Abstract
The nature of selection responsible for the maintenance of the economically and ecologically important Picea glauca × Picea engelmannii hybrid zone was investigated. Genomic, phenotypic and climatic data were used to test assumptions of hybrid zone maintenance and to model future scenarios under climate change.
Genome-wide estimates of admixture based on a panel of 86 candidate gene single nucleotide polymorphisms were combined with long-term quantitative data on growth and survival (over 20 yr), as well as one-time assessments of bud burst and bud set phenology, and cold hardiness traits. A total of 15 498 individuals were phenotyped for growth and survival.
Our results suggest that the P. glauca × P. engelmannii hybrid zone is maintained by local adaptation to growing season length and snowpack (exogenous selection). Hybrids appeared to be fitter than pure species in intermediate environments, which fits expectations of the bounded hybrid superiority model of hybrid zone maintenance. Adaptive introgression from parental species has probably contributed to increased hybrid fitness in intermediate habitats.
While P. engelmannii ancestry is higher than P. glauca ancestry in hybrid populations, on average, selective breeding in managed hybrid populations is shifting genomic composition towards P. glauca, potentially pre-adapting managed populations to warmer climates.
Keywords: adaptive introgression, bounded hybrid superiority, breeding, climate change, exogenous selection
Introduction
The role of hybridization in adaptive evolution has been a contentious issue in evolutionary biology. Some researchers have argued that hybridization is a potent evolutionary force that facilitates adaptive evolution and can lead to new species (Anderson, 1949; Arnold, 1997; Abbott et al., 2013). According to this perspective, new gene combinations resulting from hybridization may promote the development of adaptations to novel or changing environments (Rieseberg et al., 2003). By contrast, natural hybridization between divergent populations has been considered an evolutionary dead end because it can lead to unfit or unviable hybrids (Barton & Hewitt, 1985). With climates changing rapidly as a result of anthropogenic warming, the effects of hybridization and introgression on the rate of adaptation are important for both natural and managed populations, particularly for species with long generation lengths such as trees (Aitken et al., 2008).
Although definitive evidence of whether hybridization more often promotes or hinders adaptation is lacking, recent studies have supported the former for many plant species (Choler et al., 2004; Campbell & Waser, 2007; Stift et al., 2008; De Carvalho et al., 2010; Thomasset et al., 2011). In sunflower (Helianthus spp.), new gene combinations generated by introgression have contributed to ecological divergence (Rieseberg et al., 2003), and adaptation in several abiotic tolerance traits (Whitney et al., 2010). Adaptive introgression has increased flooding tolerance in Iris (Martin et al., 2005, 2006) and Rorippa (Stift et al., 2008), drought tolerance in Pinus (Ma et al., 2010) and light tolerance in Silene (Goulson, 2009).
The maintenance of hybrid zones has been the subject of considerable debate, mainly because of different points of view about the relative powers of natural selection as a divergent force and gene flow as a homogenizing force (Harrison, 1993; Barton, 2001). Environment-independent models describe hybrid zones maintained by a balance between dispersal and selection against hybrids, with selection being independent of the environment (Mayr, 1942; Barton, 1979). The tension zone model assumes that hybrids are less fit than their parents regardless of location (endogenous selection; Gay et al., 2008; Carling & Zuckerberg, 2011). Hybrid inferiority is thought to result from the break-up of epistatic co-adapted gene complexes that affect fitness traits (Barton & Hewitt, 1985; Hewitt, 1988). Environment-dependent models involve genotype-by-environment interactions, where hybrid zones are maintained through selection gradients resulting from environmental heterogeneity (Endler, 1973, 1977; Slatkin, 1973; Harrison, 1986). In these models, hybrid fitness varies with the environment (exogenous selection). The bounded hybrid superiority model (Moore, 1977) is an important environment-dependent model, which postulates that hybrid individuals are fitter than either parental species in environments that are intermediate to parental habitats, but are less fit than parental species in their respective native habitats (Wang et al., 1997; Milne et al., 2003; Miglia et al., 2005; Goulson, 2009).
Picea glauca (Moench) Voss (white spruce) and Picea engelmannii Parry ex Engelm. (Engelmann spruce) are closely related, wind-dispersed, long-lived tree species that hybridize extensively in areas where their ranges overlap, mostly in British Columbia and the western part of Alberta, Canada. Picea glauca and P. engelmannii inhabit different ecological niches separated primarily by elevation. Picea engelmannii is a subalpine species that has relatively low tolerance of high temperatures and drought, but can withstand short growing seasons and high snowfall (Alexander & Shepperd, 1990). Picea glauca is primarily a boreal species that grows under highly variable conditions and can tolerate both low winter temperatures and summer drought, but is restricted to low elevations. Hybrids occupy ecological and elevational niches intermediate to those of the parental species. Hybridization followed by backcrossing has produced hybrids with intermediate characteristics in morphology and phenotypic traits, and clinal variation along elevational gradients (Roche, 1969; Daubenmire, 1974; Rajora & Dancik, 2000; Ledig et al., 2006). Neutral molecular marker studies have found clines in admixture along elevational and latitudinal gradients, with hybrids on average having a higher contribution from P. engelmannii than from P. glauca (A. R. De La Torre & S. Aitken, unpublished data). Although it has been suggested that hybrids are fitter than parentals in intermediate environments (Roche, 1969; Daubenmire, 1974; Xie et al., 1998; Ledig et al., 2006), this has never been tested.
Picea glauca and P. engelmannii are economically important tree species in Canada. The complex of these two species and their hybrids (collectively managed as ‘interior spruce’) represents the second most planted tree in British Columbia. Tens of millions of trees are planted annually for reforestation in the province, the majority of which are products of a large breeding programme that was initiated in the early 1960s. With British Columbia getting warmer and in some areas drier as a result of climate change (Mbogga et al., 2009), there is a need to develop climate-informed strategies to manage the spruce genetic resource. In order to develop these management strategies, it is important to have a thorough understanding of the hybrid zone complex and the effects of artificial selection on the genomic architecture of this zone.
Our study is the first to assess the evolutionary dynamics responsible for the maintenance of the P. glauca × P. engelmannii hybrid zone by combining genome-wide estimates of admixture, quantitative data from long-term common garden experiments, and environmental data. By doing so, we expect to contribute valuable information for current and future management of this species complex. Our objectives are: to test the bounded hybrid superiority model of hybrid zone maintenance by classifying individuals using a single nucleotide polymorphism (SNP)-based hybrid index, comparing the fitnesses of hybrids and parental species in extensive common garden experiments, and elucidating the effects of climate on the distribution of genetic variation within the hybrid zone; and to assess the effects of ongoing selective breeding and projected climate change on genetic composition within the hybrid zone.
Materials and Methods
Genetic materials
Individuals genotyped and phenotyped in this study were collected and established by the interior spruce breeding programme of the British Columbia Ministry of Forests, Lands and Natural Resources Operations. Seed planning zones (SPZs) are local geographical units for genetic management based on ecological and quantitative trait variation. Trees in four SPZs were sampled, genotyped and phenotyped: West Kootenay, East Kootenay, Quesnel Lakes, and Mount Robson (Table1; Fig.1). Seed parents of these progeny originated from known locations within natural stands, and had a diffuse distribution across SPZs in terms of latitude, longitude, elevation and climate. Within each SPZ, a few hundred open-pollinated families are represented in several progeny tests. We selected a subset of 200 families (50 in each SPZ) to obtain a relatively uniform distribution of seed parent origins along elevational gradients within zones. Newly flushed needles were sampled from up to four randomly selected progeny from each family in a single progeny test for genotyping, with a total of 745 individuals sampled. In addition, 22 putatively pure P. glauca individuals from Fort Nelson (FN) and 40 putatively pure P. engelmannii from southwestern USA (populations E1, E2, and E3) were obtained for genotyping from grafts of mature trees sampled from natural populations and archived in clone banks. Trees from a low-elevation test site (P. glauca habitat) in Fort Nelson were also phenotyped.
Table 1.
Geographical coordinates and climatic variables for Picea glauca × Picea engelmannii common garden experiments studied
| SPZ | Site | Reps | N | Elevation (m) | Latitude (°) | Longitude (°) | MAT (°C) | MAP (mm) | PAS (mm) | DD < 0 | AH:M | Hab. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EK | Bloom Ck | 2 | 374 | 1676 | 49.0 | 115.4 | 2.1 | 1081 | 627 | 1111 | 11.2 | hyb |
| Perry Ck | 3 | 542 | 1463 | 49.5 | 116.0 | 2.1 | 703 | 372 | 1138 | 17.2 | hyb | |
| Red Rock | 9 | 979 | 762 | 53.7 | 122.7 | 3.5 | 599 | 217 | 1008 | 22.5 | hyb | |
| WK | Hall Ck | 10 | 1050 | 1200 | 49.2 | 116.3 | 3.8 | 820 | 393 | 895 | 16.8 | hyb |
| Duhamel | 10 | 1099 | 1475 | 49.6 | 117.2 | 2.1 | 1103 | 608 | 1082 | 11 | hyb | |
| Cortiana | 10 | 1207 | 1830 | 49.9 | 118.3 | 1.3 | 1100 | 562 | 1171 | 10.3 | P. eng | |
| QL | Little Benson | 8 | 1744 | 960 | 52.5 | 122.3 | 3 | 590 | 212 | 996 | 22 | hyb |
| Camp Ck | 8 | 1727 | 1080 | 51.4 | 120.3 | 3.3 | 638 | 260 | 936 | 20.8 | hyb | |
| Ketcham Ck | 8 | 1760 | 1380 | 53.1 | 121.4 | 1.4 | 864 | 382 | 1141 | 13.2 | hyb | |
| MR | Red Rock | 8 | 1600 | 762 | 53.7 | 122.7 | 3.5 | 599 | 217 | 1008 | 22.5 | hyb |
| FN | Fort Nelson | 8 | 3416 | 600 | 58.7 | 123.7 | −0.7 | 571 | 158 | 2113 | 16.3 | P. glau |
The number of individuals (N) represents a subset of the total number of individuals planted at each site.
EK, East Kootenay; WK, West Kootenay; QL, Quesnel Lakes; FN, Fort Nelson; MR, Mount Robson (not included in fitness analysis); SPZ, seed planning zone; Reps, number of replicates; N, number of individuals; MAT, mean annual temperature; MAP, mean annual precipitation; PAS, precipitation as snow; DD < 0, degree-days below 0°C; AH: M, annual heat: moisture index (MAT + 10)/(MAP/1000); Hab, habitat; hyb, hybrid; P. eng, P. engelmannii; P. glau, P. glauca.
Figure 1.
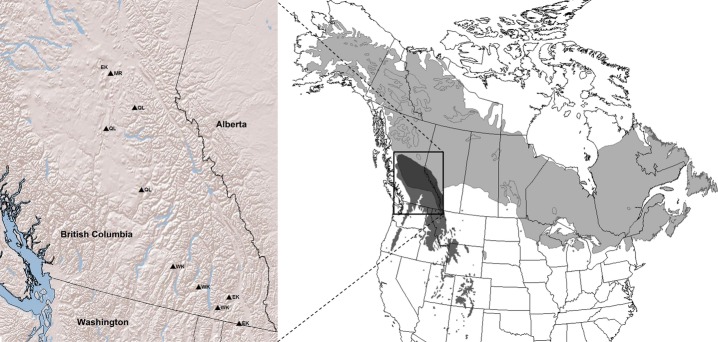
Geographical location of Picea glauca × Picea engelmannii common garden experiments in each of the four geographical regions (seed planning zones (SPZ)) studied in British Columbia, Canada. EK, East Kootenay; WK, West Kootenay; MR, Mouth Robson; QL, Quesnel Lakes.
DNA extraction and genotyping
Needles were stored at −80°C before DNA isolation. Each sample was isolated using a modified cetyltrimethyl ammonium bromide (CTAB) protocol (Doyle & Doyle, 1987). The DNA quality and concentration of each sample was assessed qualitatively using 0.8% agarose gels, and quantitatively using Nanodrop 2000C Spectrophotometer readings (Thermo Fisher Scientific Inc., Waltham, MA, USA). All DNA samples were SNP genotyped at the Genome Quebec/McGill Innovation Centre (Quebec, Canada) using an Illumina bead array chip (Illumina Inc., San Diego, CA, USA) in conjunction with the GoldenGate allele-specific assay in a 96-well, 768-SNP format (Fan et al., 2003; Shen et al., 2005). Samples from allopatric pure species populations and from hybrid populations were assayed in two different SNP arrays. In the first SNP array, allopatric P. glauca and P. engelmannii samples were tested using 1536 SNPs from a large panel of genes putatively involved in cold hardiness (Holliday et al., 2008, 2010), insect herbivory resistance (Porth et al., 2011) and growth and bud set timing (Namroud et al., 2008; Pavy et al., 2008; Pelgas et al., 2011). SNP selection and quality criteria are described in detail in A. R. De La Torre et al., submitted. In the second SNP array (96-well, 386-SNP format), SNPs selected from the first array (384) were used to genotype 745 samples from the hybrid zone. Of 384 SNPs selected, 86 were successfully genotyped in both pure species and hybrid samples and met both genotyping quality and data normalization criteria (De La Torre et al., 2013).
Hybrid index and hybrid classes
The hybrid index was calculated using 86 SNP loci with the introgress 1.1 (Gompert & Buerkle, 2010) package in R 2.13.1 (R Development Core Team, 2011). To estimate the hybrid index, the Fort Nelson (FN) population was used as a parental P. glauca population and E1, E2 and E3 were used as parental P. engelmannii populations. Individuals were divided into four genotypic categories according to their hybrid index values as follows: 0–0.199, P. glauca; 0.2–0.499, P. glauca like-hybrids; 0.5–0.799, P. engelmannii like-hybrids; 0.8–1, P. engelmannii.
Genotypic classes were also estimated using the program NewHybrids 1.1 (Anderson & Thompson, 2002). NewHybrids uses the Markov chain Monte Carlo (MCMC) simulation for computing the posterior distribution of hybrid categories into which individual samples fall. Runs were tested using 20 000 sweeps. Individuals representing pure species were identified a priori and originated from populations outside of the hybrid zone. The default genotype frequency classes were used for the calculations. Following the NewHybrids assignment, individuals were divided into four groups: P. glauca, P. engelmannii, F1 hybrids, and Fn hybrids (advanced generation hybrids, including backcrosses and F2).
The phenotyped progeny tests described above (Genetic materials section) lacked test sites in P. glauca habitats. To address this shortcoming for our phenotypic analysis, we included data from the low-elevation test site at Fort Nelson. As we lacked genotypic data for these individuals, we assigned individuals to genotypic classes based on our earlier analysis of the hybrid index along elevational and latitudinal clines (A. R. De La Torre et al., unpublished data) as follows: individuals from below 600 m or originating from northern British Columbia, Alberta or Eastern Canada were classified as P. glauca (213 individuals); individuals from above 1800 m (mainly southwestern USA) were classified as P. engelmannii (224 individuals); all other individuals were classified as hybrids (2680 individuals).
Phenotypic data
Phenotypic data were available for three progeny test sites in each of the West Kootenay, East Kootenay and Quesnel SPZs, and for one test site for Mount Robson (Table1). Each site was planted as a randomized complete block experiment with two to ten blocks. Tree height was measured and survival recorded at each site in most or all SPZs at ages 3, 6, 10 and 20 yr. Phenotypic data were also available for the Fort Nelson test site for growth traits (height and survival at age 6 yr). A total of 15 498 individuals were phenotyped and analysed in the five SPZs. The sites between 762 and 1676 m elevation were classified as hybrid sites, the site above 1800 m in the West Kootenay SPZ was classified as a P. engelmannii site, and the Fort Nelson site at 600 m elevation was classified as a P. glauca site (Table1).
Timing of bud burst was assessed on a subset of trees in the common garden experiments in spring of 2009 at the time of sampling foliage for DNA extraction and genotyping. Bud break was recorded for the terminal bud of lateral branches using a system that classifies bud burst stages from one to eight according to the amount of bud swelling, colour and elongation (Alfaro et al., 2000). Bud set data were not recorded in these common gardens, but data on the timing of bud set were available for 2-yr-old seedlings from a subset of the same East Kootenay open-pollinated families from an earlier seedling common garden (O′Neill & Aitken, 2004).
Needles from the current year′s growth from the same families in East Kootenay, Mount Robson, Quesnel, and West Kootenay were sampled in late August 2010 to artificially test fall cold hardiness. A total of 931 individuals from 184 families (four to eight progeny per family) were tested. Cold hardiness was measured using electrolytic leakage as a proxy for cell death. Details of the freeze-testing methodology are described by Hannerz et al. (1999). Briefly, freeze-testing temperatures were chosen a priori based on the results of a cold-hardiness pre-test using five temperatures (−10, −20, −30, −40 and −50°C) and 15 interior spruce individuals. Five-millimetre needle segments from each tree sampled were tested at −25, −35 and −45°C in a temperature chamber, with an unfrozen sample for a control. Samples were frozen in 0.2 ml of deionized water with a small trace amount of AgI to nucleate freezing, and an additional 2 ml of deionized water was added following freezing. Electrolytic leakage was measured for each sample after freezing, and again after being heat killed. An index of injury (It) for each of the frozen samples was calculated as the percentage of injury that occurred, ranging from zero (control) to 100 (heat-killed), as described by Flint et al. (1967):
(Rt = Lt/Lk; Ro = Lo/Ld; Lt, the conductance of leachate from the sample frozen at temperature t; Lk, the conductance of leachate from the sample frozen at temperature t and heat-killed; Lo, the control (unfrozen sample); Ld, the conductance of the leachate from the heat-killed control (unfrozen sample; Hannerz et al., 1999).
Fitness analysis
Height, survival, cold hardiness, bud burst, and bud set were used as fitness proxies to compare the performance of pure species and hybrids at different ages and between different environments within SPZs. Differences between pure species and hybrids for height at different ages were assessed by ANOVA using the following linear model:
(yijkl, the phenotype of individual l in hybrid class k in replication j of site i; si, the effect of site I; r(s)ij, the effect of replication j within site I; hk, the effect of hybrid class k; hsik, the interaction between hybrid class k and site I; eijkl, the error.) A simplified regression model was used for bud burst, bud set, and cold hardiness traits.
Least square means with corresponding F tests and P values were estimated for each genotypic class in each SPZ. Variance components were estimated using the restricted maximum likelihood method with type III sum of squares. For bud burst, bud set and cold hardiness traits, population means were used in regression analyses. Bonferroni multiple comparison tests were used to detect differences between means of genotypic classes. Survival data were analysed using binomial tests for contingency tables. All procedures were conducted using either sas enterprise guide 4.2 or sas 9.2 (SAS Institute Inc, 1989) and graphed with R 2.13.1 (R Development Core Team, 2011).
Climate analysis
ClimateWNA (Wang et al., 2012) was used to generate climate data for each sampling site and for a spatial grid (1 × 1 km) of the study areas. ClimateWNA downscales PRISM (parameter-elevation regressions on independent slopes model) (Daly et al., 2002) monthly data (2.5 × 2.5 arcmin) for the reference period (1961–1990), and calculates a large number of additional climate variables for specific locations based on latitude, longitude and elevation for western North America. Twenty annual climate variables were used to test for relationships between hybrid index, geography and climate (Supporting Information Table S7). Two climate data sets were generated for the reference period 1961–1990 (‘1970s’) and the future period 2041–2070 (‘2050s’).
To relate the hybrid index to climate, all climate variables, their combinations and transformations (quadratic and inverse forms) were screened using univariate and stepwise multivariate regression procedures in sas (SAS Institute Inc., Cary, NC, USA). In the multivariate analysis, samples from different SPZs were pooled. Hybrid indices were predicted based on the climate–hybrid index relationships developed in this study. Predicted hybrid indices were then mapped using ArcGIS (v. 10) for the two geographical regions where sampling sites were located (Fig. 5). To demonstrate the impact of climate change on the climatic niche for the hybrids, hybrid index was projected for a middle-of-the-road future climate scenario for the 2050s using the CGCM3 A2 run4.
Figure 5.
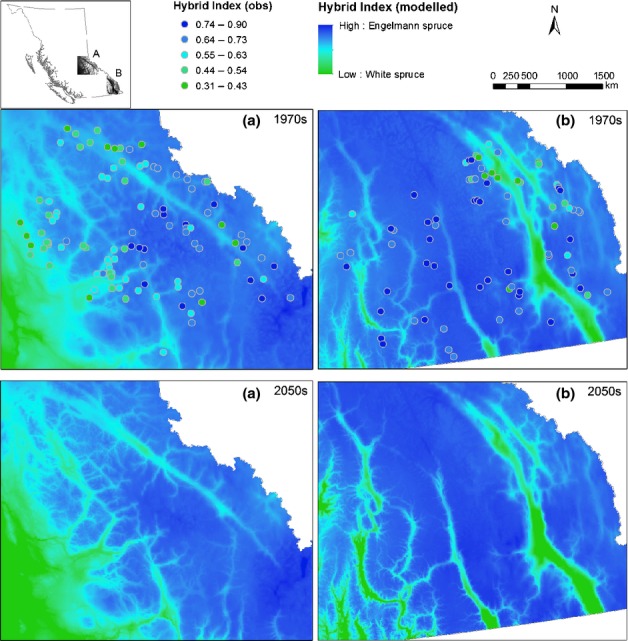
Observed (circles) and predicted (surface) hybrid index in the two sampling areas. The predicted hybrid index for the 2050s (lower two maps) was based on projected climate for this period by the Canadian third-generation coupled global climate model CGCM3 A2 run4. Blue colour indicates Picea engelmannii and green colour, Picea glauca.
Effects of artificial selection on hybrid index
In order to assess the effect of artificial selection on the hybrid index, parental breeding values (Falconer & Mackay, 1996) for height growth were correlated with mean hybrid index estimates based on genotypic data for their open-pollinated progeny for each SPZ. The Best Linear Prediction method (White & Hodge, 1989) was used to calculate the breeding values of each parent in each SPZ, according to:
(C, a vector of genetic covariance between the observed half-sib family means and the breeding value being predicted; V, an s × s matrix of the phenotypic variances and covariances of family means; y, the progeny mean of the parent (family mean); α, the progeny mean of all parents (population mean or overall family mean).
Results
Hybrid index
The distribution of genotypic classes was asymmetrical, weighted towards Engelmann spruce, with the hybrid genotypic class between 0.5 and 0.799 accounting for nearly 60% of all individuals sampled. This asymmetry towards Engelmann spruce was observed in previous studies in the same hybrid complex (Haselhorst & Buerkle, 2013; A. R. De La Torre et al., unpublished data). Individuals with genotypic classes between 0 and 0.199 (putatively pure P. glauca) accounted for just 4% of the individuals.
Fitness analysis
Significant differences in height were found among genotypic classes for all ages measured in the East Kootenay, Quesnel, and Fort Nelson test sites. In the West Kootenay hybrid habitat test sites, height differences were significant at ages 3 and 6 yr, but not at age 10 yr (Table2, Fig.2). Differences among genotypic classes were not significant for height at any ages assessed for the high-elevation Engelmann habitat West Kootenay site. introgress and NewHybrids showed similar results, with the exception of height at age 6 yr in the West Kootenay SPZ hybrid test sites (Table S1).
Table 2.
Differences among genotypic classes for phenotypic traits based on ANOVA results
| Traits | P. engelmannii environment | Hybrid environment | P. glauca environment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| West Kootenay | East Kootenay | West Kootenay | Quesnel | Fort Nelson | ||||||
| F-value (df = 2) | P-value | F-value (df = 3) | P-value | F-value (df = 2) | P-value | F-value (df = 3) | P-value | F-value (df = 2) | P-value | |
| Height age 3 yr | 1.89 | 0.1697 | 22.56 | < 0.0001 | 3.96 | 0.01 | 20.73 | < 0.0001 | – | – |
| Height age 6 yr | 1.34 | 0.2464 | – | – | 3.17 | 0.04 | 23.68 | < 0.0001 | 81.35 | < 0.0001 |
| Height age 10 yr | 1.43 | 0.2326 | 13.71 | < 0.0001 | 1.9 | 0.15 | 46.56 | < 0.0001 | – | – |
| Height age 20 yr | – | – | 20.27 | < 0.0001 | – | – | 32.05 | < 0.0001 | – | – |
| Frost injury −25°C | 0.01 | 0.9332 | 1.23 | 0.2982 | – | – | 0.19 | 0.829 | – | – |
| Bud burst | 0.66 | 0.5182 | 1.63 | 0.1983 | – | – | 3.28 | 0.0223 | – | – |
| Bud set | – | – | 25.09 | < 0.0001 | – | – | – | – | – | – |
Genotypic classes are classified as pure Picea engelmannii, pure Picea glauca, advanced generation hybrids (Fn) and first-generation hybrids (F1), based on the NewHybrids assignment. In the Fort Nelson test site, pure species and hybrids are differentiated by elevation and geographical location of populations of origin. All genotypic classes are present at East Kootenay, Quesnel and Fort Nelson sites; at West Kootenay there is no pure P. glauca. Traits showing significant differences are indicated in bold text. Results of the introgress assignment can be found in Supporting Information Table S1. Number of individuals per genotypic class, means and standard errors for individual traits are provided in Tables S2 and S3.
Figure 2.
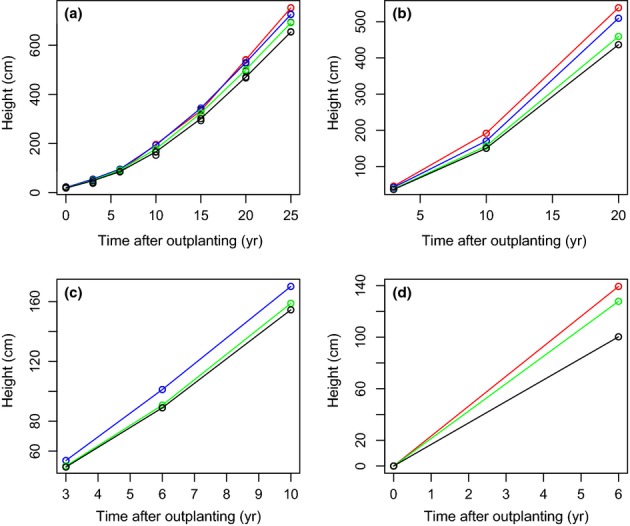
Differences in height among Picea glauca, Picea engelmannii , and their hybrids tested in sites of contrasting elevation. Intermediate-elevation tests sites are represented by (a) Quesnel and (b) East Kootenay; a high-elevation test site by (c)West Kootenay; and a low-elevation test site by (d) Fort Nelson. Red lines and dots indicate height measurements from P. glauca; blue, P. glauca-like hybrids; green, P. engelmannii-like hybrids; and black, P. engelmannii.
Picea glauca and P. glauca-like hybrids grew faster on average than P. engelmannii and P. engelmannii-like hybrids in intermediate- and low-elevation environments (Fig.2, Tables S2 and S3). In some hybrid sites, however, P. glauca and P. glauca-like hybrids did not show greater height growth than P. engelmannii until 10–15 yr of age. Picea engelmannii and P. engelmannii-like hybrids may, in fact, show faster growth at early seedling and sapling stages. In the P. engelmannii habitat of the WK high-elevation test site, there were no significant differences between the heights of P. engelmannii and hybrid individuals. There were no pure P. glauca individuals planted at this site.
Trees classified as first-generation hybrids (F1) by NewHybrids had extremely variable phenotypes (Table S3). For example, F1 hybrids grew taller on average than advanced generation hybrids and P. engelmannii in Quesnel, but showed the opposite trend in West Kootenay. In East Kootenay, F1s grew similarly to P. engelmannii, but then at age 20 yr surpassed P. engelmannii and reached sizes similar to those of the advanced generation hybrids.
Binomial tests indicated there were significant differences in survival at different ages among P. glauca, P. engelmannii and their hybrids (Table S4). On average across all hybrid habitat sites, hybrids had a higher survival rate than pure species in intermediate environments at all ages with the exception of age 20 yr (Fig.3, Table S5). At age 20 yr, the pattern is maintained in all the hybrid sites with the exception of Little Benson and Ketcham Creek (Table S5). Picea engelmannii had a higher survival rate than hybrids in the high-elevation site, and a lower survival rate than P. glauca and hybrids in the low-elevation Fort Nelson test site (Fig.3, Table S5).
Figure 3.
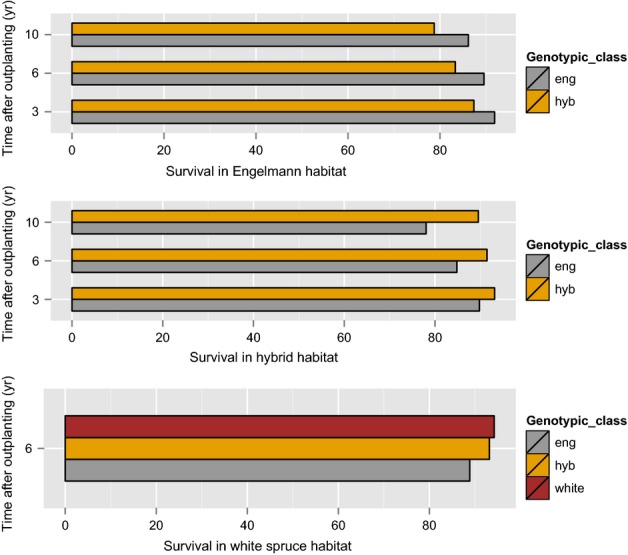
Results of the survival analysis for Picea glauca, Picea engelmannii and hybrids suggest that pure species and hybrids have better survival in their own habitat. Percentage survival was compared between hybrid and pure species in the high-elevation test site in Cortiana, West Kootenay (WK; P. engelmannii habitat); the low-elevation test site in Fort Nelson (P. glauca habitat); and across nine intermediate-elevation sites in East Kootenay (EK), Quesnel Lakes (QL) and WK (P. glauca × P. engelmannii hybrids habitat). Differences between pure species and hybrids are significant at all ages. Survival estimates for all tests sites can be found in Supporting Information Table S5.
Bud burst timing was negatively correlated with hybrid index in Quesnel (R2 = 0.28; P < 0.0001) and East Kootenay (R2 = 0.103; P = 0.021), with P. engelmannii and P. engelmannii-like hybrids having an earlier bud burst than P. glauca and P. glauca-like hybrids in the same environment. Bud burst phenology was also negatively correlated with elevation in Quesnel (R2 = 0.12; P = 0.009) and in East Kootenay (R2 = 0.2557; P = 0.0002). Bud burst timing was significantly different among P. glauca, P. engelmannii and their hybrids in East Kootenay and Quesnel but not in West Kootenay based on the introgress classification, but differed among NewHybrids genotypic classes only in Quesnel (Table2, Table S1).
Bud set timing, which was only recorded in the East Kootenay SPZ, was also negatively correlated with hybrid index (R2 = 0.126; P = 0.0003), elevation (R2 = 0.231; P < 0.0001) and latitude (R2 = 0.025; P = 0.035) in East Kootenay. Picea engelmannii and P. engelmannii-like hybrids had earlier bud set timing than P. glauca and P. glauca-like hybrids. Although hybrids were generally more cold-hardy, on average, than pure species in intermediate environments, differences between hybrids and pure species were significant only in Quesnel (F = 3.58; P = 0.0297). Injury at −25°C was significantly but weakly correlated with elevation (R2 = 0.128; P = 0.0072) in Quesnel, and with longitude (R2 = 0.108; P = 0.019) in East Kootenay.
Relationships between climate and hybrid index
The hybrid index showed strong clines corresponding to geographical and climatic gradients in temperature and precipitation (Table S7). The main climatic variables associated with variation in the hybrid index across all SPZs were precipitation as snow (PAS), mean annual precipitation (MAP) and the summer heat-moisture index (SHM). SHM is a measure of aridity calculated using mean warmest month temperature (MWMT) divided by adjusted mean summer precipitation (MSP/1000).
A stepwise multivariate regression of the hybrid index on climate variables found the best fit for a model that included 1/PAS and SHM. This model explained 54% of the total variation in the hybrid index (Table S6). The hybrid index was positively correlated with PAS (R2 = 0.445; P < 0.0001; Fig.4) and SHM added modestly to model fit (partial R2 = 0.077; P < 0.0001). Univariate regressions for SHM accounted for relatively little of the total variation (R2 = 0.108; P < 0.0001). SHM is negatively correlated with the hybrid index in the univariate model, but positively when combined with PAS.
Figure 4.
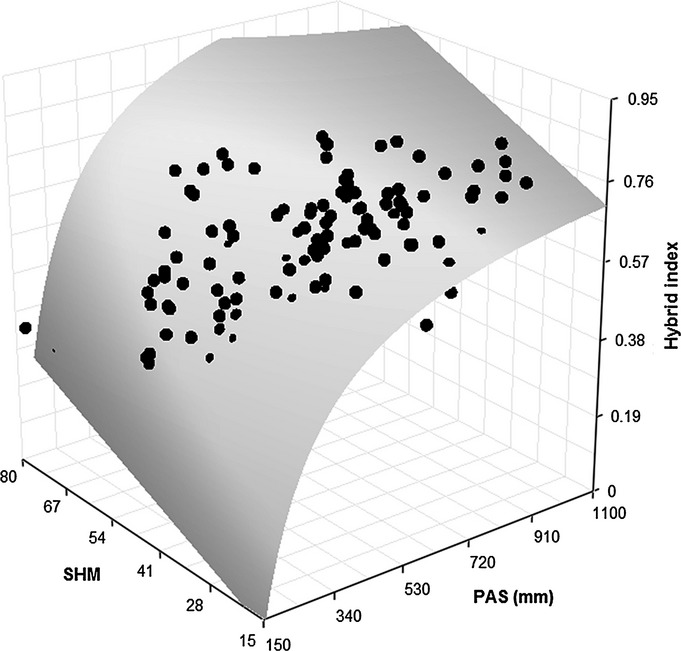
Observed (circles) and predicted (surface) spruce hybrid index against precipitation as snow (PAS) and summer heat-moisture index (SHM). Picea glauca × Picea engelmannii predicted hybrid index was generated based on the multiple linear relationships between hybrid index and climate variables shown in Table S7.
The relatively strong relationship between the hybrid index and the two most explanatory climate variables, PAS and SHM, enabled the prediction of hybrid index values over all SPZs combined for the reference period 1961–1990. Maps of predicted current distribution of the hybrid index showed good matches with observed ones (Fig.5). However, the matches were better in Mount Robson and Quesnel SPZ than in the East Kootenay and West Kootenay SPZs. Optimal hybrid index values for projected climates in 2050s were expected to decline to favour P. glauca as a result of the forecasted decrease in PAS. However, the decline in optimal hybrid index was predicted to be more rapid in areas with low PAS, while the change was slight at high PAS because of the nonlinear relationship between the two climate variables and the hybrid index (Fig.4).
Effects of artificial selection on hybrid index
Breeding values were negatively correlated with the hybrid index in three of the four SPZs studied. Pearson correlation coefficients (r) were −0.64 for East Kootenay, −0.65 for Mount Robson, and −0.53 for Quesnel (Fig.6). In these three SPZs, families with higher breeding potential were mainly composed of P. glauca-like hybrids because pure P. glauca tends to grow faster than P. engelmannii. It is important to note, however, that there is a fairly wide range of BVs for any given hybrid index value, and that these estimates were based on different progeny tests in each zone. In the West Kootenay SPZ, correlations between hybrid index and breeding values were not significant, probably as a consequence of the small range in hybrid index (between 0.6 to 1), where all individuals were either pure P. engelmannii or advanced generation P. engelmannii-like hybrids, with a correspondingly small range in breeding values (−2 to 8).
Figure 6.
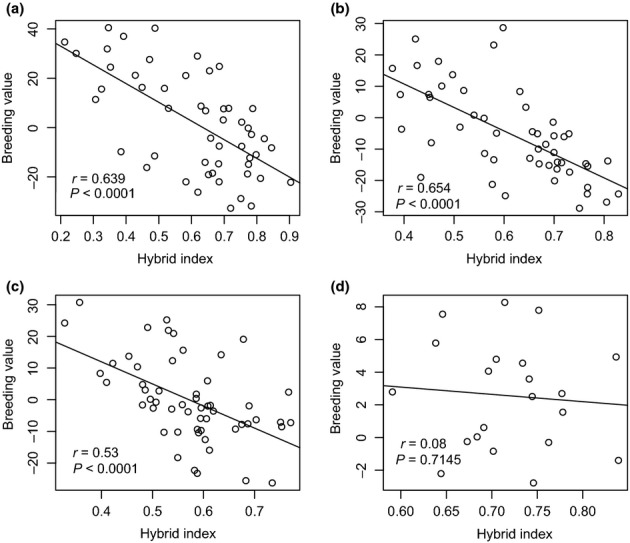
Breeding values versus family mean hybrid index for spruce in four seed planning zones (SPZs) represented in this study: (a) East Kootenay (EK), (b) Mount Robson (MR), (c) Quesnel (QL) and (d) West Kootenay (WK). Circles represent families with the potential for breeding based on stem volume (positive and high breeding values) or with no potential for breeding (negative or low breeding values). Individuals with a lower hybrid index are more Picea glauca-like, while those with higher hybrid indices are more Picea engelmannii-like.
Discussion
Fitness analysis
Boreal woody species like P. glauca have an outstanding capacity to survive deep winter freezing temperatures. Their relative fitness depends on the ability of individuals to maximize their growth to be able to compete for light and yet avoid frost and drought injury by synchronizing growth phenology with the local climate (Howe et al., 2003). Individuals with a higher fitness will be those that grow taller and yet stop growing (i.e., set a terminal bud, enter dormancy, and initiate cold acclimation) before the first frost in that environment. In subalpine environments, species like P. engelmannii need the ability to survive a long winter beneath a deep and persistent snowpack, and accomplish growth in a short available window in cool temperatures and cold soil. Thus, while both species inhabit cold environments, the climatic selection pressures in these environments differ substantially.
Hybrids have a combination of P. engelmannii and P. glauca genomes; therefore, they have inherited a combination of genes that may provide them with an adaptive advantage in intermediate environments. Hybrids will generally grow taller than pure P. engelmannii, but will set bud earlier than P. glauca. This combination of faster growth and earlier bud set gives the hybrids an environment-specific survival advantage during seedling stages. This probably explains the higher hybrid survival rates at early ages found in this study. These conditions hold in intermediate environments (hybrid habitats); however, the story is different in pure species habitats.
In low-elevation environments (P. glauca habitats), hybrids cannot compete with faster growing P. glauca (Table2). In high-elevation environments (P. engelmannii habitats), hybrids probably cannot withstand the heavy snow loads or long, cold winters to which pure P. engelmannii is adapted. The minimum winter temperatures experienced are moderated by the snowpack, but persistence under the snow presents other challenges by shortening growing season length and exposing trees to pathogens. This could explain why hybrids showed lower survival rates than P. engelmannii in the high-elevation test site, and lower survival rates than P. glauca in the low-elevation test site.
Although it was already well known that P. glauca had faster growth than P. engelmannii, what was not previously known is that this trend in growth rate is not evident at all life history stages. This study indicates that P. glauca does not attain a size advantage until later in life, around age 10 yr. During the first years as seedlings and saplings, P. glauca sometimes grows less than hybrids or P. engelmannii. This growth lag at early ages may be related to a lower capacity to withstand heavy snow loads and shorter growing seasons than P. engelmannii and hybrids. Once a tree is well established, it will be less susceptible to frost injury and therefore more energy resources can be allocated to greater growth (Sakai & Larcher, 1987). Drought hardiness also plays a role in the relative performance and growth trajectories of these species and their hybrids. Picea engelmannii grew more slowly and suffered higher mortality at drier sites in provenance trials in East Kootenays (Xie et al., 1998).
Bud set timing is generally under strong divergent selection, exhibits strong local adaptation, and occurs as a genetically determined response to photoperiod, whereas bud burst is triggered by the accumulation of a genetically determined heat sum after the exposure to chilling temperatures and shows high phenotypic plasticity and weaker local adaptation (Howe et al., 2003). While these traits are determined by different environmental cues, the timing of bud burst followed the same clinal pattern as bud set in this study, as P. engelmannii and P. engelmannii-like hybrids both burst and set buds earlier than P. glauca and P. glauca-like hybrids.
Climate analysis
Our climate analyses suggest that the hybrid zone is primarily maintained by local adaptation to winter temperatures, winter precipitation, and summer aridity, with the most important variables being PAS and SHM. PAS is based on monthly precipitation and monthly mean temperature (Wang et al., 2006). A larger value of PAS, or a deeper snowpack, can reflect a larger amount of winter precipitation, cooler temperatures, or longer winters. PAS explained 46% of the total variation in hybrid index, and it is likely to be the main factor limiting P. glauca survival in P. engelmannii habitats (high-elevation environments). The complex interaction between summer moisture (SHM) and early cold events has a strong effect on fitness and certainly on species distribution.
Exogenous selection as mechanism of maintenance of the hybrid zone
Our results indicate that the P. glauca × P. engelmannii hybrid zone is maintained by elevational climatic selection gradients resulting from environmental heterogeneity (exogenous selection), where hybrids are fitter than parental species in intermediate habitats. Adaptive introgression from P. engelmannii to P. glauca has produced hybrids with increased fall cold tolerance as a result of an earlier bud set. At the same time, adaptive introgression from P. glauca to P. engelmannii has resulted in hybrids with faster growth rates. This combination of faster growth and increased cold tolerance has given the hybrids an adaptive advantage over parental species (pure P. glauca and pure P. engelmannii) in intermediate environments. This type of adaptive introgression has been reported in other plant species. Introgression from the flood-tolerant Iris fulva into the dry-adapted Iris brevicaulis has increase flooding tolerance in I. fulva backcrosses (Martin et al., 2005, 2006). Also, adaptive introgression from Helianthus debilis into Helianthus annuus has increased hervibory resistance (Whitney et al., 2006) and abiotic tolerance in several traits (Whitney et al., 2010).
Hybrids usually appear fitter than parentals in intermediate environments, and less fit than both parentals in their respective native environments. This suggests that the P. glauca × P. engelmannii zone is maintained according to the bounded hybrid superiority model (Moore, 1977). The most well-characterized example of bounded hybrid superiority is the hybrid zone formed between subspecies of the long-lived, wind-dispersed big sagebrush, Artemisia tridentata (Wang et al., 1997; Miglia et al., 2005). The Artemisia hybrid zone occurs along a much narrower elevational gradient than that in this study. Artemisia reciprocal transplant experiments have shown that hybrids are fitter than parentals in intermediate environments. Strong elevational and habitat differentiation among pure species and hybrids habitats have also been found in Aquilegia (Hodges & Arnold, 1994), Ipomopsis (Campbell & Waser, 2007) and Carex (Choler et al., 2004).
The most likely explanation for the low numbers of P. glauca backcrosses is the higher local and regional population density of P. engelmannii than P. glauca. Our previous molecular marker studies have found that pure P. engelmannii appears to be present at a higher frequency than P. glauca within the contact zone, assuming that the samples genotyped provide a proportional representation of the parental species and their hybrids (A. R. De La Torre & S. Aitken, unpublished data). If P. glauca individuals were more common in this area, then there would be more opportunities for the hybrids to backcross with them. The low numbers of P. glauca backcrosses may also be explained by endogenous selection, for example, as a result of Dobzhansky–Muller genetic incompatibilities, which we cannot test using our data but cannot be completely ruled out. It may be that introgression from P. glauca into P. engelmannii, besides generating fit hybrids (hybrids with higher growth potential), also generates some unfavourable epistatic combinations that lead to unfit hybrids. Artificial crosses for at least two generations would be required to draw definitive conclusions about this.
Given the complexity of this hybrid zone, it is difficult to differentiate between F2 hybrids and backcrosses. Therefore, they were included in the same hybrid category in the NewHybrids analysis. This lack of differentiation between hybrid classes, however, did not have a big impact, as only a small number of F1 hybrids were identified. This is not surprising, considering that the physical distance between parental habitats is not that great relative to pollen dispersal capabilities (Kremer et al., 2012), and that the hybrid zone is not of recent origin (Haselhorst & Buerkle, 2013). Even if F1s have low fitness, on average, they may mate with nonhybrid individuals to form early backcrosses that will then mate again with pure species to form advanced generation hybrids. This process would allow the transfer of adaptations from one species to the other (Arnold et al., 2012). In our study, apparent F1 hybrids showed high variability in the traits studied and did not show, on average, increased hybrid fitness. Advanced generation hybrids, however, were the most abundant genotypic class in the zone, showing, on average, higher hybrid fitness than F1s. These differences in fitness between hybrid classes are consistent with previous reports in natural hybrid zones (Arnold et al., 2012, but see Milne et al., 2003).
In contrast to artificial hybrid zones, in which only one or two generations are tested, individuals in this study comprised a vast array of recombinant hybrid genotypes, formed as a result of several to many generations of intercrossing (Rieseberg et al., 2000). Long-term phenotypic data (collected over a 20-yr period) also provided a rare opportunity to study the adaptation of these long-lived woody species. However, because populations were highly admixed, this study failed to elucidate the processes that occurred early in hybrid zone formation, as is expected with studies using ancient natural hybrid zones, and some remaining questions about fitness of early-generation hybrids will require multiple generations of artificial crosses to answer (Carney et al., 2000; Kirk et al., 2005).
Effects of tree breeding and climate change on the hybrid zone
Picea glauca and P. engelmannii are economically important species in Canada, with tens of millions of trees planted annually for reforestation. In British Columbia, the complex of P. glauca, P. engelmannii and their hybrids is managed as a single ‘interior spruce’ species group within SPZs, based on geography and similarities in adaptive traits from provenance trials, and within seed planning units (SPUs), based on elevational bands within SPZs (Xie et al., 1998). SPZ classification is used for managing reforestation using seed collected from natural stands, with latitudinal, longitudinal and elevational limits on transfer distances from the collection site to the reforestation site. SPUs are used for managing reforestation with seed orchard lots from selective breeding programmes. Although this classification captures and manages most of the genetic variation at the macro-scale, it does not reflect the finer scale picture. Detailed knowledge of population structure and differences in adaptive traits among P. glauca, P. engelmannii and their hybrids fills this gap, and also provides key information for the basis of genetic gains from artificial selection for volume growth within this hybrid zone.
By 2050, the interior of British Columbia is expected to get warmer and drier, with a projected increase in mean annual temperature of 2–3°C according to projections of multiple General Circulation Models and glasshouse gas emissions scenarios (Mbogga et al., 2009). As a consequence of a predicted decrease in PAS, new climates should favour hybrids with higher P. glauca ancestry (hybrid index closer to 0) over those with more P. engelmannii ancestry (hybrid index closer to 1). However, as a consequence of the nonlinear relationship between hybrid index and PAS (Fig.4), and the moderate correlation between PAS and elevation (R2 = 0.416; P < 0.0001), this trend favouring P. glauca will be most evident at low and intermediate elevations. At high elevations (elevation > 1700 m and PAS > 700), the hybrid index is not expected to change much. However, because of the uncertainty of future climates, accurate projections for these changes will, at least, require the use of multiple climate change scenarios and even a more sophisticated model to better capture this relationship in future studies.
Future climatic conditions will affect both natural and managed populations, but there is more opportunity with managed forests to shift genotypes to those better adapted to new conditions through practices such as climate-based seed transfer and assisted gene flow (Aitken et al., 2008; Aitken & Whitlock, 2013). However, our data suggest that managed populations may in fact be somewhat pre-adapted to climate change compared with natural populations. Our results indicate that artificial selection for breeding is currently selecting individuals that have more P. glauca ancestry because of their faster growth compared with P. engelmannii-like hybrids and pure P. engelmannii. This asymmetry towards P. glauca in managed populations may confer an adaptive advantage over natural populations in future warmer and drier climates, especially at low and intermediate elevations. This pattern of artificial selection in breeding programmes may counter the historical effects of natural selection on populations in the hybrid zone. Other studies using neutral and adaptive molecular markers in this hybrid zone have identified a strong asymmetry in introgression, with P. engelmannii-like hybrids much more abundant than the P. glauca-like hybrids (Haselhorst & Buerkle, 2013; A. R. De La Torre & S. Aitken, unpublished data). Picea engelmannii-like hybrids are adapted to shorter, cooler growing seasons and are slower growing than P. glauca-like hybrids; therefore they may be poorly adapted to new, warmer climates in British Columbia. However, the high levels of genetic variation found at the contact zone (intermediate elevations and intermediate latitudes) may increase the ability of hybrid populations to adapt to new climates in comparison with single species populations (Aitken et al., 2008).
This study represents a significant step forward in our understanding of the evolutionary dynamics that maintain the P. glauca × P. engelmannii hybrid zone and contributes valuable information for breeding and management of the species under climate change. Our ongoing research seeks to identify the genes responsible for adaptation to climate investigation of allele–environment associations and association mapping of genes underlying climate-related phenotypic traits.
Acknowledgments
We thank G. Kiss, D. Wallden, G. Phillips and B. Hooge from the BC Ministry of Forests for the establishment and management of the common garden experiments; M. Whitlock and L. Rieseberg for valuable comments on the manuscript; and P. Smets, L. Erdle and J. Bemmels for help with sample collection. This work was supported by Genome British Columbia, Genome Canada, Natural Science and Engineering Research Council of Canada (NSERC), and BC Forest Genetics Council (grants to S.N.A.); and by UBC University Graduate Fellowships (UGF), UBC Four Year Fellowship, and B.C. Government Pacific Century Graduate Scholarship to A. R. De La Torre.
Supporting Information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Differences among genotypic classes for phenotypic traits based on introgress hybrid index estimates
Table S2 Least square means (lsmeans) and standard errors (stderr) for height measurements (cm) at different ages among genotypic classes tested in seed planning zones based on introgress hybrid index estimates
Table S3 Least square means and standard errors for height measurements (cm) at different ages among hybrid classes tested in seed planning zones based on NewHybrids assignment
Table S4 Binomial tests for differences in survival at different ages among Picea glauca, P. engelmannii and their hybrids
Table S5 Differences in survival between Picea engelmannii, P. glauca and hybrids at different ages for all sites and regions studied in field common garden experiments
Table S6 Results of stepwise regression of hybrid index on precipitation as snow (PAS) and summer heat-moisture index (SHM)
Table S7 Results of the univariate regressions of hybrid index on geographical and climate variables in each seed planning zone
References
- Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJE, Bierne N, Boughman J, Brelsford A, Buerkle CA, Buggs R, et al. Hybridization and speciation. Journal of Evolutionary Biology. 2013;26:229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- Aitken SN, Whitlock MC. Assisted gene flow to facilitate local adaptation to climate change. Annual review of Ecology, Evolution and Systematics. 2013;44 : in press. [Google Scholar]
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RR, Shepperd WD. Silvics of North America vol. I: conifers. Agricultural handbook 654. In: Burns RM, Honkala BH, editors. Picea engelmannii. Washington DC, USA: USDA Forest Service; 1990. pp. 187–203. Parry ex. Engelm. Engelmann spruce. In: , eds. [Google Scholar]
- Alfaro RI, Lewis KG, King JN. Budburst phenology of Sitka spruce and its relationship to white pine weevil attack. Forest Ecology and Management. 2000;127:19–29. [Google Scholar]
- Anderson E. Introgressive hybridization. New York, NY, USA: Wiley; 1949. [Google Scholar]
- Anderson EC, Thompson EA. A model-based method for identifying species hybrids using multilocus genetic data. Genetics. 2002;160:1217–1229. doi: 10.1093/genetics/160.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold ML. Natural hybridization and evolution. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- Arnold ML, Ballerini ES, Brothers AN. Hybrid fitness, adaptation and evolutionary diversification: lessons learned from Louisiana irises. Heredity. 2012;108:159–166. doi: 10.1038/hdy.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH. The dynamics of hybrid zones. Heredity. 1979;43:341–359. [Google Scholar]
- Barton NH. The role of hybridization in evolution. Molecular Ecology. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- Barton NH, Hewitt GM. Analysis of hybrid zones. Annual Review of Ecology and Systematics. 1985;16:113–148. [Google Scholar]
- Campbell DR, Waser NM. Evolutionary dynamics of an Ipomopsis hybrid zone: confronting models with lifetime fitness data. The American Naturalist. 2007;169:298–310. doi: 10.1086/510758. [DOI] [PubMed] [Google Scholar]
- Carling MD, Zuckerberg B. Spatio-temporal changes in the genetic structure of the Passerina bunting hybrid zone. Molecular Ecology. 2011;20:1166–1175. doi: 10.1111/j.1365-294X.2010.04987.x. [DOI] [PubMed] [Google Scholar]
- Carney SE, Gardner KA, Rieseberg LH. Evolutionary changes over the 50-year history of a hybrid population of sunflowers (Helianthus. Evolution. 2000;54:462–474. doi: 10.1111/j.0014-3820.2000.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Choler P, Erschbamer B, Tribsch A, Gielly L, Taberlet P. Genetic introgression as a potential to widen a species′ niche: Insights from alpine Carex curvula. Proceedings of the National Academy of Sciences, USA. 2004;101:171–176. doi: 10.1073/pnas.2237235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Gibson WP, Taylor GH, Johnson GL, Pasteris P. A knowledge-based approach to the statistical mapping of climate. Climate Research. 2002;22:99–113. [Google Scholar]
- Daubenmire R. Taxonomic and ecologic relationships between Picea glauca and Picea engelmannii. Canadian Journal of Botany. 1974;52:1545–1560. [Google Scholar]
- De Carvalho D, Ingvarsson P, Joseph J, Suter L, Sedivy C, Macaya-Sanz D, Cottrell J, Heinze B, Schanzer I, Lexer C. Admixture facilitates adaptation from standing variation in the European aspen (Populus tremula L.), a widespread forest tree. Molecular Ecology. 2010;19:1638–1650. doi: 10.1111/j.1365-294X.2010.04595.x. [DOI] [PubMed] [Google Scholar]
- De La Torre A, Roberts D, Aitken SN. Data from: genome-wide admixture and ecological niche modeling reveal the maintenance of species boundaries despite long history of interspecific gene flow. Dryad Digital Repository. 2013 doi: 10.1111/mec.12710. doi: 10.5061/dryad.7h65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1987;12:13–15. [Google Scholar]
- Endler JA. Gene flow and population differentiation. Science. 1973;179:243–250. doi: 10.1126/science.179.4070.243. [DOI] [PubMed] [Google Scholar]
- Endler JA. Geographic variation, speciation and clines. Princeton, NJ, USA: Princeton University Press; 1977. [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. Harlow, UK: Pearson, Prentice Hall editions; 1996. [Google Scholar]
- Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Hansen M, Steemers F, Butler SL, Deloukas P, et al. Highly parallel SNP genotyping. Cold Spring Harbor Symposia on Quantitative Biology. 2003;68:69–78. doi: 10.1101/sqb.2003.68.69. [DOI] [PubMed] [Google Scholar]
- Flint HL, Boyce BR, Beattie DJ. Index of injury, a useful expression of freezing injury to plant tissues as determined by the electrolytic method. Canadian Journal of Plant Science. 1967;47:229–230. [Google Scholar]
- Gay L, Crochet PA, Bell DA, Lenormand T. Comparing clines on molecular and phenotypic traits in hybrid zones: a window on tension zone models. Evolution. 2008;62:2789–2806. doi: 10.1111/j.1558-5646.2008.00491.x. [DOI] [PubMed] [Google Scholar]
- Gompert Z, Buerkle CA. Introgress: a software package for mapping components of isolation in hybrids. Molecular Ecology Resources. 2010;10:378–384. doi: 10.1111/j.1755-0998.2009.02733.x. [DOI] [PubMed] [Google Scholar]
- Goulson D. Evaluating the role of ecological isolation in maintaining the species boundary between Silene dioica and S. latifolia. Plant Ecology. 2009;205:201–211. [Google Scholar]
- Hannerz M, Aitken SN, King JN, Budge S. Effects of genetic selection for growth on frost hardiness in western hemlock. Canadian Journal of Forest Research. 1999;29:509–516. [Google Scholar]
- Harrison RG. Pattern and process in a narrow hybrid zone. Heredity. 1986;56:337–349. [Google Scholar]
- Harrison RG. Hybrids and hybrid zones: historical perspective. In: Harrison RG, editor. Hybrid zones and the evolutionary process. Oxford, UK: Oxford University Press; 1993. pp. 3–12. [Google Scholar]
- Haselhorst M, Buerkle CA. Population genetic structure of Picea engelmannii P. glauca and their previously unrecognized hybrids in the central Rocky Monutains. Tree Genetics & Genomes. 2013;9:669–681. [Google Scholar]
- Hewitt GM. Hybrid zones-natural laboratories for evolutionary studies. Trends in Ecology and Evolution. 1988;3:158–167. doi: 10.1016/0169-5347(88)90033-X. [DOI] [PubMed] [Google Scholar]
- Hodges SA, Arnold ML. Floral and ecological isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the National Academy of Sciences, USA. 1994;91:2493–2496. doi: 10.1073/pnas.91.7.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday JA, Ralph SG, White R, Bohlmann J, Aitken SN. Global monitoring of autumn gene expression within and among phenotypically divergent populations of Sitka spruce (Picea sitchensis. New Phytologist. 2008;178:103–122. doi: 10.1111/j.1469-8137.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- Holliday JA, Ritland K, Aitken SN. Widespread, ecologically relevant genetic markers developed from association mapping of climate-related traits in Sitka spruce (Picea sitchensis. New Phytologist. 2010;188:501–514. doi: 10.1111/j.1469-8137.2010.03380.x. [DOI] [PubMed] [Google Scholar]
- Howe GT, Aitken SN, Neale DB, Jermstad KD, Wheeler NC, Chen THH. From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Canadian Journal of Botany. 2003;81:1247–1266. [Google Scholar]
- Kirk H, Vrieling K, Klinhamer PGL. Maternal effects and heterosis influence the fitness of plant hybrids. New Phytologist. 2005;166:685–694. doi: 10.1111/j.1469-8137.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, Guillaume F, Bohrer G, Nathan R, Bridle JR, Gomulkiewicz R, Klein EK, Ritland K, et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters. 2012;15:378–392. doi: 10.1111/j.1461-0248.2012.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig FT, Hodgkiss PD, Johnson DR. The structure of genetic diversity in Engelmann spruce and a comparison with blue spruce. Canadian Journal of Botany. 2006;84:1806–1828. [Google Scholar]
- Ma F, Zhao C, Milne R, Ji M, Chen L, Liu J. Enhanced drought-tolerance in the homoploid hybrid species Pinus densata: implications for its habitat divergence from two progenitors. New Phytologist. 2010;185:204–216. doi: 10.1111/j.1469-8137.2009.03037.x. [DOI] [PubMed] [Google Scholar]
- Martin NH, Bouck AC, Arnold ML. Loci affecting long-term hybrid survivorship in Louisiana irises: implications for reproductive isolation and introgression. Evolution. 2005;59:2116–2124. [PubMed] [Google Scholar]
- Martin NH, Bouck AC, Arnold ML. Detecting adaptive trait introgression between Iris fulva and I. brevicaulis in highly selective field conditions. Genetics. 2006;172:2481–2489. doi: 10.1534/genetics.105.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E. Systematics and the origin of species. New York, NY, USA: Columbia University Press; 1942. [Google Scholar]
- Mbogga MS, Hamann A, Wang T. Historical and projected climate data for natural resource management in western Canada. Agricultural and Forest Meteorology. 2009;149:881–890. [Google Scholar]
- Miglia KJ, McArthur ED, Moore WS, Wang H, Graham JH, Freeman DC. Nine-year reciprocal transplant experiment in the gardens of the basin and mountain big sagebrush (Artemisia tridentata: Asteraceae) hybrid zone of Salt Creek Canyon: the importance of multiple-year tracking of fitness. Biological Journal of the Linnean Society. 2005;86:213–225. [Google Scholar]
- Milne RI, Terzioglu S, Abbott RJ. A hybrid zone dominated by fertile F1s: maintenance of species barriers in Rhododendron. Molecular Ecology. 2003;12:2719–2729. doi: 10.1046/j.1365-294x.2003.01942.x. [DOI] [PubMed] [Google Scholar]
- Moore WS. An evaluation of narrow hybrid zones in vertebrates. Quarterly Review of Biology. 1977;52:263–277. [Google Scholar]
- Namroud MC, Beaulieu J, Juge N, Laroche J, Bousquet J. Scanning the genome for gene single nucleotide polymorphisms involved in adaptive population differentiation in white spruce. Molecular Ecology. 2008;17:3599–3613. doi: 10.1111/j.1365-294X.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O′Neill GA, Aitken SN. Area-based breeding zones to minimize maladaptation. Canadian Journal of Forest Research. 2004;34:695–704. [Google Scholar]
- Pavy N, Pelgas B, Beauseigle S, Blais S, Gagnon F, Gosselin I, Lamothe M, Isabel N, Bousquet J. Enhancing genetic mapping of complex genomes through the design of highly-multiplexed SNP arrays: application to the large and unsequenced genomes of white spruce and black spruce. BMC Genomics. 2008;9:21. doi: 10.1186/1471-2164-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgas B, Bousquet J, Meirmans PG, Ritland K, Isabel N. QTL mapping in white spruce: gene maps and genomic regions underlying adaptive traits across pedigrees, years and environments. BMC Genomics. 2011;12:145. doi: 10.1186/1471-2164-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porth I, Hamberger B, White R, Ritland K. Defense mechanisms against herbivory in Picea: sequence evolution and expression regulation of gene family members in the phenylpropanoid pathway. BMC Genomics. 2011;12:608. doi: 10.1186/1471-2164-12-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Rajora O, Dancik B. Population genetic variation, structure, and evolution in Engelmann spruce, white spruce, and their natural hybrid complex in Alberta. Canadian Journal of Botany. 2000;78:768–780. [Google Scholar]
- Rieseberg LH, Baird SJE, Gardner KA. Hybridization, introgression and linkage evolution. Plant Molecular Biology. 2000;42:205–224. [PubMed] [Google Scholar]
- Rieseberg LH, Raymond O, Rosenthal D, Lai Z, Livingstone K, Nakasato T, Durphy J, Schwarzbach A, Donovan L, Lexer C. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301:1211–1216. doi: 10.1126/science.1086949. [DOI] [PubMed] [Google Scholar]
- Roche L. A genecological study of genus Picea in British Columbia. New Phytologist. 1969;68:505–554. [Google Scholar]
- Sakai A, Larcher W. Frost survival of plants. Berlin, Germany: Springer-Verlag; 1987. [Google Scholar]
- SAS-Institute-Inc. SAS/STAT(R) user′s guide, version 6. 4thedn. Cary, NC, USA: SAS Institute; 1989. [Google Scholar]
- Shen R, Fan JB, Campbell D, Chang W, Chen J, Doucet D, Yeakley J, Bibikova M, Wickam Garcia E, McBride C, et al. High-throughput SNP genotyping on universal bead arrays. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2005;573:70–82. doi: 10.1016/j.mrfmmm.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Slatkin M. Gene flow and selection in a cline. Genetics. 1973;75:733–756. doi: 10.1093/genetics/75.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stift M, Luttikhuinzen PC, Visser EJW, Van Tienderen PH. Different flooding responses in Rorippa amphibia and Rorippa sylvestris, and their modes of expression in F1 hybrids. New Phytologist. 2008;180:229–239. doi: 10.1111/j.1469-8137.2008.02547.x. [DOI] [PubMed] [Google Scholar]
- Thomasset M, Fernandez-Manjarres JF, Douglas GC, Frascaria-Lacoste N, Raquin C, Hodkinson TR. Molecular and morphological characterization of reciprocal F1 hybrid ash (Fraxinus excelsior x Fraxinus angustifolia, Oleaceae) and parental species reveals asymmetric character inheritance. The International Journal of Plant Sciences. 2011;172:423–433. [Google Scholar]
- Wang H, McArthur ED, Sanderson SC, Graham JH, Freeman DC. Narrow hybrid zone between two subspecies of big sagebrush (Artemisia tridentata: Asteraceae). IV. Reciprocal transplant experiments. Evolution. 1997;51:95–102. doi: 10.1111/j.1558-5646.1997.tb02391.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Hamann A, Spittlehouse DL, Aitken SN. Development of scale-free climate data for western Canada for use in resource management. International Journal of Climatology. 2006;26:383–397. [Google Scholar]
- Wang T, Hamann A, Spittlehouse DL, Murdock T. ClimateWNA - high-resolution spatial climate data for western North America. Journal of Applied Meteorology and Climatology. 2012;51:16–29. [Google Scholar]
- White TL, Hodge GR. Predicting breeding values with applications in forest tree improvement. Boston, MA, USA: Kluwer Academic Publishers; 1989. [Google Scholar]
- Whitney KD, Randell RA, Rieseberg LH. Adaptive introgression of herbivore resistance traits in the weedy sunflower Helianthus annuus. The American Naturalist. 2006;167:794–807. doi: 10.1086/504606. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Randell RA, Rieseberg LH. Adaptive introgression of abiotic tolerance traits in the sunflower Helianthus annuus. New Phytologist. 2010;187:230–239. doi: 10.1111/j.1469-8137.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- Xie CY, Yanchuk AD, Kiss GK. Genetics of interior spruce in British Columbia: performance and variability of open-pollinated families in the East Kootenays. Victoria, Canada: Forestry Division Services Branch; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences among genotypic classes for phenotypic traits based on introgress hybrid index estimates
Table S2 Least square means (lsmeans) and standard errors (stderr) for height measurements (cm) at different ages among genotypic classes tested in seed planning zones based on introgress hybrid index estimates
Table S3 Least square means and standard errors for height measurements (cm) at different ages among hybrid classes tested in seed planning zones based on NewHybrids assignment
Table S4 Binomial tests for differences in survival at different ages among Picea glauca, P. engelmannii and their hybrids
Table S5 Differences in survival between Picea engelmannii, P. glauca and hybrids at different ages for all sites and regions studied in field common garden experiments
Table S6 Results of stepwise regression of hybrid index on precipitation as snow (PAS) and summer heat-moisture index (SHM)
Table S7 Results of the univariate regressions of hybrid index on geographical and climate variables in each seed planning zone


