern (Dobbs, Furnham, & McClelland, 2011; Furnham & Strbac, 2002; Furnham, Trew, & Sneade, 1999; Miyahara & Goshiki, 2004). Dobbs et al. (2011) reported that the disruptive effects of background music on reading comprehension are smaller in magnitude in extraverts than in introverts. However, they also found a positive correlation between extraversion and intelligence, which suggests that the smaller distractibility in extraverts reflects the benefit of a greater WMC.
Interim conclusion
A previous review (Sörqvist, 2010c) concluded that individual differences in WMC are generally associated with individual differences in susceptibility to cross-modal distraction based on a large set of studies (e.g., Beaman, 2004; Ellermeier & Zimmer, 1997; Elliott, Barrilleaux, & Cowan, 2006; Elliott & Cowan, 2005; Macken et al., 2009; Sörqvist, 2010a, 2010b). The present review shows (a) that this general pattern still holds for more recent results, (b) that the pattern of results seems extendable to within-modal forms of distraction (i.e., when both the target and the to-be-ignored stimuli is presented in the auditory modality), and (c) that individual differences in WMC seem to be responsible for differences seen between a wider range of populations (age groups, clinical populations, and various personality traits) than previously considered. We now turn to a discussion of the possible responsible mechanisms for these individual differences in susceptibility.
The mechanisms of the relation between WMC and cross-modal auditory distraction
Increasing task difficulty reduces the effects of sound on that particular task (e.g., Halin, Marsh, Haga, Holmgren, & Sörqvist, 2013; Kim, Kim, & Chun, 2005), as sound loses its ability to capture attention away from the visual task (Hughes et al., 2013; SanMiguel, Corral, & Escera, 2008) and the neural processing of the background sound is constrained (Regenbogen et al., 2012; Sörqvist, Stenfelt, & Rönnberg, 2012). This happens whether task difficulty is increased as a consequence of higher cognitive load (e.g., a greater memory load; Sörqvist, Stenfelt, & Rönnberg, 2012) or higher perceptual load (e.g., a greater difficulty identifying the task materials; Halin et al., 2013; Hughes et al., 2013).
Variations in WMC appear to have a functionally similar effect on distractibility as variations in task difficulty: Higher WMC is associated with a more steadfast locus of attention and less processing of background sound. Strong support for a more steadfast locus of attention in high-WMC individuals comes from studies demonstrating that high-WMC individuals are less susceptible to the deviation effect (Hughes et al., 2013; Sörqvist, 2010b; Sörqvist et al., 2013; Sörqvist, Stenfelt, & Rönnberg, 2012). Thus, high-WMC individuals are more able to resist the call for attention from deviating background sound. Support for the assumption that high-WMC individuals have a more efficient auditory-sensory gating (i.e., the degree to which the background sound is processed) comes from neurometric studies. When sound reaches the ear, it is transformed into a neural signal at the outer hair cells. Then it passes through the brainstem and the thalamus before it eventually ends up in the auditory cortex. The brainstems' responsiveness to task-irrelevant background sound depends on individual differences in WMC. In a study by Sörqvist, Stenfelt, & Rönnberg (2012), participants were requested to undertake a visual version of the n-back task wherein sequences of letters were presented and the participants' task was to decide whether the presented letter was identical to that presented one, two, or three steps back in the sequence. The participants were also concurrently presented with a task-irrelevant sound they were instructed to ignore. The experiment revealed that the amplitude of the brainstem response (i.e., the number of neurons that respond to the sound) gets smaller in magnitude when the task difficulty increases. Moreover, the difference in magnitude between the one-back condition and the three-back condition covaried with individual differences in WMC. The difference was greater in high-WMC individuals, which suggests that they are more able to constrain processing of task-irrelevant sound. Similar results have been found for the primary auditory cortex: When visual-verbal task load is high the primary auditory cortex is deactivated (Regenbogen et al., 2012). Taken together, high-WMC individuals appear to have a superior ability to modulate the auditory-perceptual filter in comparison with their low capacity counterparts at early (subcortical) and at late (cortical) processing stages.
A neurocognitive task-engagement/distraction trade-off model
Variations in task difficulty and variations in WMC seem to have functionally similar consequences for distractibility. We argue that they both operate on the same psychological constructs: the steadfastness of the locus of attention and the sensory gating of task-irrelevant information. However, an important difference between task difficulty manipulations and individual differences in WMC is that a manipulation of task difficulty influences the current “state” of these constructs (e.g., a high task difficulty leads to a more steadfast locus of attention and a more constrained gating of task-irrelevant information) whilst individual differences in WMC reflect a “trait” characteristic of the participants (Ilkowska & Engle, 2010). Individual differences in WMC set the limit for how steadfast the locus of attention and how constrained sensory gating can be for a specific person.
WMC as a person-specific “trait” must be distinguished from other views of the “working memory” concept in order to understand the role of WMC in distractibility. One definition of working memory is that it is the ability to maintain and manipulate information in immediate memory (D'Esposito, 2007). Working memory, defined this way, plays a role in distractibility (de Fockert, 2013). For instance, loading the contents of working memory (i.e., requesting participants to maintain items in immediate memory) while simultaneously performing another unrelated task (e.g., visual search) makes people more susceptible to distraction (as measured by the cost of presenting distracters in the visual search task to the time it takes to find a target) in comparison with a condition with lower working memory load (Lavie, 2010). Here, we argue that the correlations observed between WMC (as measured using complex-span tasks or similar tasks) and individual differences in distractibility are not reflecting working memory load. The advantage in high-WMC individuals is, hence, not that they more easily can handle difficult tasks (i.e., because they are less cognitively loaded), but rather that they can reach higher states of focal-task engagement (i.e., a more steadfast locus of attention and a more constrained sensory gating of irrelevant materials). For example, it is difficult to explain why WMC predicts habituation rate in the context of the classic oddball paradigm (Sörqvist et al., 2012b)—a task that requires speeded classification of the direction of visually presented arrows, which involves very little load on working memory even for low-WMC individuals—from the perspective that the advantage of high-WMC individuals is in lower working memory load. Likewise, it is difficult to see why the difference in magnitude of the auditory brainstem response to a task-irrelevant background sound, between a one-back version and a three-back version of a visual-verbal n-back task, is larger in high-WMC individuals (Sörqvist, Stenfelt, & Rönnberg, 2012). From a working memory load perspective, the difference in task difficulty (or cognitive load) between the one- and the three-back version should be larger for low-WMC individuals.
On a further note, individual differences in WMC may be associated with distractibility in its relation to selective attention abilities (i.e., the steadfastness of the locus of attention and sensory gating of task-irrelevant information) by overruling the manifestation of distraction, and in its relation to compensation processes after distraction has already been manifested. For example, high-WMC individuals are better able to search for items in secondary memory that have been lost from primary memory due to distraction (Unsworth & Engle, 2007). The relation to postdistraction compensatory abilities may explain why WMC supports listening in noisy environments in which lost information in the signal has to be compensated for by, for example, access to long-term memory representations (i.e., postdictive processes that support listening). Moreover, predictions about the target speech can be helped by a working memory system that can hold hypotheses online (i.e., predictive processes that support listening). In all, the ability to process (and comprehend) a target signal (such as speech) is working memory dependent, particularly in noisy environments (Rönnberg et al., 2013). A key difference between this perspective and the objectives of the present article is the role for WMC in target processing, on the one hand, and the role for WMC in distracter processing on the other. Here, we restrict our discussion of the role for WMC in selective attention abilities and the suppression of the task-irrelevant information.
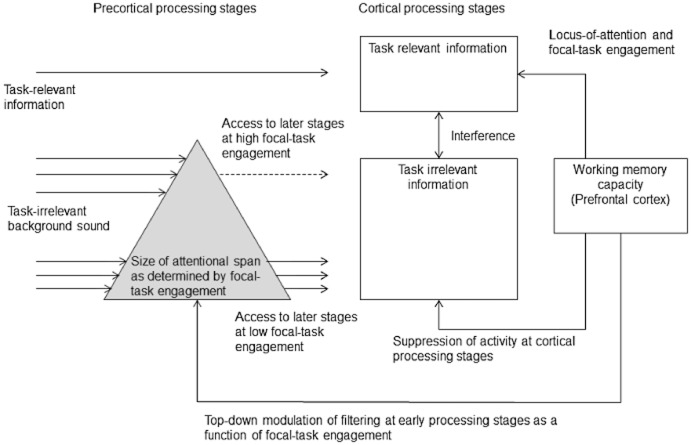
Figure 1 illustrates a neurocognitive task-engagement/distraction trade-off (TEDTOFF) model that summarizes what has been said up until this point about the role for WMC in selective attention. The model defines focal-task engagement as a continuum across which the steadfastness of the locus of attention and sensory gating of task-irrelevant information can vary. Focal-task engagement can be manipulated by changing task difficulty (cognitive load and perceptual load) and the person-specific cap for focal-task engagement is set by individual differences in WMC (e.g., Halin et al., 2013; Hughes et al., 2013; Sörqvist, Stenfelt, & Rönnberg, 2012). As mentioned above, WMC is typically assessed using a complex-span task that combines storage and recall of a set of items with a distracter activity. Accordingly, both storage (e.g., maintaining items in short-term memory) and cognitive control abilities (e.g., suppression of items from previous trials) contribute to the person-specific task score, and the WMC construct is a conglomeration of these abilities (Sörqvist, Ljungberg, & Ljung, 2010). Whilst the storage component of WMC seems to have its neural basis in the parietal areas (e.g., Braver et al., 1997; Rönnberg, Rudner, & Ingvar, 2004), the cognitive control component of WMC has its neural basis in the prefrontal cortex (Cabeza & Nyberg, 2000; D'Esposito et al., 1995; Kane & Engle, 2002). However, they are related. For instance, higher storage load (i.e., a need to maintain more items simultaneously in short-term memory) increases prefrontal cortex activity (Braver et al., 1997). The TEDTOFF model assumes that the cognitive control component of WMC (i.e., the prefrontal cortex) is responsible for individual differences in the ability to shield oneself from distraction as the prefrontal cortex is involved in top-down modulation and preparation of stimulus-selective sensory cortices (e.g., Gazzaley & Nobre, 2011; Hopfinger, Buonocore, & Mangun, 2000; Woldorff et al., 1993; Zanto, Rubens, Thangavel, & Gazzaley, 2011). More specifically, WMC orchestrates networks that (a) lock attention to the focal target material in other cortex areas (e.g., parietal cortex) and (b) influences auditory-sensory gating by modulating subcortical (i.e., the brainstem) and cortical (i.e., the auditory cortex) neural responsiveness to external stimuli.
Figure 1.
The neurocognitive task-engagement/distraction trade-off (TEDTOFF) model of working memory capacity and cross-modal auditory distraction. Task difficulty and individual differences in working memory capacity determine the state of focal-task engagement (i.e., the size of the attentional span and the steadfastness of the locus of attention). The filtering of the task-irrelevant information takes place at early (and late) processing stages. A narrower attentional span makes background sound gain less access to later, cortical processing stages.
Some criticism and methodological considerations
Resource theories have long since been questioned for their circularity (e.g., Navon, 1984). The TEDTOFF model also belongs to the family of resource theories and, hence, it can be argued that the model is ultimately circular and therefore logically flawed. For example, if participants are less distracted by background sound in a specific experimental condition (e.g., when reading a text with a hard-to-read font), wherein it is assumed that the task is difficult, in comparison with another experimental condition (e.g., when reading a text in an easy-to-read font), wherein it is assumed that the task is easy, the results would support the model. Yet, if there is no independent measure of task difficulty that ensures that the difficulty manipulation was successful, the reasoning is circular. Because of this, it is methodologically important to measure the success of the experimental manipulation when conducting experiments to test these ideas. This can be done, for example, through self-reports (e.g., asking the participants to rate the difficulty of the tasks in the various task difficulty conditions) and through pupilometric techniques (because an increase in pupilometric size indicates increased effort; Koelewijn, Zekveld, Festen, Rönnberg, & Kramer, 2012). As the basic idea is that high task difficulty protects against the disruptive effects of background sound on people's ability to carry out that particular task, it is also desirable to obtain independent measures of background sound processing rather than relying entirely on task performance. One way is to measure sound processing by event-related potentials (Sörqvist, Stenfelt, & Rönnberg, 2012).
It could also be mentioned that many influential theories of working memory and selective attention have been questioned for their circularity, such as Baddeley's working memory model (Baddeley & Larsen, 2007; Jones, Hughes, & Macken, 2007), Craik and Lockhart's (1972) levels-of-processing ideas (Baddeley, 1978), and Lavie's (2010) load theory (Benoni & Tsal, 2013). Moreover, circular theories may not be as problematic as once believed (e.g., Brown, 1993; Shogenji, 2000), particularly when Bayesian statistics are used to analyze the results, as it can quantify the support for the null hypothesis (Hahn & Oaksford, 2007). Indeed, in the Bayesian meta-analysis mentioned above, WMC was found to be unrelated to the changing-state effect (Sörqvist et al., 2013), a finding that (at least at first glance) questions the TEDTOFF model. Whether this conclusion holds against more crucial experimental manipulations (outlined below) still remains to be tested.
Research directions
One research direction for future studies is to identify associations between WMC and neural auditory processing stages other than the brainstem (Sörqvist, Stenfelt, & Rönnberg, 2012) and the auditory cortex (Regenbogen et al., 2012; Tsuchida, Katayama, & Murohashi, 2012; Yurgil & Golob, 2013). Sound is processed in the inner ear before it reaches the brainstem and thus WMC could perhaps operate on auditory processing at even earlier stages. In support for this assumption, selectively attending a specific sound signal while deliberately ignoring another, or focusing on a visual aspect of the environment while ignoring the sound, modulates the outer hair cells' responsiveness to the to-be-ignored sound (Bauer & Bayles, 1990; de Boer & Thornton, 2007; Giard, Collet, Bouchet, & Pernier, 1994; Meric & Collet, 1992; but see Michie, LePage, Solowij, Haller, & Terry, 1996). The outer hair cells are less responsive to ignored sound than to attended sound. Hence, one hypothesis that could be tested in future experiments is whether greater focal-task engagement in the visual modality, as a consequence of higher WMC, is associated with lower activity in the outer hair cells.
The specifics of the TEDTOFF model outlined in Figure 1 describe how WMC is involved in the processing of background sound while a visual-verbal task is carried out. One research direction for the future is to delineate how this model may be expanded to explain the role for WMC when the task is auditory in nature. As is well-known, WMC supports listening and speech comprehension in noisy environments (e.g., Conway et al., 2001; Rönnberg et al., 2013; Sörqvist & Rönnberg, 2012) and has been linked to indices of task difficulty, such as arousal level, in these conditions (Koelewijn et al., 2012). Furthermore, high WMC protects against cross-modal (Beaman, 2004) as well as within-modal semantic auditory distraction (Sörqvist & Rönnberg, 2012). Also, WMC appears to modulate neural responses to to-be-ignored sound when the task is to attend to another sound source. Tsuchida et al. (2012) asked participants to either attend to or ignore specific tones in a sound stream. They found that the primary auditory cortex is more responsive to to-be-attended tones in high-WMC individuals and less responsive to to-be-ignored tones in high-WMC individuals, in comparison with their low WMC counterparts. Very similar results have also been reported by Yurgil and Golob (2013). Thus, the role for WMC in cross-modal auditory distraction appears to be quite similar to its role in within-modal auditory distraction (i.e., when both the to-be-attended and the to-be-ignored information is sound). One starting point could be to use a dichotic listening paradigm (Conway et al., 2001) and manipulate the perceptual load in the to-be-attended channel (e.g., by manipulating the signal-to-noise ratio) and measure responsiveness (both neural and behavioral) to information presented in the to-be-ignored ear. Higher perceptual load (i.e., higher task difficulty) should decrease responsiveness to the stimuli in the to-be-ignored channel, and the magnitude of this effect should be related to individual differences in WMC.
Another interesting line of research would be to investigate the domain-generality and the direction of the trade-off between task engagement and distractibility. In the typical cross-modal paradigm, the task is visual and the sound is to be ignored (e.g., Macdonald & Lavie, 2011; Zhang, Chen, Yuan, Zhang, & He, 1980). There are some notable exceptions, however, where the task is auditory and the visual modality is to be ignored. It has, for instance, been shown that the activity in cortex areas that serve visual processing decreases when auditory working memory load is increased (Klemen, Büchel, Bühler, Menz, & Rose, 2010). A more general version of the TEDTOFF model would predict that higher cognitive load in the auditory task (e.g., an auditory version of the n-back task) would protect against the potentially disruptive effects of unexpected information presented in the visual modality.
In this context, it would be useful to discuss differences and similarities between the TEDTOFF model and Lavie's (2010) load theory. According to the load theory, cognitive load (i.e., maintaining items in working memory) increases distractibility, whereas perceptual load (i.e., difficulty identifying the target materials) decreases distractibility. Whilst the load theory and the TEDTOFF model agree on the assumption that perceptual load should decrease distractibility, the models disagree on the assumption that cognitive load makes people more susceptible to distraction, because the TEDTOFF model assumes that higher task difficulty—whether it is manipulated through perceptual load or through cognitive load—protects against distraction. An experiment that would differentiate between the two views is to use a dichotic listening paradigm and manipulate cognitive load by presenting an auditory version of an n-back task in the to-be-attended channel and measure responsiveness to the stimuli in the to-be-ignored channel. The TEDTOFF model predicts that distractibility should become smaller when cognitive load is increased. It should be noted, though, that studies demonstrating that cognitive load can increase distractibility typically involve a dual-task setting wherein cognitive load is manipulated by requesting participants to maintain items in working memory (e.g., one item in the low load condition and four items in the high load condition) while they simultaneously carry out an unrelated task (e.g., visual search) and the cost of cognitive load is measured by the effects of attention capture on the unrelated task (e.g., Lavie, Hirst, de Fockert, & Viding, 2004). The TEDTOFF model assumes that higher cognitive load protects against distraction to the particular task for which cognitive load is high, because the higher cognitive load (i.e., higher task difficulty) increases focal-task engagement (i.e., locks attention to the task materials and attenuates extratask information processing).
Another research direction is to test the generality of the TEDTOFF model to various forms of cross-modal auditory distraction. Hughes et al. (2013) have shown that the deviation effect is abolished when the to-be-recalled items are masked by visual noise. Warning the participants prior to the trial has the same effect: they abolish the deviation effect. The authors' interpretation of these findings is that visual noise (i.e., perceptual load) and warnings increase focal-task engagement, which results in a more steadfast locus of attention and, consequently, a smaller susceptibility to the deviation effect. In contrast, the changing-state effect resisted the focal-task engagement manipulations. This is in line with the assumption that the changing-state effect is not a consequence of sound capturing attention but rather a consequence of automatic interference as an inevitable byproduct of perceptual organization processes (Hughes et al., 2007; Macken et al., 2009). However, these results challenge the TEDTOFF model, as the model predicts that higher task difficulty modulates auditory-sensory gating. A more constrained processing of the background sound should influence how much (order) information is abstracted from the sound and thus attenuate the magnitude of the changing-state effect. One possibility is that the state of focal-task engagement, induced by masking visual noise or warning manipulations, was too lenient to abolish the changing-state effect. If combined, however, visual noise and warnings may, together, make the participant reach a high enough state of focal-task engagement to overrule the changing-state effect, particularly in high-WMC individuals (as the task-engagement ceiling is higher in these individuals).
The TEDTOFF model may also have implications for research fields other than distraction. For instance, higher task difficulty should abolish (or at least attenuate) incidental learning from information presented in the background environment, as the background information is filtered/suppressed. For example, people have better (incidental) memory of irrelevant words spoken in the background that are related to the current processing intentions (Marsh, Cook, Meeks, Clark-Foos, & Hicks, 2007). Memory of the task-irrelevant words should be abolished with higher task difficulty, particularly in high-WMC individuals. Similarly, when undertaking a visual task in the presence of background sound, people form expectations of future sound events as a result of incidental auditory sequence learning (e.g., Nöstl, Marsh, & Sörqvist, 2012; Parmentier, Elsley, Andrés, & Barceló, 2011). The TEDTOFF model predicts that learning should be abolished when engagement in the visual task increases and suggests that high-WMC individuals may be learning less from the background sound.
Finally, the TEDTOFF model delineates several applied lines of research. One is clinical research. It may, for instance, be used as a framework for understanding distractibility in persons with attentional engagement deficits as in schizophrenia (Reilly, Harris, Khine, Keshavan, & Sweeney, 2007) and ADHD (Gumenyuk et al., 2005) and the model points toward techniques for how to help these individuals overcome distraction. A second area of applied research concerns human factors. Mind-wandering is a major source of accidents (e.g., He, Becic, Lee, & McCarley, 2011), but can be prevented through perceptual load (Forster & Lavie, 2009) and top-down executive control (Kane et al., 2007). The TEDTOFF model provides a framework for understanding how task difficulty manipulations can be used to prevent mind wandering and, in extension, potential accidents. A third field of applied research is the consequences of environmental noise. Noise is a pervasive source of stress and performance decrements (A. Smith, 2012; Szalma & Hancock, 2011) as it impairs many office- and school-related abilities such as writing (Sörqvist, Nöstl, & Halin, 2012a), reading comprehension (Ljung, Sörqvist, & Hygge, 2009; Sörqvist, Halin, & Hygge, 2010), and long-term memory (Hygge, Evans, & Bullinger, 2002; Sörqvist, 2010a). A generally accepted view is that high cognitive load is bad for learning and performance (Sweller, 1994). However, disfluency can sometimes facilitate learning (Diemand-Yauman, Oppenheimer, & Vaughan, 2011) and cognitive load can have other positive effects such as facilitating physiological restoration processes (Bosch et al., 2012). In line with the positive effects of cognitive load, the TEDTOFF model predicts that high cognitive load should facilitate learning, particularly in noisy environments, as higher task difficulty attenuates distraction (Halin et al., 2013). In general, the TEDTOFF model provides a framework for investigating individual differences in desirable difficulties in noisy and potentially distracting environments.
Acknowledgments
The preparation and writing of this paper was financially supported by a grant from Stiftelsen Riksbankens Jubileumsfond (P11-0617:1) awarded to Patrik Sörqvist.
References
- Andrés P, Parmentier FBR. Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia. 2006;44:2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. The trouble with levels: A reexamination of Craik and Lockhart's framework for memory research. Psychological Review. 1978;85:139–152. doi: 10.1037/0033-295X.85.3.139. [Google Scholar]
- Baddeley AD. Larsen JD. The phonological loop: Some answers and some questions. The Quarterly Journal of Experimental Psychology. 2007;60:512–518. doi: 10.1080/17470210601147572. doi: 10.1080/17470210601147663. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Bayles RL. Precortical filtering and selective attention: An evoked potential analysis. Biological Psychology. 1990;30:21–33. doi: 10.1016/0301-0511(90)90088-e. doi: 10.1016/0301-0511(90)90088-E. [DOI] [PubMed] [Google Scholar]
- Beaman CP. The irrelevant sound phenomenon revisited: What role for working memory capacity? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:1106–1118. doi: 10.1037/0278-7393.30.5.1106. doi: 10.1037/0278-7393.30.5.1106. [DOI] [PubMed] [Google Scholar]
- Beaman P. Irrelevant sound effects amongst younger and older adults: Objective findings and subjective insights. European Journal of Cognitive Psychology. 2005;17:241–265. doi: 10.1080/09541440440000023. [Google Scholar]
- Bell R, Buchner A. Mund I. Age-related differences in irrelevant-speech effects. Psychology and Aging. 2008;23:377–391. doi: 10.1037/0882-7974.23.2.377. doi: 10.1037/0882-7974.23.2.377. [DOI] [PubMed] [Google Scholar]
- Benoni H. Tsal Y. Conceptual and methodological concerns in the theory of perceptual load. Frontiers in Psychology. 2013;4:522. doi: 10.3389/fpsyg.2013.00522. doi: 10.3389/fpsyg.2013.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman E, Enmarker I. Hygge S. Strength of noise effects on memory as a function of noise source and age. Noise and Health. 2005;7:11–26. doi: 10.4103/1463-1741.31636. doi: 10.4103/1463-1741.31636. [DOI] [PubMed] [Google Scholar]
- Bosch T, Mathiassen SE, Hallman D, de Looze MP, Lyskov E, Visser B. van Dieën JH. Temporal strategy and performance during a fatiguing short-cycle repetitive task. Ergonomics. 2012;55:863–873. doi: 10.1080/00140139.2012.682739. doi: 10.1080/00140139.2012.682739. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE. Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brown HI. A theory-laden observation can test the theory. British Journal for the Philosophy of Science. 1993;44:555–559. doi: 10.1093/bjps/44.3.555. [Google Scholar]
- Cabeza R. Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cao X, Li Z, Metcalfe HM, Yang T, Tan S, Wang Y. Chan RCK. The nature and extent of working memory dysfunction in schizophrenia. PsyCh Journal. 2013;2:175–182. doi: 10.1002/pchj.30. doi: 10.1002/pchj.30. [DOI] [PubMed] [Google Scholar]
- Christopher G. MacDonald J. The impact of clinical depression on working memory. Cognitive Neuropsychiatry. 2005;10:379–399. doi: 10.1080/13546800444000128. doi: 10.1080/13546800444000128. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N. Bunting MF. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin and Review. 2001;8:331–335. doi: 10.3758/bf03196169. doi: 10.3758/BF03196169. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O. Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin and Review. 2005;12:769–786. doi: 10.3758/bf03196772. doi: 10.3758/BF03196772. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. doi: 10.1016/S0022-5371(72)80001-X. [Google Scholar]
- de Boer J. Thornton ARD. Effect of subject task on contralateral suppression of click evoked otoacoustic emissions. Hearing Research. 2007;233:117–123. doi: 10.1016/j.heares.2007.08.002. doi: 10.1016/j.heares.2007.08.002. [DOI] [PubMed] [Google Scholar]
- de Fockert JW. Beyond perceptual load and dilution: A review of the role of working memory in selective attention. Frontiers in Psychology. 2013;4:287. doi: 10.3389/fpsyg.2013.00287. doi: 10.3389/fpsyg.2013.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S. Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Diemand-Yauman C, Oppenheimer DM. Vaughan EB. Fortune favors the boldand the italicized): Effects of disfluency on educational outcomes. Cognition. 2011;118:111–115. doi: 10.1016/j.cognition.2010.09.012. doi: 10.1016/j.cognition.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Dobbs S, Furnham A. McClelland A. The effect of background music and noise on the cognitive test performance of introverts and extraverts. Applied Cognitive Psychology. 2011;25:307–313. doi: 10.1002/acp.1692. [Google Scholar]
- Doyle M. Furnham A. The distracting effects of music on the cognitive test performance of creative and non-creative individuals. Thinking Skills and Creativity. 2012;7:1–7. doi: 10.1016/j.tsc.2011.09.002. [Google Scholar]
- Ellermeier W. Zimmer K. Individual differences in susceptibility to the “irrelevant speech effect.”. Journal of the Acoustical Society of America. 1997;102:2191–2199. doi: 10.1121/1.419596. doi: 10.1121/1.419596. [DOI] [PubMed] [Google Scholar]
- Elliott E, Barrilleaux K. Cowan N. Individual differences in the ability to avoid distracting sounds. European Journal of Cognitive Psychology. 2006;18:90–108. doi: 10.1080/09541440500216044. [Google Scholar]
- Elliott EM. The irrelevant-speech effect and children: Theoretical implications of developmental change. Memory and Cognition. 2002;30:478–487. doi: 10.3758/bf03194948. doi: 10.3758/BF03194948. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Bhagat SP. Lynn SD. Can children with (central) auditory processing disorders ignore irrelevant sounds? Research in Developmental Disabilities. 2007;28:506–517. doi: 10.1016/j.ridd.2006.06.005. doi: 10.1016/j.ridd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Elliott EM. Briganti AM. Investigating the role of attentional resources in the irrelevant speech effect. Acta Psychologica. 2012;140:64–74. doi: 10.1016/j.actpsy.2012.02.009. doi: 10.1016/j.actpsy.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Elliott EM. Cowan N. Coherence of the irrelevant-sound effect: Individual profiles of short-term memory and susceptibility to task-irrelevant materials. Memory and Cognition. 2005;33:664–675. doi: 10.3758/bf03195333. doi: 10.3758/BF03195333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EM, Hughes RW, Macken WJ, Briganti AM. Kytola KL. Role of serial order in auditory distraction in children and adults. Minneapolis, MN: Poster presented at the Annual meeting of the Psychonomic Society,; 2012. November. [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. doi: 10.1111/1467-8721.00160. [Google Scholar]
- Eysenck MW. Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50:955–960. doi: 10.1016/j.paid.2010.08.019. [Google Scholar]
- Foo C, Rudner M, Rönnberg J. Lunner T. Recognition of speech in noise with new hearing instrument compression release settings requires explicit cognitive storage and processing capacity. Journal of the American Academy of Audiology. 2007;18:618–631. doi: 10.3766/jaaa.18.7.8. doi: 10.3766/jaaa.18.7.8. [DOI] [PubMed] [Google Scholar]
- Forster S. Lavie N. Harnessing the wandering mind: The role of perceptual load. Cognition. 2009;111:345–355. doi: 10.1016/j.cognition.2009.02.006. doi: 10.1016/j.cognition.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnham A. Strbac L. Music is as distracting as noise: The differential distraction of background music and noise on the cognitive test performance of introverts and extraverts. Ergonomics. 2002;45:203–217. doi: 10.1080/00140130210121932. doi: 10.1080/00140130210121932. [DOI] [PubMed] [Google Scholar]
- Furnham A, Trew S. Sneade I. The distracting effects of vocal and instrumental music on the cognitive test performance of introverts and extraverts. Personality and Individual Differences. 1999;27:381–392. doi: 10.1016/S0191-8869(98)00249-9. [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B. Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J. D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A. Nobre AC. Top-down modulation: Bridging selective attention and working memory. Trends in Cognitive Sciences. 2011;16:129–135. doi: 10.1016/j.tics.2011.11.014. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard M-H, Collet L, Bouchet P. Pernier J. Auditory selective attention in the human cochlea. Brain Research. 1994;633:353–356. doi: 10.1016/0006-8993(94)91561-x. doi: 10.1016/0006-8993(94)91561-X. [DOI] [PubMed] [Google Scholar]
- Guerreiro MJS, Murphy DR. Van Gerven PWM. Making sense of age-related distractibility: The critical role of sensory modality. Acta Psychologica. 2013;142:184–194. doi: 10.1016/j.actpsy.2012.11.007. doi: 10.1016/j.actpsy.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Guerreiro MJS. Van Gerven PWM. Now you see it, now you don't: Evidence for age-dependent and age-independent cross-modal distraction. Psychology and Aging. 2011;26:415–426. doi: 10.1037/a0021507. doi: 10.1037/a0021507. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V, Korzyukov O, Escera C, Hämäläinen M, Huotilainen M, Häyrinen T. Alho K. Electrophysiological evidence of enhanced distractibility in ADHD children. Neuroscience Letters. 2005;374:212–217. doi: 10.1016/j.neulet.2004.10.081. doi: 10.1016/j.neulet.2004.10.081. [DOI] [PubMed] [Google Scholar]
- Hahn U. Oaksford M. The rationality of informal argumentation: A Bayesian approach to reasoning fallacies. Psychological Review. 2007;114:704–732. doi: 10.1037/0033-295X.114.3.704. doi: 10.1037/0033-295X.114.3.704. [DOI] [PubMed] [Google Scholar]
- Halin N, Marsh JE, Haga A, Holmgren M. Sörqvist P. Effects of speech on proofreading: Can task-engagement manipulations shield against distraction? Journal of Experimental Psychology: Applied. 2013 doi: 10.1037/xap0000002. Advance online publication. doi: 10.1037/xap0000002. [DOI] [PubMed] [Google Scholar]
- He J, Becic E, Lee Y-C. McCarley JS. Mind wandering behind the wheel: Performance and oculomotor correlates. Human Factors. 2011;53:13–21. doi: 10.1177/0018720810391530. doi: 10.1177/0018720810391530. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH. Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hua H, Karlsson J, Widén S, Möller C. Lyxell B. Quality of life, effort and disturbance perceived in noise: A comparison between employees with aided hearing impairment and normal hearing. International Journal of Audiology. 2013;52:642–649. doi: 10.3109/14992027.2013.803611. doi: 10.3109/14992027.2013.803611. [DOI] [PubMed] [Google Scholar]
- Hughes RW, Hurlstone MJ, Marsh JE, Vachon F. Jones DM. Cognitive control of auditory distraction: Impact of task difficulty, foreknowledge, and working memory capacity supports duplex-mechanism account. Journal of Experimental Psychology: Human Perception and Performance. 2013;39:539–553. doi: 10.1037/a0029064. doi: 10.1037/a0029064. [DOI] [PubMed] [Google Scholar]
- Hughes RW, Vachon F. Jones DM. Disruption of short-term memory by changing and deviant sounds: Support for a duplex-mechanism account of auditory distraction. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:1050–1061. doi: 10.1037/0278-7393.33.6.1050. doi: 10.1037/0278-7393.33.6.1050. [DOI] [PubMed] [Google Scholar]
- Hygge S, Evans GW. Bullinger M. A prospective study of some effects of aircraft noise on cognitive performance in schoolchildren. Psychological Science. 2002;13:469–474. doi: 10.1111/1467-9280.00483. doi: 10.1111/1467-9280.00483. [DOI] [PubMed] [Google Scholar]
- Ilkowska M. Engle RW. Trait and state differences in working memory capacity. In: Szymura B, editor; Gruszka A, Matthews G, editors. Handbook of individual differences in cognition: Attention, memory, and executive control. New York, NY: Springer; 2010. pp. 295–320. doi: 10.1007/978-1-4419-1210-7_18. [Google Scholar]
- Jahncke J. Halin N. Performance, fatigue and stress in open-plan offices: The effects of noise and restoration on hearing impaired and normal hearing individuals. Noise and Health. 2012;14:260–272. doi: 10.4103/1463-1741.102966. doi: 10.4103/1463-1741.102966. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liu X, Li K. Lan Y. Effect of working memory load on filtering novel distracters in children with attention deficit hyperactivity disorder. Acta Psychologica Sinica. 2013;45:961–969. doi: 10.3724/SP.J.1041.2013.00961. [Google Scholar]
- Johansson CR. Effects of low intensity, continuous and intermittent noise on mental performance and writing pressure of children with different intelligence and personality characteristics. Ergonomics. 1983;26:275–288. doi: 10.1080/00140138308963341. doi: 10.1080/00140138308963341. [DOI] [PubMed] [Google Scholar]
- Jones DM, Hughes RW. Macken WJ. The phonological store abandoned. The Quarterly Journal of Experimental Psychology. 2007;60:505–511. doi: 10.1080/17470210601147598. doi: 10.1080/17470210601147598. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA. Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. doi: 10.1037/0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I. Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Kane MJ. Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin and Review. 2002;9:637–671. doi: 10.3758/bf03196323. doi: 10.3758/BF03196323. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Kim M-S. Chun MM. Concurrent working memory load can reduce distraction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16524–16529. doi: 10.1073/pnas.0505454102. doi: 10.1073/pnas.0505454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte M, Bergström K. Lachmann T. Does noise affect learning? A short review on noise effects on cognitive performance in children. Frontiers in Developmental Psychology. 2013;4:578. doi: 10.3389/fpsyg.2013.00578. doi: 10.3389/fpsyg.2013.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemen J, Büchel C, Bühler M, Menz MM. Rose M. Auditory working memory load impairs visual ventral stream processing: Toward a unified model of attentional load. Journal of Cognitive Neuroscience. 2010;22:437–446. doi: 10.1162/jocn.2009.21204. doi: 10.1162/jocn.2009.21204. [DOI] [PubMed] [Google Scholar]
- Koelewijn T, Zekveld AA, Festen JM, Rönnberg J. Kramer SE. Processing load induced by informational masking is related to linguistic abilities. International Journal of Otolaryngology. 2012;2012:865731. doi: 10.1155/2012/865731. doi: 10.1155/2012/865731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. Attention, distraction, and cognitive control under load. Current Directions in Psychological Science. 2010;19:143–148. doi: 10.1177/0963721410370295. [Google Scholar]
- Lavie N, Hirst A, de Fockert JW. Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lederer A, Tegelbeckers J, Schares L, Bonath B, Flechtner H-H. Krauel K. The influence of auditory novelty on the attentional performance in children with ADHD. Milan, Italy: Poster presented at the 4th World Congress on ADHD,; 2013. June. [Google Scholar]
- Lee J. Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Soininen M, Čeponienė R, Almqvist F, Näätänen R. Aronen ET. Auditory event-related potential indices of increased distractibility in children with major depression. Clinical Neurophysiology. 2004;115:620–627. doi: 10.1016/j.clinph.2003.10.020. doi: 10.1016/j.clinph.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Ljung R, Sorqvist P. Hygge S. Effects of road traffic noise and irrelevant speech on children's reading and mathematical performance. Noise and Health. 2009;11:194–198. doi: 10.4103/1463-1741.56212. doi: 10.4103/1463-1741.56212. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L. Zacks RT. Inhibitory deficit theory: Recent developments in a “new view.”. In: MacLeod CM, editor; Gorfein DS, editor. Inhibition in cognition. Washington, DC: American Psychological Association; 2008. pp. 145–162. [Google Scholar]
- Lyxell B. Rönnberg J. Information-processing skill and speech-reading. British Journal of Audiology. 1989;23:339–347. doi: 10.3109/03005368909076523. doi: 10.3109/03005368909076523. [DOI] [PubMed] [Google Scholar]
- Macdonald JSP. Lavie N. Visual perceptual load induces inattentional deafness. Attention, Perception, and Psychophysics. 2011;73:1780–1789. doi: 10.3758/s13414-011-0144-4. doi: 10.3758/s13414-011-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macken WJ, Phelps FG. Jones DM. What causes auditory distraction? Psychonomic Bulletin and Review. 2009;16:139–144. doi: 10.3758/PBR.16.1.139. doi: 10.3758/PBR.16.1.139. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Cook GI, Meeks JT, Clark-Foos A. Hicks JL. Memory for intention-related material presented in a to-be-ignored channel. Memory and Cognition. 2007;35:1197–1204. doi: 10.3758/bf03193593. doi: 10.3758/BF03193593. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S. Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Meric C. Collet L. Visual attention and evoked otoacoustic emissions: A slight but real effect. International Journal of Psychophysiology. 1992;12:233–235. doi: 10.1016/0167-8760(92)90061-f. doi: 10.1016/0167-8760(92)90061-F. [DOI] [PubMed] [Google Scholar]
- Michie PT, LePage EL, Solowij N, Haller M. Terry L. Evoked otoacoustic emissions and auditory selective attention. Hearing Research. 1996;98:54–67. doi: 10.1016/0378-5955(96)00059-7. doi: 10.1016/0378-5955(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Miyahara M. Goshiki T. Individual differences in the irrelevant speech effect. Psychologia. 2004;47:178–190. doi: 10.2117/psysoc.2004.178. [Google Scholar]
- Muenssinger J, Sting KT, Matuz T, Binder G, Ehehalt S. Preissl H. Auditory habituation to simple tones: Reduced evidence for habituation in children compared to adults. Frontiers in Human Neuroscience. 2013;7:377. doi: 10.3389/fnhum.2013.00377. doi: 10.3389/fnhum.2013.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D. Resources—a theoretical soup stone? Psychological Review. 1984;91:216–234. doi: 10.1037/0033-295X.91.2.216. [Google Scholar]
- Ng EHN, Classon E, Larsby B, Arlinger S, Lunner T, Rudner M. Rönnberg J. Dynamic relation between working memory capacity and speech recognition in noise during the first six months of hearing aid use. 2013. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- Ng EHN, Rudner M, Lunner T, Pedersen MS. Rönnberg J. Effects of noise and working memory capacity on memory processing of speech for hearing-aid users. International Journal of Audiology. 2013;52:433–441. doi: 10.3109/14992027.2013.776181. doi: 10.3109/14992027.2013.776181. [DOI] [PubMed] [Google Scholar]
- Nöstl A, Marsh JE. Sörqvist P. Expectations modulate the magnitude of attentional capture by auditory events. PLoS ONE. 2012;7:e48569. doi: 10.1371/journal.pone.0048569. doi: 10.1371/journal.pone.0048569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier FBR. Andrés P. The involuntary capture of attention by sound: Novelty and postnovelty distraction in young and older adults. Experimental Psychology. 2010;57:68–76. doi: 10.1027/1618-3169/a000009. doi: 10.1027/1618-3169/a000009. [DOI] [PubMed] [Google Scholar]
- Parmentier FBR, Elsley JV, Andrés P. Barceló F. Why are auditory novels distracting? Contrasting the roles of novelty, violation of expectation and stimulus change. Cognition. 2011;119:374–380. doi: 10.1016/j.cognition.2011.02.001. doi: 10.1016/j.cognition.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Pelletier M-F, Hodgetts HM, Lafleur MF, Vincent A. Tremblay S. Vulnerability to the irrelevant sound effect in adult ADHD. Journal of Attention Disorders. 2013 doi: 10.1177/1087054713492563. Advance online publication. doi: 10.1177/1087054713492563. [DOI] [PubMed] [Google Scholar]
- Perham N. Sykora M. Disliked music can be better for performance than liked music. Applied Cognitive Psychology. 2012;26:550–555. doi: 10.1002/acp.2826. [Google Scholar]
- Perham N. Vizard J. Can preference for background music mediate the irrelevant sound effect? Applied Cognitive Psychology. 2011;25:625–631. doi: 10.1002/acp.1731. [Google Scholar]
- Regenbogen C, De Vos M, Debener S, Turetsky BI, Mößnang C, Finkelmeyer A. Kellermann T. Auditory processing under cross-modal visual load investigated with simultaneous EEG-fMRI. PLoS ONE. 2012;7:e52267. doi: 10.1371/journal.pone.0052267. doi: 10.1371/journal.pone.0052267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Harris MSH, Khine TT, Keshavan MS. Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biological Psychiatry. 2007;63:776–783. doi: 10.1016/j.biopsych.2007.11.009. doi: 10.1016/j.biopsych.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg J, Lunner T, Zekveld A, Sörqvist P, Danielsson H, Lyxell B. Rudner M. The ease of language understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience. 2013;7:31. doi: 10.3389/fnsys.2013.00031. doi: 10.3389/fnsys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg J, Rudner M, Foo C. Lunner T. Cognition counts: A working memory system for ease of language understanding (ELU) International Journal of Audiology. 2008;47:S99–S105. doi: 10.1080/14992020802301167. doi: 10.1080/14992020802301167. [DOI] [PubMed] [Google Scholar]
- Rönnberg J, Rudner M. Ingvar M. Neural correlates of working memory for sign language. Cognitive Brain Research. 2004;20:165–182. doi: 10.1016/j.cogbrainres.2004.03.002. doi: 10.1016/j.cogbrainres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Rudner M, Foo C, Rönnberg J. Lunner T. Cognition and aided speech recognition in noise: Specific role for cognitive factors following nine-week experience with adjusted compression settings in hearing aids. Scandinavian Journal of Psychology. 2009;50:405–418. doi: 10.1111/j.1467-9450.2009.00745.x. doi: 10.1111/j.1467-9450.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- Ruhnau P, Wetzel N, Widmann A. Schröger E. The modulation of auditory novelty processing by working memory load in school age children and adults: A combined behavioral and event-related potential study. BMC Neuroscience. 2010;11:126. doi: 10.1186/1471-2202-11-126. doi: 10.1186/1471-2202-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel I, Corral M-J. Escera C. When loading working memory reduces distraction: Behavioral and electrophysiological evidence from an auditory-visual distraction paradigm. Journal of Cognitive Neuroscience. 2008;20:1131–1145. doi: 10.1162/jocn.2008.20078. doi: 10.1162/jocn.2008.20078. [DOI] [PubMed] [Google Scholar]
- Schlittmeier SJ, Hellbrück J. Klatte M. Does irrelevant music cause an irrelevant sound effect for auditory items? European Journal of Cognitive Psychology. 2008;20:252–271. doi: 10.1080/09541440701427838. [Google Scholar]
- Shelton JT, Elliott EM, Matthew RA, Hill BD. Gouvier WD. The relationships of working memory, secondary memory, and general fluid intelligence: Working memory is special. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:813–820. doi: 10.1037/a0019046. doi: 10.1037/a0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogenji T. Self-dependent justification without circularity. British Journal of Philosophy of Science. 2000;51:287–298. doi: 10.1093/bjps/51.2.287. [Google Scholar]
- Sikström S. Söderlund G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychological Review. 2007;114:1047–1075. doi: 10.1037/0033-295X.114.4.1047. doi: 10.1037/0033-295X.114.4.1047. [DOI] [PubMed] [Google Scholar]
- Smith A. An update on noise and performance: Comment on Szalma and Hancock (2011) Psychological Bulletin. 2012;138:1262–1268. doi: 10.1037/a0028867. doi: 10.1037/a0028867. [DOI] [PubMed] [Google Scholar]
- Smith A, Waters B. Jones H. Effects of prior exposure to office noise and music on aspects of working memory. Noise and Health. 2010;12:235–243. doi: 10.4103/1463-1741.70502. doi: 10.4103/1463-1741.70502. [DOI] [PubMed] [Google Scholar]
- Smith PA. Ageing, auditory distraction, and grammaticality judgement. Aphasiology. 2010;24:1342–1353. doi: 10.1080/02687030903490533. [Google Scholar]
- Smucny J, Rojas DC, Eichman LC. Tregellas JR. Neural effects of auditory distraction on visual attention in schizophrenia. PLoS ONE. 2013;8:e60606. doi: 10.1371/journal.pone.0060606. doi: 10.1371/journal.pone.0060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund GBW, Sikström S, Loftesnes JM. Sonuga-Barke EJ. The effects of background white noise on memory performance in inattentive school children. Behavioral and Brain Functions. 2010;6:55. doi: 10.1186/1744-9081-6-55. doi: 10.1186/1744-9081-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörqvist P. Effects of aircraft noise and speech on prose memory: What role for working memory capacity? Journal of Environmental Psychology. 2010a;30:112–118. doi: 10.1016/j.jenvp.2009.11.004. [Google Scholar]
- Sörqvist P. High working memory capacity attenuates the deviation effect but not the changing-state effect: Further support for the duplex-mechanism account of auditory distraction. Memory and Cognition. 2010b;38:651–658. doi: 10.3758/MC.38.5.651. doi: 10.3758/MC.38.5.651. [DOI] [PubMed] [Google Scholar]
- Sörqvist P. The role of working memory capacity in auditory distraction: A review. Noise and Health. 2010c;12:217–224. doi: 10.4103/1463-1741.70500. doi: 10.4103/1463-1741.70500. [DOI] [PubMed] [Google Scholar]
- Sörqvist P, Halin N. Hygge S. Individual differences in susceptibility to the effects of speech on reading comprehension. Applied Cognitive Psychology. 2010;24:67–76. doi: 10.1002/acp.1543. [Google Scholar]
- Sörqvist P, Ljungberg JK. Ljung R. A sub-process view of working memory capacity: Evidence from effects of speech on prose memory. Memory. 2010;18:310–326. doi: 10.1080/09658211003601530. doi: 10.1080/09658211003601530. [DOI] [PubMed] [Google Scholar]
- Sörqvist P, Marsh JE. Nöstl A. High working memory capacity does not always attenuate distraction: Bayesian evidence in support of the null hypothesis. Psychonomic Bulletin and Review. 2013;20:897–904. doi: 10.3758/s13423-013-0419-y. doi: 10.3758/s13423-013-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörqvist P, Nöstl A. Halin N. Disruption of writing processes by the semanticity of background speech. Scandinavian Journal of Psychology. 2012a;53:97–102. doi: 10.1111/j.1467-9450.2011.00936.x. doi: 10.1111/j.1467-9450.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Sörqvist P, Nöstl A. Halin N. Working memory capacity modulates habituation rate: Evidence from a cross-modal auditory distraction paradigm. Psychonomic Bulletin and Review. 2012b;19:245–250. doi: 10.3758/s13423-011-0203-9. doi: 10.3758/s13423-011-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörqvist P. Rönnberg J. Episodic long-term memory of spoken discourse masked by speech: What is the role for working memory capacity? Journal of Speech, Language, and Hearing Research. 2012;55:210–218. doi: 10.1044/1092-4388(2011/10-0353). doi: 10.1044/1092-4388(2011/10-0353) [DOI] [PubMed] [Google Scholar]
- Sörqvist P, Stenfelt S. Rönnberg J. Working memory capacity and visual–verbal cognitive load modulate auditory–sensory gating in the brainstem: Toward a unified view of attention. Journal of Cognitive Neuroscience. 2012;24:2147–2154. doi: 10.1162/jocn_a_00275. doi: 10.1162/jocn_a_00275. [DOI] [PubMed] [Google Scholar]
- Sweller J. Cognitive load theory, learning difficulty, and instructional design. Learning and Instruction. 1994;4:295–312. doi: 10.1016/0959-4752(94)90003-5. [Google Scholar]
- Szalma JL. Hancock PA. Noise effects on human performance: A meta-analytic synthesis. Psychological Bulletin. 2011;137:682–707. doi: 10.1037/a0023987. doi: 10.1037/a0023987. [DOI] [PubMed] [Google Scholar]
- Tsuchida Y, Katayama J. Murohashi H. Working memory capacity affects the interference control of distractors at auditory gating. Neuroscience Letters. 2012;516:62–66. doi: 10.1016/j.neulet.2012.03.057. doi: 10.1016/j.neulet.2012.03.057. [DOI] [PubMed] [Google Scholar]
- Unsworth N. Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114:104–132. doi: 10.1037/0033-295X.114.1.104. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Van Gerven PWM. Murphy DR. Aging and distraction by irrelevant speech: Does emotional valence matter? Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2010;65B:667–670. doi: 10.1093/geronb/gbq048. doi: 10.1093/geronb/gbq048. [DOI] [PubMed] [Google Scholar]
- Wiley J. Jarosz AF. Working memory capacity, attentional focus, and problem solving. Current Directions in Psychological Science. 2012;22:258–262. doi: 10.1177/0963721412447622. [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D. Bloom FE. Modulation of early sensory processing in human auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgil KA. Golob EJ. Cortical potentials in an auditory oddball task reflect individual differences in working memory capacity. Psychophysiology. 2013;50:1263–1274. doi: 10.1111/psyp.12140. doi: 10.1111/psyp.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A. Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;14:656–661. doi: 10.1038/nn.2773. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekveld AA, Rudner M, Johnsrude IS, Festen JM, van Beek JH. Rönnberg J. The influence of semantically related and unrelated text cues on the intelligibility of sentences in noise. Ear and Hearing. 2011;32:16–25. doi: 10.1097/AUD.0b013e318228036a. doi: 10.1097/AUD.0b013e318228036a. [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Rudner M, Johnsrude IS. Rönnberg J. The effects of working memory capacity and semantic cues on the intelligibility of speech in noise. Journal of the Acoustical Society of America. 2013;134:2225–2234. doi: 10.1121/1.4817926. doi: 10.1121/1.4817926. [DOI] [PubMed] [Google Scholar]
- Zentall SS. Shaw JH. Effects of classroom noise on performance and activity of second-grade hyperactive and control children. Journal of Educational Psychology. 72:830–840. doi: 10.1037/0022-0663.72.6.830. [PubMed] [Google Scholar]
- Zhang P, Chen X, Yuan P, Zhang D. He S. The effect of visuospatial attentional load on the processing of irrelevant acoustic distractors. NeuroImage. 2006;33:715–724. doi: 10.1016/j.neuroimage.2006.07.015. doi: 10.1016/j.neuroimage.2006.07.015. [DOI] [PubMed] [Google Scholar]