Abstract
Background/purpose
The superficial layer on the skin surface, known as the acid mantle, comprises a mixture of sebum, sweat, corneocyte debris and constituents of natural moisturizing factor. Thus, the phrase ‘residual skin surface components’ (RSSC) is an appropriate term for the mixture of substances recovered from the skin surface. There is no general agreement about the effects of ethnicity, gender and age on RSSC. The aim of this human volunteer study was to evaluate RSSC in relation to ethnicity, gender and age. A suitable acquisition medium for RSSC collection was identified and samples of RSSC were subsequently analysed using gas chromatography-mass spectrometry (GC-MS) and gravimetry.
Methods
A total of 315 volunteers participated in the study from a range of self-declared ethnic backgrounds. Six acquisition media were compared to determine the most suitable media for RSSC collection. The effect of age, gender and ethnicity on RSSC collection was evaluated by gravimetric analysis while GC-MS was used to determine the composition of RSSC.
Results
Of the six candidate materials assessed, cigarette paper provided the most practical and reproducible sample acquisition medium. There was no significant difference in the amount of RSSC collected when based on gender and ethnicity and no significant correlation between RSSC recovery and age. Up to 49 compounds were detected from human RSSC when analysed by GC-MS.
Conclusions
The results of the present study suggest that RSSC can be effectively collected using cigarette paper and analysed by GC-MS. Ethnicity, gender and age had no significant impact on the quantity of RSSC recovered from the skin surface.
Keywords: age, cigarette paper, ethnicity, gas chromatography-mass spectrometry, gender, sebum, skin surface lipids
The superficial layer on the skin surface is known as the acid mantle and comprises a mixture of sebum, sweat and corneocyte debris. Additionally, proteolytic products from filagrin (which act as a natural moisturizing factor) are present within the stratum corneum and may form part of this mixture 1. The predominant component of the superficial layer is sebum, which imparts low pH on the skin surface due to the presence of free fatty acids 2. Sebum is a secretion of the sebaceous glands which are holocrine, multilobular glands mainly associated with hair follicles 3. Freshly secreted sebum is a clear, oily substance which consists mainly of squalene, wax esters and triglycerides with a small proportion of cholesterol and cholesterol esters 4–7. The actual composition is believed to vary according to the anatomical location 7. Although the purpose of sebaceous glands in humans has been the subject of some debate 8, the fact that they are controlled by a complex hormonal mechanism and are well developed in particular areas such as the face, upper back and chest suggests that these glands are not vestigial 9. Indeed, sebum may be involved in thermoregulation 10, control of Gram-positive bacterial colonization 11, maintenance of the skin surface 12, excretion of lipophilic compounds 13–15 and delivery of superficial antioxidant (vitamin E) to the skin surface 16.
Previous studies have reported the collection of sebum or skin surface lipids by various methods such as solvent extraction 7,17,18, cigarette paper 19–21, polyurethane (PU) foam 22 and Sebutape® 23 with subsequent analysis by thin layer chromatography 5,24, gas chromatography 25,26 or infrared spectroscopy 27. However, there is no general agreement about the quantity of sebum present on the skin 19,21,28,29 or its composition 30. As sebum and sweat are present on the skin surface in the form of a complex mixture 31,32, material acquired from the skin surface may not necessarily be representative of sebum but rather a mixture of sweat and sebum along with the lipids of the stratum corneum and proteolytic products from filagrin. Thus, the phrase ‘residual skin surface components’ (RSSC) is a more appropriate term for the mixture of substances recovered from the skin surface.
An in vitro study using artificial sebum deposited on pig skin has demonstrated that sebum can absorb and retain vapours of organic chemicals after topical exposure 33. This property of sebum could potentially be exploited to utilize sebum as bio-monitoring matrix to identify human exposure to harmful chemicals. Alternatively, sebum or RSSC may have diagnostic utility. For example, Sakai et al. reported specific perturbations in the recovery of certain RSSC components relative to total lipid content in hairless mice with experimentally induced diabetes 34 and the quantity of skin surface lipids on the forehead of diabetic patients is significantly lower than control populations 35. The compositional changes in the skin surface lipids of humans with other clinical disorders are awaiting investigation. However, in order to utilize RSSC for bio-monitoring or diagnostic applications, it is first necessary to establish its ‘normal’ characteristics in populations. Thus, the aim of this human volunteer study was to evaluate the effect of ethnicity, gender and age on RSSC accumulation and composition. An optimal method for acquiring cumulative RSSC samples was identified and composition of RSSC from subsequent samples was determined by gas chromatography-mass spectrometry (GC-MS).
Materials and Methods
Cigarette paper (Rizla+™ red, density: 17.5 g/m2, thickness: 27 μm, composition: 14% calcium carbonate, 86% eucalyptus cellulose fibres), absorbent cotton (Boots UK Ltd., Nottingham, UK), PU foams (pore size: type A 300 μm, type B 75 μm; Boots UK Ltd) and Scotch® tape (3M UK PLC., Bracknell, UK) were purchased from a local supplier. Sebutape® was purchased from CuDerm Corporation (Dallas, TX, USA). Diethyl ether and hexane (GC grade) were obtained from Sigma-Aldrich (Dorset, UK). A Mettler Toledo AX205 (Mettler-Toledo Ltd., Leicester, UK) series balance was used for gravimetric analysis and gas chromatography analysis was performed on Varian 450 GC with Varian 240 MS (Varian UK Inc., Oxford, UK).
Volunteers
Ethical approval to perform this study was granted by the School of Pharmacy and Postgraduate Medicine Ethics Committee with Delegated Authority, University of Hertfordshire (ethics number: PHAEC/10-25). All participants provided informed consent. Personal data and details of health conditions of volunteers were collected using a questionnaire. Volunteers were then categorized into different ethnic groups based on self-declared information according to the UK ‘Household Questionnaire Census 2011’ 36. For the purpose of this study, the ‘Asian’ group comprised individuals from India, Pakistan and Bangladesh. A total of 315 volunteers participated in the study.
Experiment 1: Comparison of collection media
The optimal sample acquisition material was determined on three volunteers (Volunteer A: African, female, 26 years old; Volunteer B: Asian, male, 29 years old; Volunteer C: African, female, 34 years old) by comparing six products (cigarette paper, PU foams type A and type B, absorbent cotton, Scotch® tape and Sebutape®). The PU foams used in this study were porous (type A) and fibrous (type B), with pore sizes of 300 and 75 μm respectively (Fig.1). Each sampling medium was cut into a 2 cm× 2 cm square, soaked in diethyl ether and fully dried before use with the exception of Sebutape® and Scotch® tape which were not solvent treated. Each pre-weighed medium was applied to five sites on the forehead of each volunteer after wiping the area with absorbent cotton soaked in diethyl ether and held in place with an elasticated head band for 3 h. Each sampling medium was dried and weighed after RSSC collection, with the difference in weight being ascribed to the amount of RSSC collected (expressed as mg/cm2).
Figure 1.
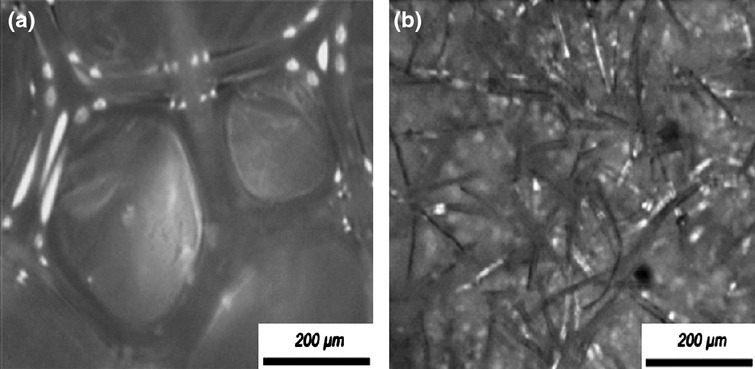
Representative light microscope images of polyurethane (PU) foam A and B. The average pore size of type A and type B foams were 300 and 75 µm, respectively.
Experiment 2: Characterization of optimal (cigarette paper) acquisition method
The putatively optimized method of RSSC collection using cigarette paper (determined from experiment 1) was adopted for subsequent RSSC collection 19 which was characterized using 10 volunteers (5 males and 5 females, 31.5 ± 11.5 years old, mean age ± SD) by collecting RSSC over a 3 h period, on two different occasions, up to 1 week apart. Cigarette papers were prepared as described above. After removal, the cigarette papers were subject to dehydration (by passive evaporative loss) in plastic sample cups covered with pierced Parafilm™ (Bemis Flexible Packaging, Neenah, WI, USA) in a fume cupboard for a minimum period of 2 h after which each sample cup was closed using a plastic cap 21. Each paper was subsequently reweighed to determine the amount of dry RSSC collected.
Experiment 3: Collection and analysis of RSSC in different sample groups (ethnicity, gender and age)
Samples of RSSC were acquired from a total of 315 volunteers using the optimized cigarette paper method as described above. A dried, pre-weighed cigarette paper was applied (in duplicate) to the forehead, for a period of 1 h. For volunteers aged ≥18 years of age, the duration of RSSC collection was extended by replacing the papers with a fresh paper each hour for up to 3 h to determine the rate of RSSC accumulation. Samples were collected within the temperature range of 18–25°C at relative humidity of 50–60%. Following dehydration and weighing, papers from each volunteer were placed in a glass vial containing 4 mL hexane 37. The vial was shaken vigorously for 5 min using a vortex mixer and allowed to stand for 20 min. The papers were then removed and the extract was filtered through a 0.2 μm pore Polytetrafluoroethylene membrane. The extract was concentrated by purging with nitrogen until approximately 1 mL of sample remained. Cigarette papers without RSSC were extracted in exactly the same manner for use as ‘blank’ samples. Gas chromatographic separation was achieved using a Varian 450 GC with a 5% phenyl–95% dimethyl polysiloxane capillary column (l = 30 m, i.d. = 0.25 mm, film thickness = 0.25 μm) using helium carrier gas (1 mL/min). The column was heated to 50°C for 2 min following sample injection and the temperature was subsequently increased (10°C/min) to 330°C which was maintained for a further 5 min. Detection was performed using a Varian 240 MS on full ion scan mode (range 40–1000 Da) following electron impact ionization. The column was cleaned by injecting hexane after each sample. This method was used for qualitative analysis of RSSC samples and did not allow quantification of the RSSC components.
Statistical analysis
A commercially available software package (Statistical Package for the Social Sciences, version 20; SPSS Inc., Chicago, IL, USA) was used to perform the statistical analysis. The Kolmogorov–Smirnov test was used to determine the normality of the data set and groups were compared either by paired t-test, Kruskal–Wallis anova or Mann–Whitney test (as appropriate). P ≤ 0.05 was considered significant. Sample size determination was calculated using G*Power freeware 38 using previously reported sebum collection values for Caucasian males and females 39,40. The calculation indicated that a minimum of 70 volunteers were required (35 males and 35 females) to determine significant gender difference within each ethnic group.
Results
Demographic presentation of volunteer population
A total of 315 volunteers participated in the study; 161 males and 154 females from a range of self-declared ethnic backgrounds (African, White or Asian; Fig.2).
Figure 2.
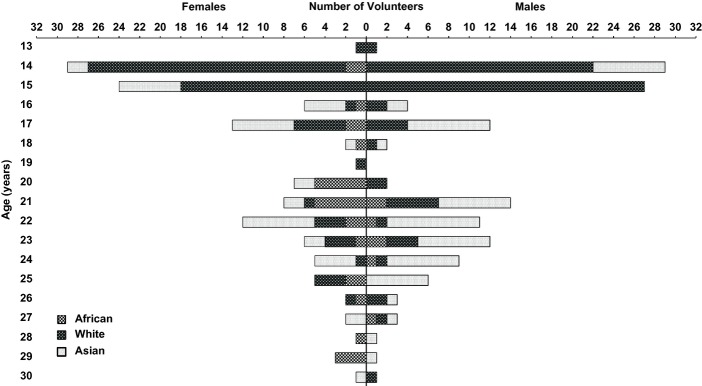
Demographic presentation of self-declared ethnic groups based on age and gender. The x-axis refers to the number of males or females expressed as function of age (y-axis) according to self-declared ethnicities (African, White and Asian).
Comparison of collection media
The quantity of RSSC recovered from cotton (n = 5 replicates from three individuals) was significantly higher than Sebutape®, cigarette paper, PU type A and B foams and Scotch® Tape (P < 0.05). However, both PU type A and type B foams exhibited high variability in the recovery of RSSC, with percentage coefficient of variance (%CV) exceeding 50% (Fig.3). The variation with Sebutape®, cigarette paper, cotton and Scotch® tape was less than 26%.
Figure 3.
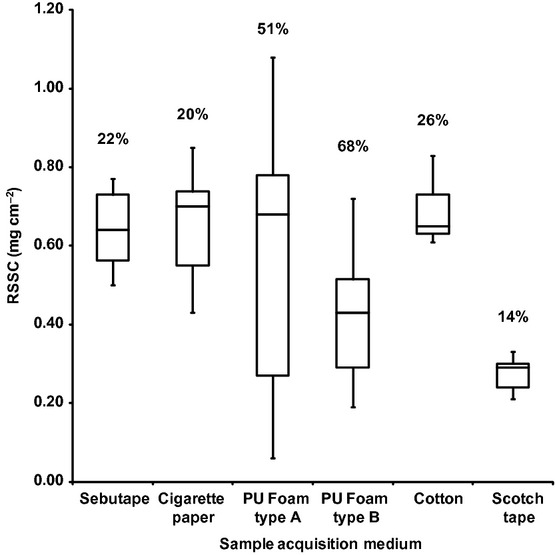
Amount of residual skin surface components (RSSC) collected (n = 5 replicates) from one volunteer (Volunteer A: African, female, 26 years old) using six collection media. Each box represents interquartile range with median, while minimum and maximum values of RSSC collection are shown by bars. The number above each boxplot denotes the coefficient of variation (CV) of RSSC collection among three volunteers (Volunteer B: Asian, male, 29 years old; Volunteer C: African, female, 34 years old).
Characterization of optimal (cigarette paper) acquisition method
The amounts of RSSC recovered from cigarette paper did not vary significantly when collected on different days within individuals (P > 0.05) and was reproducible between individuals (r2 = 0.739; Fig.4).
Figure 4.
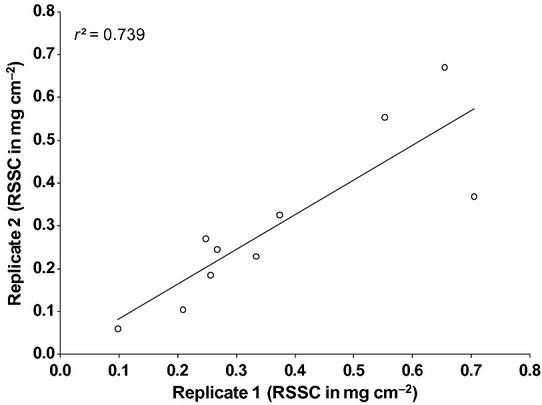
Reproducibility of residual skin surface components (RSSC) collected on two different occasions from 10 randomly selected volunteers. No statistically significant difference was observed in RSSC accumulation on two different occasions (paired t-test, P > 0.05).
Gravimetric analysis of RSSC
The amount of RSSC collected in the first hour from males (0.11 ± 0.06 mg/cm2; mean ± SD) was not significantly different to females (0.12 ± 0.07 mg/cm2, P > 0.05). There was no significant correlation between RSSC recovery and age (Fig.5) and no significant difference in RSSC recovery between ethnic groups (Table1; P > 0.05). Furthermore, RSSC recovery did not vary between males and females of White and Asian ethnic groups (P > 0.05). However, the recovery of RSSC was dependent on the sampling time: the amounts recovered at 2 and 3 h were not significantly different from each other but significantly less than at 1 h (Table2; P < 0.05).
Figure 5.
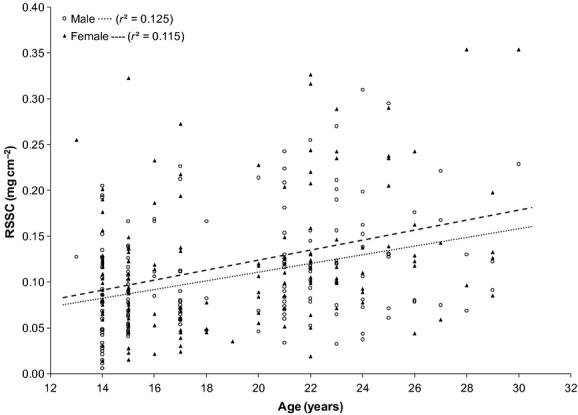
Residual skin surface components (RSSC) collection from 161 males and 154 females after 1 h. No change in RSSC accumulation with increasing age was observed for either gender.
Table 1.
Summary of residual skin surface components (RSSC) collection in three population subgroups based on ethnicity
| Groups | n | RSSC (mg/cm2), mean ± SD | ||
|---|---|---|---|---|
| Males | Females | All | ||
| African | 34 | 0.18 ± 0.07 | 0.12 ± 0.07 | 0.13 ± 0.07 |
| White | 135 | 0.09 ± 0.05 | 0.10 ± 0.07 | 0.10 ± 0.06 |
| Asian | 97 | 0.12 ± 0.07 | 0.13 ± 0.08 | 0.12 ± 0.07 |
Table 2.
Amount of residual skin surface components (RSSC) collected in each hour using cigarette paper method. There was a significant difference in the rate of accumulation between the first and the second and third hours
| Time (hour) | RSSC (mg/cm2) |
|---|---|
| 1 | 0.13 ± 0.07 |
| 2 | 0.09 ± 0.05 |
| 3 | 0.08 ± 0.04 |
Values are expressed as mean ± SD.
P < 0.05.
GC-MS analysis of RSSC
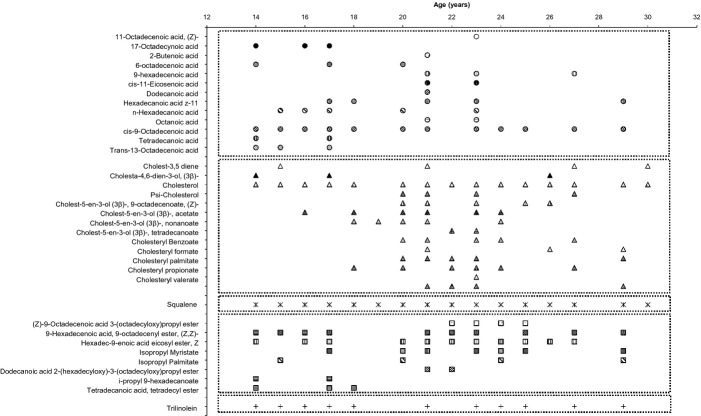
Up to 49 compounds were detected from human RSSC when analysed by GC-MS (Fig.6) and were grouped into five classes: (i) squalene, (ii) cholesterol and cholesterol esters, (iii) wax esters, (iv) free fatty acids and (v) triglycerides. Squalene was constitutively present in all RSSC samples while trilinolein was the only triglyceride detected. Various free fatty acids, wax esters and cholesterol esters were present over the age range of 14–30 years. However, less cholesterol esters were present below 18 years old whereas the number of fatty acids decreased after the age of 23 years.
Figure 6.
Components of residual skin surface components (RSSC) identified by GC-MS analysis. Detected compounds were classified into five groups: free fatty acids, cholesterol and cholesterol esters, squalene, wax esters and triglycerides.
Discussion
This study has demonstrated that there is no significant difference in the quantity of RSSC based on ethnicity, gender or age. In contrast to previous studies 7,28,29,39,41,42, this study was based on a relatively large number of volunteers and can be considered statistically robust (Table3).
Table 3.
Comparison of results from current and previous studies
| Ref. | Number of volunteers, age, ethnicity | Site and method of collection | Measurements | Results | Comments |
|---|---|---|---|---|---|
| 19 | Four volunteers, all males | Forehead, cigarette papers | Sebum (mean ± SD) 316 ± 58.9 μg/cm2/3 h |
Cigarette paper is a suitable collection medium for sebum. | Smaller number of volunteers than present study Cigarette paper as suitable collection medium is in agreement with the current study |
| 21 | 43 volunteers | Forehead, cigarette paper | Sebum, 20–790 μg/cm2/2.75 h | Cigarette paper is a suitable collection medium for sebum. | Smaller number of volunteers than present study Cigarette paper as suitable collection medium is inagreement with the current study |
| 7 | Five volunteers, all males, age: 20–40 years | Forehead, extraction (hexane) | Skin surface lipids 160 μg/cm2/3 h |
The epidermal lipids contribute about 3–6% of the surface lipid on the forehead | Smaller number of volunteers than present study |
| 41 | 20 volunteers 10 young: 29.8 ± 3.9 years 10 old: 73.6 ± 17.4 years |
Forehead Sebumeter 810PC |
Sebum (mean ± SD) Young: 62.9 ± 28.8 μg/cm2 Old 74.7 ± 51.9 μg/cm2 |
No statistically significant differences in the sebum content between two age groups | Smaller number of volunteers than present study Age-specific results are in agreement with the current study |
| 29 | 29 volunteers 14 males and 15 females, Age: 14 young: 26.7 ± 2.8 years and 15 old: 70.5 ± 13.8 years |
Forehead Sebumeter 810PC, Skin pH meter pH 900 | Sebum On the forehead (mean ± SEM) Young: 137.9 ± 19.9 μg/cm2 Old: 127.4 ± 23.2 μg/cm2 |
No significant difference in casual sebum level between genders No significant difference in casual sebum level between age groups except for the ankle Higher pH in the aged group on the forehead than young group |
Smaller number of volunteers than present study Gender- and age-specific results are in agreement with the current study |
| 39 | 12 volunteers, six males (mean age: 24.2 ± 0.4 years) and six females (mean age: 24.3 ± 0.8 years) Skin photo type II and III |
Forearm Sebumeter 810PC |
Sebum rate (mean ± SD) Male: 3.0 ± 4.6 μg/cm2 Female: 0.7 ± 0.5 μg/cm2 |
No significant difference in sebum rate between genders | Smaller number of volunteers than present study. However, only one skin type was studied Gender-specific results are in agreement with the current study |
| 51 | 1360 volunteers, all females Ethnicity Caucasian (1353) Asian Indian (474) African-American (435) Latino/Hispanics (310) East Asian (207) Japanese (381) |
Forehead Sebumeter SM 810 |
Sebum | Sebum excretion increases during the early decades, peaking in the third and fourth decade after which it declines African-Americans showed significantly more secretion than east Asians and Hispanics Hispanics had the lowest sebum secretion, significantly less than both Caucasian and African-Americans |
Larger number of volunteers than present study. However, only female subjects were studied Ethnicity specific results are not in agreement with the current study |
| 42 | 193 volunteers 75 males and 118 females Age: 4–60 years |
Forehead Absorbent paper |
Sebum | After the age of 15, the sebum excretion rate is greater in males than females and rate rises to maximal levels in both genders between the age of 26 and 40 declining thereafter but to greater degree in females | Smaller number of volunteers than present study Gender- and age-specific results are not in agreement with the current study |
| 28 | 60 volunteers 30 males (mean age 29 years) and 30 females (mean age 32 years) |
Cheek Sebumeter SM 810 |
Sebum Male: 152.0 μg/cm2 Female: 102.9 μg/cm2 |
The sebum output level was significantly higher in males than in females. Sebum output increases with increasing age and increased pore size | Smaller number of volunteers than present study Gender- and age-specific results are not in agreement with the current study |
| 50 | 713 volunteers 328 males and 385 females Age: 0.5–94 years Ethnicity: Chinese |
Forehead Sebumeter pH meter pH 905 |
Sebum and pH | Males tended to have higher sebum content than females Sebum secretion peaks at around the age of 40 years in females and 50 years in males. Sebum secretion declines earlier in females than in males. Higher pH in the aged group on the forehead than young group |
Larger number of volunteers and broader age range studied than present study. However, limited to a Chinese population Gender- and age-specific results are not in agreement with the current study |
| 26 | One volunteer Female Age: 26 years |
Absorbent papers, six body areas | Skin surface lipids | More than 200 compounds have been identified using GC-MS analysis | Skin surface lipids were collected from only one subject GC-MS results are in agreement with present study |
Clearly, the selection of an appropriate sample acquisition medium is pivotal to the conduct of such a large-scale volunteer study. Although the percentage coefficient of variation of RSSC collection using cotton (26%) was not high (Fig.3), retaining integrity of this material after application on the forehead was found to be practically difficult, as a proportion adhered to the application strap and thus prevented full recovery (and so compromised the gravimetric analysis). The PU foams were unsuitable as an acquisition medium due to high variability between replicates during RSSC collection compared to Sebutape®, cigarette paper, cotton and Scotch® tape (Fig.3). The adhesive coating on the collecting surface of Scotch® tape and Sebutape® presented a higher risk of corneocyte collection from the upper layer of skin surface, as it has potential to strip the skin surface and thus potentially contaminate skin surface lipids with epidermal lipids 43. In addition, the use of adhesive tape may interfere with chemical analysis due to extraction of the adhesive components along with RSSC 37. Cigarette paper provided the most practical collection media, in that its physical integrity was maintained during the collection process, it was economical, produced reproducible data (on different days), was readily available and free of adhesive on the collecting surface. The GC-MS analysis of blank cigarette paper showed that any endogenous impurities were adequately removed by pre-washing with diethyl ether (data not shown). Moreover, it has previously been demonstrated that cigarette paper tends to absorb fewer epidermal components and so this sampling medium should present the purest form of RSSC for analysis 44,45. Thus overall, cigarette papers represent the most pragmatic and robust option for acquisition of forehead RSSC samples. The successful analysis of RSSC by GC-MS in the absence of derivatization following a single-step extraction contributed to a relatively short analysis time and so represents a practical and cost-effective method for the analysis of large numbers of samples acquired using cigarette paper.
In a study of 193 volunteers (aged 4–60 years old), Cotterill et al. reported that sebum secretion rates start to increase around the age of 15 and are higher in males than females, continuing to increase gradually until 40 years of age before decreasing in both genders (but to a greater degree in females than males) 42. However, no gradual increase in RSSC secretion was observed in either gender in the current study over the age range of 14–30 years (Fig.5). It has been reported that sebum excretion varies according to age and gender as a consequence of the diversity of hormonal signals 46–48, particularly androgenic stimulation 49, although other factors (such as target tissue response) may also influence sebum secretion rates 24. Although it has been hypothesized that age-dependent variations in sebum secretion are most probably due to changes in sex hormone levels, the results of this current study do not support this hypothesis: no correlation between age (between 14 and 30 years) and RSSC accumulation was identified. This is in agreement with two other studies 29,41 where no significant age-specific differences in sebum secretion were noted at multiple skin locations between younger (~29 years) and older (~72 years) individuals with the exception of sebum collected from ankle skin 29 (Table3).
When RSSC data from male and female volunteers (pooled from different racial groups) were compared in this study, there was no significant difference in the amount of RSSC collected. Previous studies have provided conflicting outcomes, both supporting 42,50 and refuting 29,39 gender-specific variation (Table3). The studies which reported a gender difference were carried out either only in a Chinese population 50 or with smaller number of volunteers than the present study 28,42. Based on previous work in a white population, it was calculated that a minimum of 35 individuals were required to identify a statistically significant difference in the RSSC collection between males and females within an ethnic group. In this study of 135 White volunteers (73 males, 62 females), no statistically significant gender difference was identified. Thus, this data is in agreement with a smaller study reported by Jacobi et al. 39.
Limited information is available in the literature regarding the effect of ethnicity on RSSC. A previous study involving only female volunteers on different skin types showed an increasing rate of sebum secretion from Hispanics to Caucasians to African-Americans. It was also reported that East Asians have lower sebum secretion rates than African-Americans 51. No statistically significant differences in the amount of RSSC were identified between African, White and Asian populations in this study (Table1). It may have been anticipated that individuals in the African ethnic group would have greater RSSC accumulation rates due to the reported presence of larger sebaceous glands 52. However, the results of this study clearly do not support this supposition.
The rate of RSSC accumulation onto the skin surface was found to peak in the 1 h before decreasing subsequently in the second and third hours (Table2). Millns and Maibach reported similar changes in sebum collection and hypothesized that the higher rate of sebum secretion in the first hour was due to follicular depletion following solvent extraction 53 while Eberhardt attributed this effect to a ‘feed-back theory’ in which secreted lipids decrease subsequent sebum excretion by their own surface tension 54. However, sebum is continuously produced by holocrine secretion of sebocytes 55 and there is a large pool of sebum in the gland and sebaceous duct compared to the skin surface 20. It has been suggested that continuous removal of skin surface sebum over the period of 14 h will deplete the accumulated reservoir of sebum 5,56. Thus, the feed-back theory does not appear to explain the time-dependent rate of sebum secretion. Instead, the follicular depletion hypothesis (which is independent of sebum production) appears to be a more convincing explanation.
The relatively small quantities of RSSC that can be recovered from the skin surface are one of the major technical challenges in skin surface lipid analysis 45. Techniques previously utilized for sebum analysis such as thin layer chromatography 5,24 and infrared spectroscopy 27 allowed identification of a chemical class (for example, wax esters or triglycerides) but were unable to detect individual components of a lipid class. This problem was alleviated to some extent in this present study by the use of a more sensitive technique (GC-MS) to identify components of RSSC. Compounds from all five reported chemical classes of sebum (squalene, cholesterol and cholesterol esters, wax esters, free fatty acids and triglycerides) were detected. Previous analysis of sebum composition using gas chromatography has employed derivatization steps 26. The method developed in the present study involved no derivatization steps and enabled RSSC to be directly analysed following extraction from cigarette paper. Although, sensitivity could be compromised to some extent due to lack of derivatization, this method is advantageous as it allowed the identification of individual components from a large number of samples in a relatively short time.
Fatty acids on the skin surface originating from the hydrolysis of triglycerides by lipase activity impart low pH on the skin surface 2. In the current study, it was observed that the number of free fatty acids decreased after the age of 23 years. A previous study has reported an increase in skin surface pH with increasing age 50 which may be explained by the decrease in number of free fatty acids found in this present study.
In the current study, younger (≤17 years of age) volunteers participated in 1 h session (instead of 3 h) in order to integrate with their school timetable. As a consequence, the total amount of RSSC collected in 1 h was less than that collected in 3 h. It is acknowledged that cholesterol and its corresponding esters account for the smallest percentage of total sebum composition 7. Thus, it seems reasonable to assume that the lower recovery of RSSC from younger participants may have resulted in the levels of cholesterol and corresponding esters being below the analytical detection limit and thus apparently absent from this age group.
In summary, the results of the present study (Table3) suggest that RSSC can be effectively collected using cigarette paper and analysed by GC-MS without the need for derivatization. Gender, age and ethnicity had no significant impact on the quantity of RSSC recovered from the skin surface, although a larger volunteer number would be required to confirm gender differences within each ethnic group. A consistency in the amount of RSSC recovered from the skin surface combined with its rapid analysis by GC-MS indicates that RSSC may provide a non-invasive matrix to identify exposure to exogenous materials present in the external environment or secreted from within the body. The use of RSSC as a bio-monitoring matrix could lead to a quick and non-invasive means of identifying exposure to harmful chemicals. Further work on the variation in the composition of RSSC in normal and diseased individuals is required in order to determine the potential use of RSSC as a diagnostic or prognostic indicator.
Acknowledgments
The authors would like to express their gratitude to all volunteers with special thanks to Norton Canes High School (Norton Canes), Rooks Heath College (Harrow) and Sir John Lawes School (Harpenden) for their participation in this study.
References
- Williams A. Transdermal and topical drug delivery: from theory to clinical practice. London, UK: Pharmaceutical Press London; 2003. [Google Scholar]
- Chilcott R. Cutaneous anatomy and function. In: Chilcott R, Price S, editors. Principles and practice of skin toxicology. West Sussex, England: Wiley Online Library; 2008. pp. 4–16. [Google Scholar]
- Clarys P, Barel A. Quantitative evaluation of skin surface lipids. Clin Dermatol. 1995;13:307–321. doi: 10.1016/0738-081x(95)00079-u. [DOI] [PubMed] [Google Scholar]
- Wertz PW, Michniak BB. Sebum. In: Elsner P, Maibach HI, editors. Cosmeceuticals: drugs vs. cosmetics. New York: Marcel and Dekker Inc; 2004. pp. 45–56. [Google Scholar]
- Stewart ME, Downing DT. Measurement of sebum secretion rates in young children. J Invest Dermatol. 1985;84:59–61. doi: 10.1111/1523-1747.ep12274825. [DOI] [PubMed] [Google Scholar]
- Stewart ME, Downing DT, Pochi PE, Strauss JS. The fatty acids of human sebaceous gland phosphatidylcholine. Biochim Biophys Acta. 1978;529:380–386. doi: 10.1016/0005-2760(78)90082-6. [DOI] [PubMed] [Google Scholar]
- Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variations in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970;54:240–247. doi: 10.1111/1523-1747.ep12280318. [DOI] [PubMed] [Google Scholar]
- Kligman AM. The uses of sebum. Br J Dermatol. 1963;75:307–319. doi: 10.1111/j.1365-2133.1963.tb13567.x. [DOI] [PubMed] [Google Scholar]
- Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416. doi: 10.1152/physrev.1989.69.2.383. [DOI] [PubMed] [Google Scholar]
- Porter AM. Why do we have apocrine and sebaceous glands? J R Soc Med. 2001;94:236. doi: 10.1177/014107680109400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kydonieusb A, Wille JJ. Palmitoleic acid isomer (C16: 1¢ 6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol Appl Skin Physiol. 2003;16:176–187. doi: 10.1159/000069757. [DOI] [PubMed] [Google Scholar]
- Rode B, Ivens U, Serup J. Degreasing method for the seborrheic areas with respect to regaining sebum excretion rate to casual level. Skin Res Technol. 2000;6:92–97. doi: 10.1034/j.1600-0846.2000.006002092.x. [DOI] [PubMed] [Google Scholar]
- Faergemann J, Zehender H, Denouel J, Millerioux L. Levels of terbinafine in plasma, stratum corneum, dermis-epidermis (without stratum corneum), sebum, hair and nails during and after 250 mg terbinafine orally once per day for four weeks. Acta Derm Venereol. 1993;73:305. doi: 10.2340/000155557300304. [DOI] [PubMed] [Google Scholar]
- Iida T, Hirakawai H, Matsueda T, Takenaka S, Yu ML, Leon Guo YL. Recent trend of polychlorinated dibenzo-p-dioxins and their related compounds in the blood and sebum of Yusho and Yu-Cheng patients. Chemosphere. 1999;38:981–993. doi: 10.1016/s0045-6535(98)00360-9. [DOI] [PubMed] [Google Scholar]
- Cauwenbergh G, Degreef H, Heykants J, Woestenborghs R, van Rooy P, Haeverans K. Pharmacokinetic profile of orally administered itraconazole in human skin. J Am Acad Dermatol. 1988;18:263–268. doi: 10.1016/s0190-9622(88)70037-7. [DOI] [PubMed] [Google Scholar]
- Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J Invest Dermatol. 1999;113:1006–1010. doi: 10.1046/j.1523-1747.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- Hodgson-Jones I, Wheatley V. Studies of sebum. 3. Methods for the collection and estimation of small amounts of sebum. Biochem J. 1952;52:460. doi: 10.1042/bj0520460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing DT, Strauss JS, Pochi PE. Variability in the chemical composition of human skin surface lipids. J Invest Dermatol. 1969;53:322–327. doi: 10.1038/jid.1969.157. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Pochi PE. The quantitative gravimetric determination of sebum production. J Invest Dermatol. 1961;36:293–298. [Google Scholar]
- Cunliffe W, Shuster S. The rate of sebum excretion in man. Br J Dermatol. 1969;81:697–704. doi: 10.1111/j.1365-2133.1969.tb16211.x. [DOI] [PubMed] [Google Scholar]
- Lookingbill D, Cunliffe W. A direct gravimetric technique for measuring sebum excretion rate. Br J Dermatol. 1986;114:75–81. doi: 10.1111/j.1365-2133.1986.tb02781.x. [DOI] [PubMed] [Google Scholar]
- Ramasastry P, Downing D, Pochi P, Strauss J. Chemical composition of human skin surface lipids from birth to puberty. J Invest Dermatol. 1970;54:139–144. doi: 10.1111/1523-1747.ep12257164. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Miller DL. Sebutape: a device for visualising and measuring human sebaceous secretion. J Soc Cosmet Chem. 1986;37:369–374. [Google Scholar]
- Jacobsen E, Billings JK, Frantz RA, Kinney CK, Stewart ME, Downing DT. Age-related changes in sebaceous wax ester secretion rates in men and women. J Invest Dermatol. 1985;85:483–485. doi: 10.1111/1523-1747.ep12277224. [DOI] [PubMed] [Google Scholar]
- James A, Wheatley V. Studies of sebum. 6. The determination of the component fatty acids of human forearm sebum by gas–liquid chromatography. Biochem J. 1956;63:269. doi: 10.1042/bj0630269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Jubeli R, Bleton J, Baillet-Guffroy A. High-temperature gas chromatography-mass spectrometry for skin surface lipids profiling. J Lipid Res. 2011;52:143–151. doi: 10.1194/jlr.D008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A, Fulton J. Sebum: analysis by infrared spectroscopy. J Invest Dermatol. 1973;60:115–120. doi: 10.1111/1523-1747.ep12682018. [DOI] [PubMed] [Google Scholar]
- Roh M, Han M, Kim D, Chung K. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890–894. doi: 10.1111/j.1365-2133.2006.07465.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm KP, Cua AB, Maibach HI. Skin aging: effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol. 1991;127:1806. doi: 10.1001/archderm.127.12.1806. [DOI] [PubMed] [Google Scholar]
- Lu GW, Valiveti S, Spence J, Zhuang C, Robosky L, Wade K, Love A, Hu LY, Pole D, Mollan M. Comparison of artificial sebum with human and hamster sebum samples. Int J Pharm. 2009;367:37–43. doi: 10.1016/j.ijpharm.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Harvey CJ, Wertz PW. Formulation and stability of a novel artificial sebum under conditions of storage and use. Int J Cosmet Sci. 2010;32:347–355. doi: 10.1111/j.1468-2494.2010.00561.x. [DOI] [PubMed] [Google Scholar]
- Buckley WR, Lewis CE. The “ruster” in industry. J Occup Med. 1960;2:23–31. [PubMed] [Google Scholar]
- Wakefield JC, Kaur, K, Chilcott RP. A preliminary study for assessing the feasibility of sebum sampling for monitoring human exposure to environmental chemicals following inadvertent or malicious release. Toxicology. 2008;253:123. [Google Scholar]
- Sakai S, Endo Y, Ozawa N, Sugawara T, Kusaka A, Sayo T, Tagami H, Inoue S. Characteristics of the epidermis and stratum corneum of hairless mice with experimentally induced diabetes mellitus. J Invest Dermatol. 2003;120:79–85. doi: 10.1046/j.1523-1747.2003.12006.x. [DOI] [PubMed] [Google Scholar]
- Seirafi H, Farsinejad K, Firooz A, Davoudi SM, Robati RM, Hoseini MS, Ehsani AH, Sadr B. Biophysical characteristics of skin in diabetes: a controlled study. J Eur Acad Dermatol Venereol. 2009;23:146–149. doi: 10.1111/j.1468-3083.2008.02950.x. [DOI] [PubMed] [Google Scholar]
- Household Questionnaire- England Census 2011. 2011. Available from: http://www.ons.gov.uk/ons/guide-method/census/2011/the-2011-census/2011-census-questionnaire-content/index.html. [accessed on 18 December 2011]
- Vaule H, Leonard S, Traber M. Vitamin E delivery to human skin: studies using deuterated [alpha]-tocopherol measured by APCI LC-MS. Free Radic Biol Med. 2004;36:456–463. doi: 10.1016/j.freeradbiomed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Jacobi U, Gautier J, Sterry W, Lademann J. Gender-related differences in the physiology of the stratum corneum. Dermatology. 2007;211:312–317. doi: 10.1159/000088499. [DOI] [PubMed] [Google Scholar]
- Prajapati B, Dunne M, Armstrong R. Sample size estimation and statistical power analyses. Optometry Today. 2010;16:123–132. [Google Scholar]
- Marrakchi S, Maibach HI. Biophysical parameters of skin: map of human face, regional, and age related differences. Contact Dermatitis. 2007;57:28–34. doi: 10.1111/j.1600-0536.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- Cotterill J, Cunliffe W, Williamson B, Bulusu L. Age and sex variation in skin surface lipid composition and sebum excretion rate. Br J Dermatol. 1972;87:333–340. doi: 10.1111/j.1365-2133.1972.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Li S, Guz NV, Sokolov I. A modified in vitro stripping method to automate the calculation of geometry of corneocytes imaged with fluorescent microscopy: example of moisturizer treatment. Skin Res Technol. 2011;17:213–219. doi: 10.1111/j.1600-0846.2010.00487.x. [DOI] [PubMed] [Google Scholar]
- Cunliffe W, Cotterill J, Williamson B. Variations in skin surface lipid composition with different sampling techniques—I. Br J Dermatol. 1971;85:40–45. doi: 10.1111/j.1365-2133.1971.tb07176.x. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Kellum R. Skin lipids. I. Sampling problems of the skin and its appendages. J Am Oil Chem Soc. 1965;42:685–690. doi: 10.1007/BF02540041. [DOI] [PubMed] [Google Scholar]
- Nouveau S, Bastien P, Baldo F, de Lacharriere O. Effects of topical DHEA on aging skin: a pilot study. Maturitas. 2008;59:174–181. doi: 10.1016/j.maturitas.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Giltay E, Gooren L. Effects of sex steroid deprivation/administration on hair growth and skin sebum production in transsexual males and females. J Clin Endocrinol Metab. 2000;85:2913. doi: 10.1210/jcem.85.8.6710. [DOI] [PubMed] [Google Scholar]
- Piérard-Franchimont C, Piérard G. Postmenopausal aging of the sebaceous follicle: a comparison between women receiving hormone replacement therapy or not. Dermatology. 2000;204:17–22. doi: 10.1159/000051804. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Pochi P. The human sebaceous gland: its regulation by steroidal hormones and its use as an end organ for assaying androgenecity in vivo. Recent Prog Horm Res. 1963;19:385. [PubMed] [Google Scholar]
- Man MQ, Xin SJ, Song SP, Cho SY, Zhang XJ, Tu CX, Feingold KR, Elias PM. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22:190–199. doi: 10.1159/000231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand GG, Levine MJ, Miyamoto K. The age dependent changes in skin condition in African Americans, Asian Indians, Caucasians, East Asians & Latinos. IFSCC Mag. 2001;4:259–266. [Google Scholar]
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79–93. doi: 10.1111/j.1467-2494.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- Millns JL, Maibach HI. Mechanisms of sebum production and delivery in man. Arch Dermatol Res. 1982;272:351–362. doi: 10.1007/BF00509067. [DOI] [PubMed] [Google Scholar]
- Eberhardt H. The regulation of sebum excretion in man. Arch Dermatol Res. 1974;251:155–164. doi: 10.1007/BF00560397. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Shelley WB. An investigation of the biology of the human sebaceous gland. J Invest Dermatol. 1958;30:99–125. [PubMed] [Google Scholar]
- Harris HH, Downing DT, Stewart ME, Strauss JS. Sustainable rates of sebum secretion in acne patients and matched normal control subjects. J Am Acad Dermatol. 1983;8:200–203. doi: 10.1016/s0190-9622(83)70023-x. [DOI] [PubMed] [Google Scholar]