Abstract
Conclusion
As a bedside test of subjective visual vertical (SVV), the ‘bucket test’ has a role as a viable and cost-effective clinical test of unilateral utricular dysfunction in older individuals.
Objective
To investigate whether the bucket test as a test of the SVV is a valid bedside test of utricular function in older individuals.
Methods
This was a diagnostic validation study at a tertiary academic medical center. Vestibular function was evaluated using sound-evoked cervical and tap-evoked ocular vestibular evoked myogenic potential (cVEMP and oVEMP, respectively) asymmetry ratios, the bucket test of SVV, and the Dizziness Handicap Index (DHI), in 51 older individuals aged 70–95 years.
Results
Bucket test scores are correlated in both magnitude and direction with utricle-selective tap-evoked oVEMP asymmetry ratios, but not with sound-evoked cVEMP asymmetry ratios, which are saccule-selective, or with the DHI. Receiver operating characteristics analysis suggests that the bucket test is more specific than sensitive for utricular dysfunction, and a bucket test SVV score of 2° may maximize diagnostic yield relative to the currently accepted score of 3.
Keywords: Ocular vestibular-evoked myogenic potential, subjective visual vertical, bucket test, utricle
Introduction
The human vestibular system consists of five end organs, including three semicircular canals (SCCs) that sense angular acceleration and two otolith organs (utricle and saccule) that sense linear acceleration. Clinical tests used to assess otolith function include vestibular-evoked myogenic potentials (VEMPs) and subjective visual vertical (SVV) or horizontal (SVH) [1]. Cervical VEMP (cVEMP) in response to air-conducted sound is a product of the sacculo-collic reflex and thought to reflect saccular function [2], and accumulating evidence suggests that vibration-evoked ocular VEMP (oVEMP) selectively measures utricular function [3].
SVV and SVH are well-studied tests that examine a subject’s perception of tilt of the external world, and abnormal test scores may reflect an imbalance in static utricular function leading to a perceived tilt in the roll plane. Abnormalities in SVH have been correlated with asymmetry in oVEMP in patients with Ménière’s disease, corroborating their potential shared physiologic basis [4]. Recently, the ‘bucket test’ has been described, and demonstrated to validly measure SVV [5]. In this test, patients attempt to align a vertical line inscribed at the inside bottom of a bucket to the true vertical in the absence of visual cues. The test is inexpensive to construct and maintain, easy to administer, and does not require specialized expertise for interpretation.
In this study, we evaluated the bucket test as a clinical test of utricular function in a sample of normative older adults using the tap-evoked oVEMP test as the reference. The study was conducted as part of a larger study investigating age-related changes in vestibular physiologic function and fall risk in community-dwelling older individuals, in whom a 20% prevalence of utricular dysfunction based on abnormal tap-evoked oVEMP testing was observed [6]. Our goal was to evaluate the potential usefulness of the bucket test as a measure of age-related utricular dysfunction.
Material and methods
Subjects
Study subjects were recruited from a registry [6] of older individuals interested in participating in clinical studies as well as from outpatient geriatrics clinics. Eligible subjects were aged 70 and above, did not have prior otologic or neurotologic diagnoses, and were able to provide informed consent to participate in study procedures. Individuals were excluded if they could not participate in study procedures due to blindness. Data on participant age, gender, race, and symptoms of dizziness and imbalance, including the Dizziness Handicap Index (DHI) [7], were collected based on prior work showing significant associations between these factors and vestibular dysfunction [8]. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Vestibular physiologic testing
All participants underwent comprehensive vestibular physiologic testing, including cVEMP, oVEMP, and bucket testing, as described below.
Cervical VEMP testing
Participants were positioned supine with their upper bodies elevated at a 30° angle from horizontal. Participants actively flexed their neck by lifting it off the pillow during cVEMP stimulation and recording to provide tonic background muscle activity. Air-conducted 500 Hz, 125 dB SPL tone bursts of positive polarity, with a linear envelope (1 ms rise/fall time, 2 ms plateau), at a repetition rate of five pulses per second were delivered monaurally via intra-auricular speakers. cVEMPs were recorded from an electrode montage consisting of a non-inverting electrode placed at the midpoint of the ipsilateral sternocleidomastoid muscle belly, an inverting electrode placed on the sternoclavicular junction, and a ground electrode placed on the manubriumsterni. The responses to 100 stimuli were averaged. The first positive and negative peaks that occurred between 13 and 23 ms after stimulus onset were designated p13 and n23, respectively. The raw peak-to-peak amplitude was calculated as the sum of the p13 and n23 amplitudes. The corrected peak-to-peak amplitude was calculated by dividing the raw peak-to-peak amplitude by the rectified background EMG activity recorded during the 10 ms interval before stimulus onset. The asymmetry ratio (AR) of the corrected p13 to n23 peak-to-peak amplitudes was calculated as:
| (1) |
Ocular VEMP testing
Participants were positioned supine with their upper bodies elevated at a 30° angle from horizontal. Maximum up-gaze was maintained during oVEMP stimulation and recording. ‘Mini taps,’ as described by Iwasaki et al. [9], were delivered by a reflex hammer at the Fz cranial site (in the midline at the hairline, 30% of the distance between the inion and nasion). Fz taps have been shown to provide an acceleration wave that propagates through the skull to the mastoid on either side, predominantly causing an outward linear acceleration of the utricles bilaterally [3]. oVEMPs were recorded from an electrode montage consisting of a non-inverting electrode placed over the contralateral inferior oblique muscle approximately 3 mm below the eye and centered beneath the pupil, an inverting electrode centered 2 cm below the non-inverting electrode, and a ground electrode placed on the manubrium sterni. The responses to 50 stimuli were averaged. Before testing with tap stimulation, 20° vertical saccades were performed to ensure that symmetrical signals were recorded from both eyes. If these saccadic responses showed >25% asymmetry, the electrodes were removed and new ones were applied. The first negative and positive peaks of the oVEMP response that occurred between 10 and 16 ms after stimulus onset were designated n1 and p1, respectively. oVEMP n1 amplitudes were evaluated, given that this is the portion of the response that is most clearly vestibular and prior data from our laboratory demonstrate a high inter-rater reliability of this measure [10]. The AR of the n1 amplitude was calculated as:
| (2) |
The oVEMP is a crossed response, and we define laterality with respect to the otolith organ. Therefore, ‘right amplitude’ is the n1 amplitude measured from the left inferior oblique, reflecting activity of the right utricle.
Bucket testing
The bucket testing protocol has been described previously [5]. Briefly, participants were seated facing forward. The examiner advanced a bucket around the participant’s head such that the bottom of the bucket was at the center of the subject’s gaze and their visual field was completely covered by the rim of the bucket. Inscribed on the inside bottom of the bucket was a straight line, which was hand-drawn and measured approximately 3 mm in width. Inscribed on the outside was the same straight line, with a protractor and weighted plumb line to indicate degrees of rotation of the inscribed line from true vertical. For each SVV measurement, the bucket was rotated by the examiner to a random position, right or left, and rotated back towards the true vertical. When the subject estimated that the true vertical had been reached, he or she said ‘stop’ and the degrees offset was recorded as the bucket score for that trial. SVV was determined under binocular vision, and a total of five trials was conducted for each subject. A clockwise rotation of the vertical from the perspective of the subject was designated as a positive deviation. Mean bucket scores were computed, and absolute bucket test scores were calculated using the formula:
| (3) |
Statistical analysis
The Student’s t test was used for comparisons of continuous variables and simple linear regression was used for correlation analyses to compute coefficients and associated p values. SPSS v. 17.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
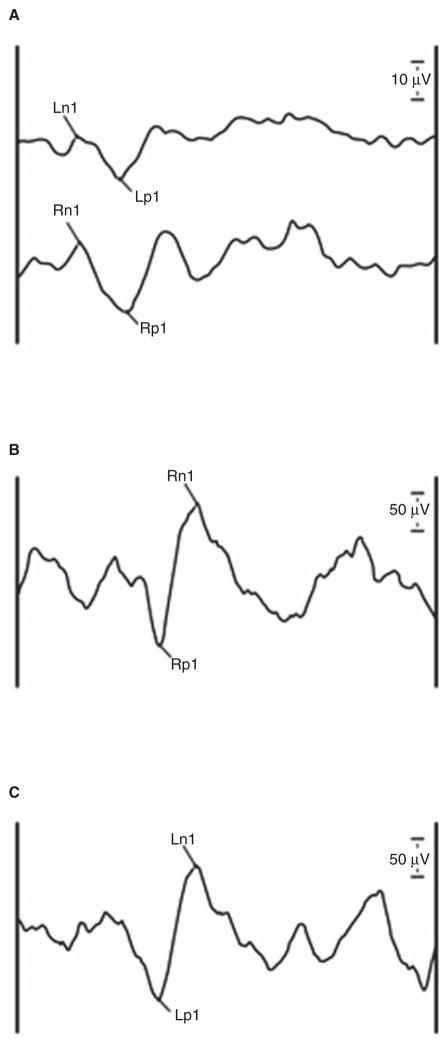
The study population comprised 51 subjects with a mean age of 77 years (standard deviation (SD) 6.1, range 70–95 years). In all, 49% of subjects were male and 51% were female, while 88% were white and 12% were black (Table I). Twenty-seven (53%) individuals reported a history of dizziness in the previous 12 months. The mean total DHI score for study subjects was 6.2 (SD 11.9, range 0–52), corresponding to no perceived dizziness-associated handicap. oVEMP asymmetry ratios were available for 46 subjects, with a mean n1 amplitude asymmetry ratio of 41.9% (SD 37.2, range 1.0–100). cVEMP asymmetry ratios were available for 44 subjects, with a mean peak-to-peak amplitude asymmetry ratio of 38.8% (SD 37.8, range 0.1–100). Figure 1 shows representative oVEMP and cVEMP waveforms for a single subject. Five and seven subjects had bilaterally absent oVEMP and cVEMP responses, respectively, so asymmetry ratios could not be calculated for these subjects. No individuals had both absent oVEMP and cVEMP. There was no statistically significant difference in the mean ages of subjects who had evaluable versus missing oVEMP (p = 0.50) or cVEMP (p = 0.80) asymmetry ratios. There were no significant associations between the oVEMP asymmetry ratios and history of dizziness (p = 0.78) or DHI score (p = 0.95) of study subjects. There was also no association between oVEMP and cVEMP asymmetry ratios (p = 0.37). The bucket test was performed on all subjects in the study population, with a mean score of 1.3° (SD 1.3, range 0–5.8). In all, 47% of subjects scored between 0 and 1, 25% between 1 and 2,16% between 2 and 3, 8% between 3 and 4, and 4% scored above 4.
Table I.
Demographic and clinical characteristics of study population (n = 51).
| Characteristic | n (%) | Mean (SD) |
|---|---|---|
| Age (years) | 77.4 (6.1) | |
| Gender | ||
| Male | 25 (49) | |
| Female | 26 (51) | |
| Race | ||
| White | 45 (88) | |
| Black | 6 (12) | |
| DHI | 6.2 (11.9) | |
| oVEMP tap n1 amplitude AR (%) | 41.9 (37.6) | |
| cVEMP sound peak-to-peak amplitude AR (%) | 38.8 (37.8) | |
| Bucket test score | ||
| 0 to <1 | 24 (47) | |
| 1 to <2 | 13 (25) | |
| 2 to <3 | 8 (16) | |
| 3 to <4 | 4 (8) | |
| >4 | 2 (4) | |
AR, asymmetry ratio.
Figure 1.
Representative VEMP tracings from a single patient. Tap-evoked oVEMP (A) tracings show bilateral n1 and p1 peaks while sound-evoked cVEMP (B and C) tracings show bilateral p1 and n1 peaks. Vertical scale bars are 10 μV and 50 μV for oVEMP and cVEMP tracings, respectively.
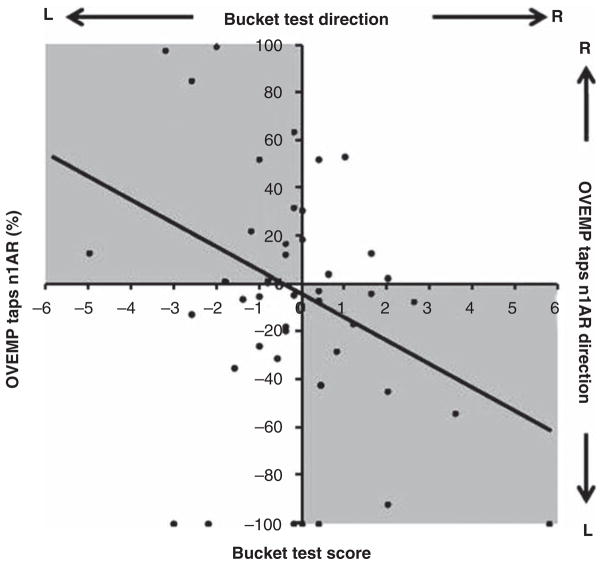
To investigate the relationship between oVEMP n1 amplitude asymmetry ratio and bucket test scores, we plotted the directional values for each subject and observed a significant linear correlation (β = −9.4, R2 = 0.096, p = 0.040) between the outcomes of these two tests (Figure 1). Increasing bucket score magnitude was associated with increasing asymmetry ratio of the oVEMP n1 amplitude. Moreover, there was concordance between the laterality of relative weakness (right vs left) as predicted by the bucket test and oVEMP asymmetry ratio (Figure 2). We coded a higher right-sided oVEMP amplitude compared with the left as a positive oVEMP asymmetry ratio, indicative of a relative left-sided utricular hypofunction. This deficit would result in torsion of the eyes toward the subject’s left (in response to perceived head tilt to the right), which manifests as a tilt of the SVV toward the left [11], which is coded as a negative bucket test score. In other words, a leftward rotation of the bucket score (scored negatively on the x-axis in Figure 2) would correspond to a positive oVEMP asymmetry ratio, which is what we observed.
Figure 2.
Correlation between bucket test scores and tap-evoked oVEMP n1 amplitude asymmetry ratio in study subjects. A leftward rotation of the bucket score (scored negatively on the x-axis) indicates a relative left-sided utricular weakness; this corresponds to a positive oVEMP asymmetry ratio and indicates greater utricular activity on the right compared with the left side. The reverse is true for a rightward rotation of the bucket score. Grey boxes indicate quadrants of concordance in lesion laterality as measured by the bucket test and oVEMP. There is a significant linear correlation (p = 0.04) between bucket scores and tap-evoked oVEMP asymmetry ratio (AR).
In current clinical practice, an absolute bucket test score of 3° or above is considered abnormal [1,12]. We investigated whether this diagnostic threshold is associated with observable differences in VEMP recordings, and whether a dichotomized bucket test score is able to segregate subjects in accordance with their oVEMP asymmetry ratios (Table II). Forty-one subjects had a bucket score of <3 with a mean oVEMP asymmetry ratio of 38.2% (SD 35.6), whereas five subjects had a bucket score of ≥3, with a mean asymmetry ratio of 72.9% (SD 38.8), representing a statistically significant difference (p = 0.049). In contrast, the bucket test score was not significantly associated with age (p = 0.87), DHI (p = 0.18), or the peak-to-peak asymmetry ratio of sound-evoked cVEMP (p = 0.12). Interestingly, a more significant association was found when the fail threshold of the bucket test was lowered to a score of 2 (Table II). Thirty-two subjects scored a passing score of <2 on the bucket test with a mean tap-evoked oVEMP n1 amplitude asymmetry ratio of 33.7% (SD 33.3), while 13 subjects scored ≥2, with a mean asymmetry ratio of 62.2% (SD 41.1, p = 0.019). Again, no statistically significant associations were found between the bucket test at a fail threshold of 2 and age (p = 0.63), DHI (p = 0.90), or cVEMP asymmetry ratio (p = 0.22).
Table II.
Association between bucket test outcome and clinical variables.
| Characteristic | Bucket test score threshold = 3
|
Bucket test score threshold = 2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pass (<3)
|
Fail (≥3)
|
p value | Pass (<2)
|
Fail (≥2)
|
p value | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |||
| Age (years) | 45 | 77.4 (6.1) | 6 | 77.0 (7.0) | 0.87 | 37 | 77.6 (6.2) | 14 | 76.7 (6.0) | 0.63 |
| DHI | 45 | 7.0 (12.4) | 6 | 0 (0) | 0.18 | 37 | 6.3 (11.0) | 14 | 5.9 (14.3) | 0.90 |
| oVEMP taps n1 AR (%) | 40 | 38.0 (36.1) | 5 | 72.9 (38.8) | 0.049 | 32 | 33.7 (33.3) | 13 | 62.2 (41.1) | 0.019 |
| cVEMP sound peak-to-peak AR (%) | 38 | 42.8 (39.3) | 6 | 17.1 (16.5) | 0.12 | 31 | 34.6 (35.5) | 13 | 50.3 (42.7) | 0.22 |
The values given in bold denote statistical significance.
AR, asymmetry ratio; DHI, Dizziness Handicap Index.
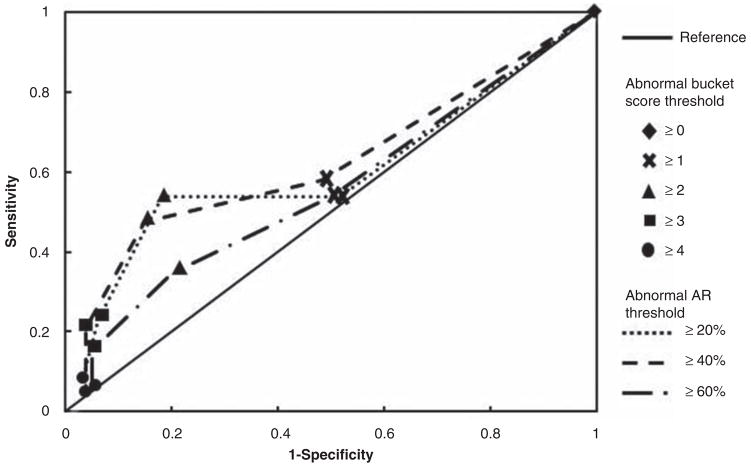
We next investigated the receiver-operating characteristics (ROC) of the bucket test as a clinical diagnostic test of utricular function. Although in our laboratory we use a tap-evoked oVEMP n1 amplitude asymmetry ratio of ≥40% as the diagnostic threshold for utricular dysfunction [4], we studied the sensitivity and specificity of the bucket test at several asymmetry ratio thresholds, including 20%, 40%, and 60% (Figure 3). Based on ROC analyses derived from this study population, the bucket test was more specific than sensitive in diagnosing utricular dysfunction. Using an asymmetry ratio of 40% or greater as diagnostic, the bucket test achieved a sensitivity of 58% even at the minimum fail threshold of score 1, whereas it was able to achieve a specificity of 96% at a maximum fail threshold of score 4. The currently accepted fail threshold score of 3 yielded sensitivity of 21% and specificity of 96% at an asymmetry ratio threshold of 40% or greater. ROC analysis showed that score 2 (sensitivity 47%, specificity 85%) is the fail threshold at which the bucket test may best be able to diagnose unilateral utricular dysfunction.
Figure 3.
Receiver operating characteristics of the bucket test at score thresholds of 0 through 4, and using oVEMP asymmetry ratio (AR) diagnostic thresholds of 20%, 40%, and 60% as gold standards.
Discussion
We found a significant correlation between SVV measured with the bucket test and the tap-evoked oVEMP asymmetry ratio in a cohort of ‘normative’ older individuals, who were observed in a prior study to have evidence of age-related vestibular loss [6]. Age-related changes in vestibular function are a subject of increasing interest given their potential contribution to the risk of falls in older individuals. This study extends prior studies in vestibulopathic patients – patients with unilateral vestibular loss [13] and Ménière’s disease [4]–that also observed significant associations between abnormal SVV and oVEMP results. Moreover, we observed that the bucket test, previously found to be a convenient, low-cost, and accurate assessment of SVV [5], appears to be a valid bedside measure of utricular function as compared to the tap-evoked oVEMP in the elderly population. Although we did not elicit any functional impairment related to unilateral utricular dysfunction in our cohort based on dizziness symptoms or DHI, studies have found an association between isolated utricular dysfunction and postural instability [14]. It is possible that the utricular dysfunction observed in our subjects was subclinical, or that neither the symptom of dizziness nor the DHI effectively captured the manifestations of utricular dysfunction.
Several lines of evidence support the proposition that both the tap-evoked oVEMP [3,15] and SVV reflect utricular function. With respect to oVEMP, although a high-frequency tap is not a selective stimulus and results in activation of both saccular and utricular afferents [3], the change in potential of the inferior oblique and inferior rectus muscles measured by oVEMP, and specifically the n1 amplitude [16], are thought to be utricle-specific [9]. With respect to SVV, in a study that used various modes of tilt and eccentric rotation to specifically vary utricular stimulation [13], patients with unilateral vestibular loss were found to have poorer SVV scores as well as greater side-to-side asymmetries in oVEMP n1 amplitude relative to controls. In the current study, SVV scores were consistent with the neurophysiological principles of utricular function [13], such that subjects experienced tilt in their SVV away from the side of greater utricular function. Unilateral utricular dysfunction has been shown to cause ocular torsion away from the side of the lesion, and a corresponding deviation of the patient’s SVV towards the side of the lesion [11], as observed in our study.
Although we observed a significant association between SVV and oVEMP asymmetry ratios the overlap was not absolute, possibly owing to mechanistic differences between SVV and the oVEMP. Methodologically, the tap-evoked oVEMP uses brief, high-frequency (100 Hz) bone-conducted vibrations to stimulate specific populations of otolith afferent neurons to measure the motor activation of a reflex pathway. There is accumulating evidence that high frequency bone-conducted stimuli selectively activate irregular otolith afferents [3], which are physiologically tuned to time-dependent changes in linear acceleration (jerk). The oVEMP is thus a dynamic test that measures utricular function in response to stimulation (linear acceleration).
In contrast, SVV is a static test that reflects tonic activity of the utricles. As a static test, the SVV may be more sensitive to adaptation and compensation. Indeed, studies [11,12,17] have shown that in patients with unilateral vestibular neuritis, the SVV is initially abnormal but almost completely normalizes 3 months after onset. Successful vestibular adaptation likely accounts for the small number of patients with abnormal bucket scores in this study, although some patients may have persistent abnormalities in SVV when visual and proprioceptive cues are minimized, or when adaptation is incomplete. Indeed, Valko et al. [13] were able to elicit abnormalities in SVV using eccentric rotation 3 months after the initial diagnosis of vestibular neuritis. oVEMP, in contrast, is an evoked stimulus that may be less susceptible to adaptation [17] and may be a more sensitive indicator of dynamic utricular dysfunction.
An additional distinction between the SVV and oVEMP is that the SVV reflects graviceptive perception at a system level. Its outcome is dependent not only on input from the afferent neuronal population in the otolith end organ but also the internal estimate of gravitational tilt derived from high-level synthesis of all perceptual inputs by the brain [1]. As such, compared with oVEMP, the bucket test may be more likely to be affected by central lesions [5]. In fact, the SVV was originally developed to screen patients for brainstem infarcts [1]. This distinction may be magnified in the older population of this study in whom central lesions may be more prevalent. As a result of these considerations, the SVV and oVEMP may differentially reflect utricular function.
Notably, we observed that the SVV was not significantly associated with the sound-evoked cVEMP, which is thought to measure saccular function [3,15]. Similarly, in another study [18] of patients with vestibular neuritis, no significant association was found between SVV and cVEMP asymmetry ratios. In a recent report, Cohen and Sangi-Haghpeykar [19] investigated the correlation of bucket test scores to benign paroxysmal positional vertigo (BPPV) and unilateral vestibular weakness and found no statistically significant associations. This may be because BPPV and unilateral vestibular weakness, defined as asymmetry on caloric testing, both represent pathology of the semicircular canals and not necessarily of the utricle.
The present study also explored the clinical threshold at which a bucket test score may be diagnostic of utricular dysfunction in older individuals. The currently accepted threshold of a tilt of 3° or more from vertical is based on the original investigation by Dieterich and Brandt [1] that studied SVV in a group of patients with brainstem infarcts and set 2.5° as the pathologic threshold. Our analysis indicates an optimum diagnostic threshold of score 2 in older subjects, which is in range of the original diagnostic cut-off and has also been termed ‘moderately abnormal’ in other reports [12]. It should be noted that based on ROC analysis, the bucket test appears to be a more specific than sensitive test of utricular dysfunction, which should be considered in its role as a screening test of utricular dysfunction.
Several limitations exist in this study. Although the directional correlation between the bucket test and oVEMP asymmetry is consistent with physiologic principles, eye position was not measured and therefore deviations in SVV could not be specifically attributed to ocular torsion alone. Moreover, the small number of subjects with pathologic bucket scores also rendered the statistical analysis vulnerable to experimental noise. Additionally, as discussed previously, confounding factors such as central pathology may have differentially affected performance in the bucket and oVEMP tests in the older study population. ROC analysis in this study uses oVEMP as the gold standard in diagnosing utricular dysfunction, although this remains controversial [20]. An oVEMP asymmetry ratio of 40% has been suggested [4] as a viable diagnostic threshold for utricular dysfunction and our sensitivity analysis is consistent with this threshold. However, it is important to note that additional data are needed in validating the optimum diagnostic threshold.
Conclusion
This study provides evidence that the bucket test, which estimates SVV, is both directionally and quantitatively associated with the tap-evoked oVEMP asymmetry ratio as a measure of unilateral utricular dysfunction in normative older individuals. As a diagnostic tool, it is more specific than sensitive, and a threshold score of 2, rather than the currently used value of 3, may be optimal for discriminating asymmetric utricular function in older adults. These data suggest that the bucket test may have the potential to be a cost-effective and clinically useful tool in detecting unilateral utricular dysfunction in older patients.
Acknowledgments
Support for this work was provided by the American Neurotology Society (Y.A.), the American Otological Society (Y.A.), and the Johns Hopkins Older Americans Independence Center (Y.A.). The authors would like to acknowledge Professor Ian S. Curthoys PhD, for his valuable input on this manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol. 1993;33:292–9. doi: 10.1002/ana.410330311. [DOI] [PubMed] [Google Scholar]
- 2.Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;64:1682–8. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- 3.Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010;121:132–44. doi: 10.1016/j.clinph.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Lin K-Y, Young Y-H. Correlation between subjective visual horizontal test and ocular vestibular-evoked myogenic potential test. Acta Otolaryngol. 2011;131:149–55. doi: 10.3109/00016489.2010.518973. [DOI] [PubMed] [Google Scholar]
- 5.Zwergal A, Rettinger N, Frenzel C, Dieterich M, Brandt T, Strupp M. A bucket of static vestibular function. Neurology. 2009;72:1689–92. doi: 10.1212/WNL.0b013e3181a55ecf. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–9. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–7. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169:938–44. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki S, Chihara Y, Smulders YE, Burgess AM, Halmagyi GM, Curthoys IS, et al. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol. 2009;120:588–93. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curthoys IS, Dai MJ, Halmagyi GM. Human ocular torsional position before and after unilateral vestibular neurectomy. Exp Brain Res. 1991;85:218–25. doi: 10.1007/BF00230003. [DOI] [PubMed] [Google Scholar]
- 12.Hirvonen TP, Jutila T, Aalto H. Subjective head vertical test reveals subtle head tilt in unilateral peripheral vestibular loss. Eur Arch Otorhinolaryngol. 2011;268:1523–6. doi: 10.1007/s00405-011-1560-8. [DOI] [PubMed] [Google Scholar]
- 13.Valko Y, Hegemann SC, Weber KP, Straumann D, Bockisch CJ. Relative diagnostic value of ocular vestibular evoked potentials and the subjective visual vertical during tilt and eccentric rotation. Clin Neurophysiol. 2011;122:398–404. doi: 10.1016/j.clinph.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Pelosi S, Schuster D, Jacobson GP, Calson ML, Haynes DS, Bennett ML, et al. Clinical characteristics associated with isolated unilateral utricular dysfunction. Am J Otolaryngol. 2013;34:490–5. doi: 10.1016/j.amjoto.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121:636–51. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki S, McGarvie LA, Halmagyi GM, Burgess AM, Kim J, Colbatch JG, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68:1227–9. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- 17.Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology. 1998;51:838–4. doi: 10.1212/wnl.51.3.838. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y, Otsuka K, Shimizu S, Inagaki T, Kondo T, Suzuki M. Subjective visual vertical perception in patients with vestibular neuritis and sudden sensorineural hearing loss. J Vestib Res. 2012;22:205–11. doi: 10.3233/VES-2012-0447. [DOI] [PubMed] [Google Scholar]
- 19.Cohen HS, Sangi-Haghpeykar H. Subjective visual vertical in vestibular disorders measured with the bucket test. Acta Otolaryngol. 2012;132:850–4. doi: 10.3109/00016489.2012.668710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colebatch JG. Sound conclusions? Netherlands Clin Neurophysiol. 2010;121:124–6. doi: 10.1016/j.clinph.2009.09.026. [DOI] [PubMed] [Google Scholar]