Abstract
Objective
To describe and profile abnormalities of the lumbar spine in a cohort of patients with enthesitis-related arthritis (ERA) as compared to a control group of adolescents with mechanical back pain.
Methods
We performed a retrospective review of magnetic resonance imaging (MRI) lumbar spine scans of 79 patients (58 cases, 21 controls). The study was covered by institutional review board approval and informed consent was obtained for review of all clinical investigations. Images were reviewed by an expert MRI reader who was blinded to clinical details. The presence or absence of morphologic features of enthesitis, apophyseal joint synovitis, and inflammation of posterior elements was assessed at each lumbar vertebral level. The apophyseal joint inflammation was graded from 0 to 3 using a grading system that was adapted from one used in adults with inflammatory facet osteoarthropathy. STATA software was used for data analysis.
Results
One or more abnormalities of the lumbar spine were found in 39 (67%) of 58 cases and sacroiliitis was present in 45 (78%) of the cases. Apophyseal joint synovitis was seen in 22 (38%) cases and in 1 (5%) control patient. This difference was highly significant (P = 0.004). Inflammatory changes in the interspinous ligaments were seen in a higher percentage of cases than controls and this observation was of statistical significance (P = 0.04).
Conclusion
Statistically significant inflammation of the lumbar apophyseal joints and interspinous ligaments was seen in our cohort of ERA patients, most of whom have concurrent sacroiliitis. This could be contributing to back pain in these patients.
INTRODUCTION
Enthesitis-related arthritis (ERA) is a subtype of juvenile inflammatory arthritis (JIA) as defined by the International League of Associations for Rheumatology (ILAR) classification criteria for childhood arthritis (1). It is characterized by enthesitis and an inflammatory arthritis most often affecting lower extremities and sacroiliac (SI) joints, with disease onset before age 16 years. It has a male propensity and is often associated with HLA–B27 positivity. Enthesitis, which refers to inflammation of the areas of attachment of ligaments, tendons, and fascia to bone, is an important feature of the disease.
Very little is known about the natural history of ERA. The pathogenesis of ERA is poorly defined, although it is likely to involve genetic (2) and immunologic (3) mechanisms that overlap with that of ankylosing spondylitis, given the similarities in clinical phenotype.
Radiologic assessment of the adolescent spine is challenging as the appearances of disease must be distinguished from the changes seen in the maturing skeleton. Imaging is used in ERA patients presenting with back pain to exclude other causes of adolescent back pain (such as Scheuermann's disease, mechanical causes, tumor, and osteoid osteoma) (4) and to assess spinal inflammation and sacroiliitis.
Magnetic resonance imaging (MRI) has emerged as the most sensitive imaging modality to detect inflammatory arthritides and to discriminate between acute and chronic inflammation. In children with ERA, contrast-enhanced MRI has been shown to be sensitive for the early detection of sacroiliitis (5–7).
To our knowledge, there are no published data on the detailed MRI features of lumbar spinal changes in patients with ERA. The aim of this case–control study was to describe and profile abnormalities of the lumbar spine in a cohort of patients with ERA as compared to a control group of adolescents with mechanical back pain.
Box Significance & Innovations.
There is a statistically significant difference (P = 0.004) in the prevalence of lumbar apophyseal joint synovitis in patients with enthesitis-related arthritis (ERA) as compared to controls.
Apophyseal joint synovitis can occur in the absence of sacroiliitis.
Inflammatory changes of the apophyseal joints could be visualized only on the postcontrast images in 70% of ERA cases. Therefore, imaging protocols should include contrast-enhanced images.
Lumbar spine inflammatory disease should be considered as a cause of back pain in patients with ERA, independent of the presence of sacroiliitis.
SUBJECTS AND METHODS
Subjects
We performed a retrospective case–control study and reviewed MRI lumbar spine scans of 79 patients (58 cases and 21 controls). The study was covered by Institutional Review Board approval from the National Research Ethics Service Committee (London-Bentham, England). Informed consent was obtained for review of all clinical investigations.
All patients underwent MRI of the lumbar spine and SI joints as part of routine clinical care at our institution between December 2006 and May 2012. In both the cases and the controls the indication for the MRI was low back pain. We analyzed the initial MRI performed at presentation.
Cases: ERA group
All cases were outpatients attending a specialist adolescent rheumatology clinic at our institution and all fulfilled the ILAR criteria for ERA (1).
Control group
The controls were adolescent patients attending the same specialist rheumatology clinic with lower back pain present for at least 1 month. All patients in this control group underwent MRI lumbar spine scans during the same time period as the cases and had a diagnosis of noninflammatory back pain.
Matching
This is an unmatched case–control study. However, the demographic characteristics of the controls are similar to those of the cases, although there is a higher preponderance of male patients in the cases (see Results).
MRI technique
MRI of the lumbar spine and SI joints was performed using a 1.5T system (Avanto). The protocol consisted of the following sequences: 1) lumbar spine: sagittal T1-weighted spin-echo (repetition time [TR] 400 msec, time to echo [TE] 10 msec, slice thickness 4.0 mm, number of slices 13, flip angle 90°, matrix 256 × 256 pixels, 2 signals averaged), sagittal STIR turbo spin-echo (TR/TE 4,000/74 msec, slice thickness 4 mm, number of slices 13, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged), and contrast-enhanced sagittal T1-weighted spin-echo with fat saturation (TR/TE 510/10 msec, slice thickness 4 mm, number of slices 13, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged); and 2) SI joints (angled to the SI joints): coronal T1-weighted turbo spin-echo (TR/TE 610/11 msec, slice thickness 3 mm, number of slices 18, flip angle 90°, matrix 256 × 256 pixels, 2 signals averaged), coronal T1 turbo inversion recovery (TR/TE 4,340/83 msec, slice thickness 4 mm, number of slices 14, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged), axial T1-weighted turbo spin-echo (TR/TE 475/11 msec, slice thickness 5 mm, number of slices 20, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged), axial STIR (TR/TE 6,070/83 msec, slice thickness 5 mm, number of slices 18, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged), contrast-enhanced axial (TR/TE 619/11 msec, slice thickness 5 mm, number of slices 20, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged), and coronal T1-weighted turbo spin-echo fat-saturated (TR/TE 795/11 msec, slice thickness 3 mm, number of slices 18, flip angle 150°, matrix 256 × 256 pixels, 2 signals averaged). The contrast agent used was gadoterate meglumine, which was administered as an intravenous bolus at a dose of 0.2 mmoles/kg.
Postcontrast imaging was obtained in 65 of the 79 patients. Contrast-enhanced images could not be obtained in 11 cases and in 3 controls as they either refused to be cannulated or the scan had to be terminated prematurely due to claustrophobia. Total imaging time of the lumbar spine was 11–12 minutes and imaging time of the SI joints was 22 minutes.
Image analysis
All images were reviewed by a consultant radiologist (MAH-C) with more than 20 years of musculoskeletal MRI experience and with expertise in adolescent spine imaging. Scans were presented randomly to the reader, who was blinded to the clinical details.
Images were analyzed on a PACS workstation (IMPAX ES, Agfa-Gevaert). The presence or absence of morphologic features of enthesitis, apophyseal joint synovitis, and inflammation of the posterior elements was assessed at each vertebral level in the lumbar spine. Specifically, the features were assessed as described below.
Apophyseal joints
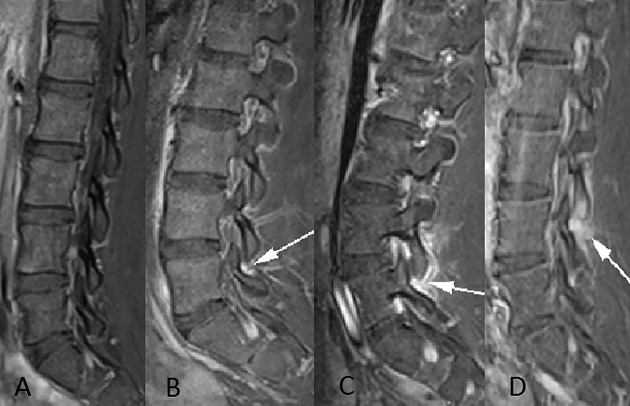
Articular processes were assessed for edema, seen as high signal (compared to muscle) on STIR and contrast-enhanced images. Contrast-enhanced images were evaluated for enhancing synovitis. Grading of the apophyseal joint inflammation (Figure 1) was adapted from a grading system used in adult patients with inflammatory facet osteoarthropathy (8) as follows: grade 0 = normal (Figure 1A), grade 1 = high signal confined to the joint capsule (Figure 1B), grade 2 = intraarticular and periarticular signal abnormality (Figure 1C), and grade 3 = grade 2 plus bone marrow edema of articular process (Figure 1D).
Figure 1.
Sagittal contrast-enhanced T1-weighted fat-saturated images of the lumbar spine in 4 different patients demonstrate the grades of apophyseal synovitis. A, Normal apophyseal joint. B, Abnormal enhancement (arrow) is confined within the apophyseal joint capsule indicating a grade 1 synovitis, clearly showing inflammation as compared to the normal apophyseal joint (A). C, Grade 2 synovitis with intraarticular synovial enhancement and periarticular high signal (arrow). D, Marked synovial enhancement and bone edema of the inferior articular process (arrow), indicating a grade 3 synovitis.
We have combined grades 2 and 3 used in the original classification as we are of the opinion that differentiating between less than or more than 50% of the joint perimeter affected by edema (as described in the study by Czervionke and Fenton 8) is too subjective and not reliable in adolescents.
End plates
Edema and enhancement of the anterior and posterior margins of the vertebral end plates were assessed. This finding indicates enthesitis and was recorded in a dichotomous manner (present/absent).
Posterior elements
Posterior structures, i.e., lamina, pedicles, spinous processes, and interspinous ligaments, were evaluated for edema on STIR and for enhancement on the postcontrast images. The presence of any of these findings in ≥1 of the posterior structures indicates inflammation. The enhancement in the interspinous ligaments was graded subjectively as no enhancement, a “blush” (defined as a faint area of increased signal on the contrast-enhanced images) (Figure 2A), or definite enhancement (defined as an intense band of confluent enhancement) (Figure 2B).
Figure 2.
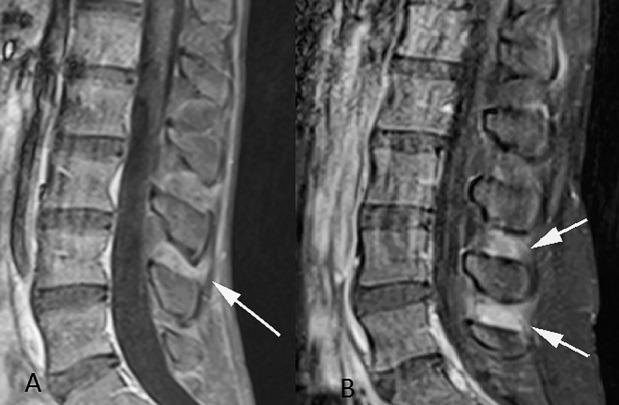
Sagittal contrast-enhanced T1-weighted fat-saturated images of the lumbar spine. A, A faint blush of enhancement (arrow) in the interspinous ligaments. B, A band of intense enhancement (arrows) of the interspinous ligaments.
Each of the inflammatory lesions described above had to be present on ≥2 consecutive sagittal slices in order to be considered as definite inflammation. The descriptions of inflammation that we have used are in agreement with definitions of spinal inflammatory lesions (on STIR images) in axial spondyloarthritis as described by the Assessment of SpondyloArthritis international Society (ASAS)/Outcome Measures in Rheumatology (OMERACT) MRI Study Group (9).
A reference sheet containing representative images of the various grades of apophyseal joint synovitis and inflammation of end plates and posterior elements was utilized during image analysis and scoring to ensure consistency of scoring. It was recorded whether the apophyseal joint changes were present at single or multiple levels and whether they were unilateral or bilateral, along with grading of the severity (as described above). Similarly, for the end-plate changes, it was recorded if the changes were present at a single level or at multiple levels. The presence or absence of sacroiliitis, as evidenced by erosions of the joint articular surface, bone marrow edema, and enhancement (10), was also recorded for each of the 79 patients.
Statistical analysis
The chi-square test was used to determine if the difference in the prevalence of inflammation between the cases and the controls was significant at a 5% level. Data analysis was done by the lead author (KV) using STATA software.
RESULTS
Patients
There were 58 ERA cases with a mean age of 16.7 years (range 8.3–21.9 years, median 16.5 years); 50 males with a mean age of 16.8 years (range 8.3–21.9 years) and 8 females with a mean age of 16.1 years (range 13.1–18.4 years). The average duration between disease diagnosis and date of MRI scan was 3.8 years (range 1 month to 13.2 years, median 3.0 years).
Only 8 (14%) of the 58 ERA cases were females, which is what is typically observed in this disease (2). The HLA–B27 status was unknown in 4 of the 58 cases as this information was not recorded in the available clinical notes. In the remainder of the 54 ERA cases, 74% (n = 40) had HLA–B27 positivity. This is similar to that reported in the literature (2). In our cohort, 95% (55 of 58) of the cases had peripheral arthritis, 47% (27 of 58) had enthesitis, 7% (4 of 58) had inflammatory bowel disease, and 9% (5 of 58) had uveitis, which was an acute anterior uveitis in 3 of the 5 cases and a chronic uveitis in 2 of the 5 cases. All of the cases had low back pain. At the time of the MRI scan, 51 of the 58 cases were taking some form of medication. Biologic agents were prescribed in 16% (9 of 58). These were all tumor necrosis factor (TNF) blockade therapies and 7 of the 9 biologic agents were prescribed in combination with methotrexate. Disease-modifying antirheumatic drugs (DMARDs) were prescribed in 34% of cases (20 of 58; of these, 40% [n = 8] were taking sulphasalazine and 60% [n = 12] were taking methotrexate) and only nonsteroidal antiinflammatory drugs were prescribed in 38% of cases (22 of 58). The remaining 12% of cases (7 of 58) were not receiving any drug treatment.
There were 21 patients in the control group, with a mean age of 15.5 years (range 10.8–18.1 years, median 15.7 years); 14 males with a mean age of 15.6 years (range 10.8–18.1 years) and 7 females with a mean age of 15.3 years (range 14.5–17.2 years).
Cases
One or more abnormalities of the lumbar spine were found in 39 (67%) of the 58 ERA cases reviewed (Table1). In 19 (33%) of the 58 cases the lumbar spine was normal. Forty-five (78%) of 58 cases had definite sacroiliitis, indeterminate changes (showing one small focus of edema only) were seen in 6 cases (10%), and in 7 cases (12%) the SI joints were normal. Five (71%) of the 7 cases with normal SI joints had abnormalities of the lumbar spine (Schmorl's nodes [n = 1], apophyseal joint synovitis [n = 3], and interspinous ligaments inflammation [n = 1]). In total, 54 (93%) of the cases had either an abnormal lumbar spine or inflammation of the SI joints. Among these, 30 cases had both sacroiliitis and abnormalities of the lumbar spine.
Table 1.
Summary of abnormalities in the lumbar spine and SI joints of cases and controls*
| Abnormality | Cases (n = 58) | Controls (n = 21) | P |
|---|---|---|---|
| Apophyseal joint synovitis | 22 (38) | 1 (5) | 0.004 |
| Inflammation in posterior elements | 15 (26) | 1 (5) | 0.04 |
| Blush in interspinous ligaments | 12 (21) | 7 (33) | 0.25 |
| Marginal end-plate edema | 4 (7) | 0 (0) | 0.22 |
| Disc abnormalities | 26 (45) | 7 (33) | 0.36 |
| Definite sacroiliitis | 45 (78) | 0 (0) | 0.0001 |
Values are the number (percentage) unless indicated otherwise. SI = sacroiliac.
By chi-square test.
Among the 19 cases with a normal lumbar spine, 15 had definite sacroiliitis and 2 had indeterminate SI joint changes. Only 2 ERA cases had an entirely normal lumbar spine and normal SI joints. Eleven of the 58 ERA cases did not have postcontrast imaging and in these patients inflammatory lesions were assessed on STIR images (Figure 3).
Figure 3.
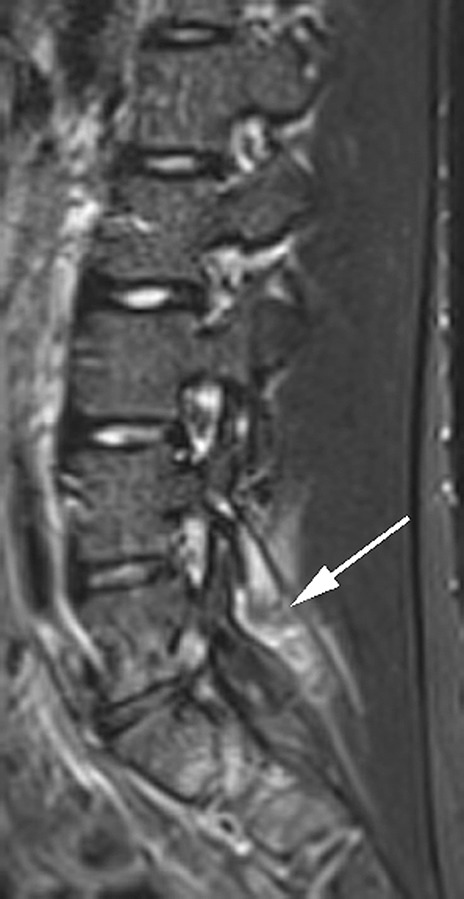
Sagittal STIR image of the lumbar spine shows grade 3 synovitis (arrow) of L4–L5 apophyseal joint.
Apophyseal joints
Of the 58 cases reviewed, 22 (38%) had apophyseal joint synovitis on MRI (Figure 1). The grading of the apophyseal abnormalities and their relationship to sacroiliitis and inflammatory lesions in the posterior elements are summarized in Table2. Among patients with abnormal apophyseal joints (n = 22), grade 1 and grade 2 lesions were the most common, comprising 77% (17 of 22) of apophyseal joint disease, compared to grade 3 synovitis, which was present in only 5 cases (23%).
Table 2.
Prevalence of apophyseal joint synovitis in ERA cases*
| Apophyseal synovitis grade | Cases, no. | Synovitis plus definite enhancement of posterior elements | Synovitis plus sacroiliitis | Synovitis plus indeterminate SI joints | Synovitis plus normal SI joints |
|---|---|---|---|---|---|
| Grade 3 | 5 | 80 (4/5) | 100 (5/5) | 0 (0/5) | 0 (0/5) |
| Grade 2 | 9 | 33 (3/9) | 89 (8/9) | 11 (1/9) | 0 (0/9) |
| Grade 1 | 8 | 38 (3/8) | 50 (4/8) | 25 (2/8) | 25 (2/8) |
| Grade 0 | 36 | 14 (5/36) | 78 (28/36) | 8 (3/36) | 14 (5/36) |
Values are the percentage (number/total number) unless indicated otherwise. ERA = enthesitis-related arthritis; SI = sacroiliac.
Apophyseal joint synovitis was present at multiple levels in 12 of 22 cases and at a single level in the remainder. Bilateral inflammatory changes, not necessarily at the same segmental level, were seen in 9 of 12 cases with multilevel disease and in 4 of 10 cases with apophyseal joint synovitis at a single level. This difference was not significant (P = 0.10). Erosion of an inferior articular process was seen in 1 ERA patient with multilevel grade 1 synovitis. Definite sacroiliitis was present in 17 (77%) of 22 cases with apophyseal synovitis. In the remaining 5 cases with apophyseal synovitis there was no MRI evidence of definite sacroiliitis (normal SI joints in 2 cases and indeterminate SI joints in 3 cases).
Contrast-enhanced images were available in 20 of 22 cases with apophyseal synovitis. In 14 (70%) of the 20 cases with contrast-enhanced images, inflammatory changes could be visualized only on the postcontrast images and were not apparent on the STIR images (Table3).
Table 3.
Comparison of apophyseal joint synovitis on STIR and postcontrast images in ERA cases with contrast-enhanced imaging (n = 20)*
| Grade of apophyseal synovitis | Apophyseal synovitis seen on STIR | Apophyseal synovitis seen on postcontrast images |
|---|---|---|
| Grade 1 (n = 8) | 13 (1/8) | 100 (8/8) |
| Grade 2 (n = 8) | 38 (3/8) | 100 (8/8) |
| Grade 3 (n = 4) | 50 (2/4) | 100 (4/4) |
Values are the percentage (number/total number). ERA = enthesitis-related arthritis.
Posterior elements
Enhancement of the posterior elements was present in 15 (26%) of 58 cases. In 10 cases there was an intense band of enhancement limited to the interspinous ligaments, in 1 case only the spinous process was enhanced, and in the other 4 cases there was enhancement of interspinous ligaments and spinous processes. A “blush” of enhancement was seen in the interspinous ligaments in 12 (21%) of 58 cases. Four of 58 cases had a pars defect with signal changes in the intervertebral disc related to this.
Discs and end plates
Intervertebral disc and end-plate changes were seen in 26 (45%) of 58 cases with ERA. Purely mechanical changes, i.e., disc desiccation, were present in 12 cases, and in 3 of these, disc desiccation was the only abnormality of the lumbar spine. Eighteen cases had end-plate defects (Schmorl's nodes) and 4 of these were edematous. Marginal end-plate edema was present in 4 of 58 cases. All 4 patients had associated sacroiliitis and inflammatory changes of the apophyseal joints and/or the posterior elements.
Controls
The most prevalent abnormality in the control group was changes in the intervertebral discs. No control patient showed definite sacroiliitis; 18 controls had normal SI joints and 3 had indeterminate changes.
Apophyseal joints
Apophyseal joint synovitis (grade 1) was present in 1 of 21 controls.
Posterior elements
Only 1 control patient showed intense enhancement of the interspinous ligaments. Another control showed a small focus of edema in a lumbar spinous process and this was considered to be indeterminate. A blush of enhancement in the interspinous ligaments was seen in 7 (33%) of 21 controls.
Discs and end plates
Seven (33%) of 21 controls showed mechanical disc abnormalities, including disc bulge, disc desiccation, torn annulus, and an isolated Schmorl's node. A transitional lumbosacral vertebra was seen in 2 controls. None of the patients in the control group showed marginal edema of the end plates.
Cases versus controls
The key findings seen in both groups are summarized in Table1. Apophyseal joint synovitis was more common in patients with ERA compared to the control group. This difference was highly significant at P = 0.004. Intense interspinous ligament enhancement was also statistically more common in ERA cases compared to the controls (P = 0.04). The difference between the cases and controls in the prevalence of disc changes and end-plate edema was not statistically significant.
DISCUSSION
To our knowledge, this is the first detailed MRI study to assess the prevalence of inflammatory lesions in the lumbar spine in patients with ERA. The onset of ERA typically occurs in late childhood and adolescence. The mean age (16.7 years) and male preponderance (86% [n = 50 of 58]) of ERA cases in our study is similar to other published cohorts (7). The prevalence of MRI-detected sacroiliitis in our series (78% [n = 45 of 58]) is similar to another recent study where sacroiliitis was found in 80% of ERA patients with inflammatory back pain (7). In our cohort the majority of ERA cases with sacroiliitis had abnormalities of the lumbar spine (67% [n = 30 of 45]).
We have shown that apophyseal joint synovitis occurs significantly more commonly in patients with ERA than in a control group (P = 0.004). Most patients with apophyseal synovitis had sacroiliitis and all patients with grade 3 apophyseal joint inflammation had sacroiliitis. We have also shown that inflammatory changes in the interspinous ligaments (attachment sites of these ligaments to spinous processes are entheses) occur in a higher percentage of cases compared to controls and this difference is statistically significant (P = 0.04). These observations suggest that lumbar spine inflammatory disease could be contributing to back pain in patients with ERA and this has not been described previously.
Our findings also indicate that contrast-enhanced sequences should be routinely performed as part of the imaging protocol in these patients. We found that contrast-enhanced images were more sensitive than STIR imaging, as inflammatory changes in the apophyseal joints could be seen only on the postcontrast images in 70% (14 of 20) of cases with apophyseal synovitis (Table3). A blush of the interspinous ligaments was seen as commonly in cases and controls, with no significant difference between the two groups. This pattern of enhancement is likely to be within normal limits.
Apophyseal joints are synovial joints and their involvement is a recognized feature of ankylosing spondylitis (AS), the prototype of spondyloarthropathies. Studies in AS patients have shown a good correlation between histologic interstitial edema and MRI edema of the apophyseal joints (11) and that involvement of zygaphopyseal joints restricts spinal mobility (12,13). Inflammation of the interspinous ligaments has also been documented in AS (14).
Currently there is no robust scoring method for evaluating inflammatory lesions of the apophyseal joints in children. The grading criteria that we have used have been adapted from a classification system in adults (8) and from the ASAS/OMERACT MRI Study Group definition of apophyseal joint arthritis in axial spondyloarthritis (10). We found our grading system simple to use and effective for quantifying inflammatory changes in the apophyseal joints.
Recently it has been observed by Fisher et al that there are possibly 2 clinical phenotypes of ERA: those with axial disease (aERA) and a high prevalence of HLA–B27 positivity and a second group that has a significantly lower prevalence of HLA–B27 positivity with peripheral arthritis and enthesitis being the dominant features (15). The authors found that extraarticular manifestations, such as acute anterior uveitis and inflammatory bowel disease, occurred only in the aERA group. Differences in management were also observed, with the aERA group requiring a higher proportion of anti-TNF treatment (15). Two distinct patterns of ERA have also been recognized and described recently by other groups (7). Larger studies are required to further characterize these 2 clinical phenotypes and to determine if there are any differences in the imaging features.
Long-term followup of children with ERA has shown poor outcome in this subtype of JIA. Flato et al found that ERA patients had higher levels of physical disability and reported a higher intensity of pain compared with other types of JIA (16). Therefore, it is important to intervene early and commence treatment. However, although there are clinical criteria as defined by ILAR to diagnose ERA, there are currently no adequate radiologic methods to measure severity of disease or for longitudinal assessment of these patients, particularly in the context of monitoring response to therapy.
In children with ERA, DMARDs do not alter axial disease progression (17), whereas anti-TNF therapy is showing some promise. Tse et al (18) reported sustained clinical improvement and radiologic remission of enthesitis and synovitis on MRI at 2-year followup in a single ERA patient on etanercept therapy. It may be that there is a window of opportunity for therapeutic intervention in early disease, which, if present, could lead to a reduction in disability and enhance the quality of life in these children. MRI may provide an invaluable tool to aid early diagnosis and, importantly, to monitor response to treatment, as well as aiding the validation of future disease activity tools.
A limitation of this study is that detailed information relating to back pain in terms of localization (e.g., buttock pain versus lumbar pain) and severity using scoring methods of inflammatory back pain was unavailable. Although there is no validated scoring system for inflammatory back pain in children or adolescents with ERA, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Bath Ankylosing Spondylitis Functional Index (BASFI) have shown good interrater reliability in children with ERA, but remain to be validated in this age group (19). It would be of interest to correlate BASDAI and BASFI scores to the abnormalities we have observed in the lumbar spine in patients with ERA and also scores of spinal mobility, such as Schober's test. A further limitation of this study is that the patient numbers are relatively small, yet this is among the largest cohorts reported.
In conclusion, in this study we have described statistically significant inflammation of the apophyseal joints and interspinous ligaments of the lumbar spine in a cohort of ERA patients, most of whom have concurrent sacroiliitis. This is a previously undescribed finding and it is possible that inflammatory changes in the lumbar spine could be contributing to back pain in these patients.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Vendhan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Vendhan, Sen, Hall-Craggs.
Acquisition of data. Vendhan, Sen, Fisher, Ioannou, Hall-Craggs.
Analysis and interpretation of data. Vendhan, Sen, Hall-Craggs.
REFERENCES
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 31:390–2. [PubMed] [Google Scholar]
- 2.Fisher C, Ioannou Y, Hall-Craggs M, Sen D. Enthesitis related arthritis: a new era of understanding. Ann Paediatr Rheum. 1:8–16. [Google Scholar]
- 3.Mahendra A, Misra R, Aggarwal A. Th1 and Th17 predominance in the enthesitis-related arthritis form of juvenile idiopathic arthritis. J Rheumatol. 2004;36:1730–6. doi: 10.3899/jrheum.081179. [DOI] [PubMed] [Google Scholar]
- 4.Faingold R, Saigal G, Azouz EM, Morales A, Albuquerque PA. Imaging of low back pain in children and adolescents. Semin Ultrasound CT MR. 2012;25:490–505. doi: 10.1053/j.sult.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Bollow M, Biedermann T, Kannenberg J, Paris S, Schauer-Petrowski C, Minden K, et al. Use of dynamic magnetic resonance imaging to detect sacroiliitis in HLA-B27 positive and negative children with juvenile arthritides. J Rheumatol. 2009;25:556–64. [PubMed] [Google Scholar]
- 6.Bollow M, Braun J, Biedermann T, Mutze S, Paris S, Schauer-Petrowskaja C, et al. Use of contrast-enhanced MR imaging to detect sacroiliitis in children. Skeletal Radiol. 2004;27:606–16. doi: 10.1007/s002560050446. [DOI] [PubMed] [Google Scholar]
- 7.Pagnini I, Savelli S, Matucci-Cerinic M, Fonda C, Cimaz R, Simonini G. Early predictors of juvenile sacroiliitis in enthesitis-related arthritis. J Rheumatol. 1998;37:2395–401. doi: 10.3899/jrheum.100090. [DOI] [PubMed] [Google Scholar]
- 8.Czervionke LF, Fenton DS. Fat-saturated MR imaging in the detection of inflammatory facet arthropathy (facet synovitis) in the lumbar spine. Pain Med. 1998;9:400–6. doi: 10.1111/j.1526-4637.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 9.Hermann KG, Baraliakos X, van der Heijde DM, Jurik AG, Landewe R, Marzo-Ortega H, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis. 2010;71:1278–88. doi: 10.1136/ard.2011.150680. [DOI] [PubMed] [Google Scholar]
- 10.Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by ASAS/ OMERACT MRI group. Ann Rheum Dis. 2008;68:1520–7. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 11.Appel H, Loddenkemper C, Grozdanovic Z, Ebhardt H, Dreimann M, Hempfing A, et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res Ther. 2012;8:R143. doi: 10.1186/ar2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simkin PA, Downey DJ, Kilcoyne RF. Apophyseal arthritis limits lumbar motion in patients with ankylosing spondylitis. Arthritis Rheum. 2009;31:798–802. doi: 10.1002/art.1780310617. [DOI] [PubMed] [Google Scholar]
- 13.Russell AS, Jackson F. Computer assisted tomography of the apophyseal changes in patients with ankylosing spondylitis. J Rheumatol. 2006;13:581–5. [PubMed] [Google Scholar]
- 14.Hermann KG, Althoff CE, Schneider U, Zuhlsdorf S, Lembcke A, Hamm B, et al. Spinal changes in patients with spondyloarthritis: comparison of MR imaging and radiographic appearances. Radiographics. 1988;25:559–69. doi: 10.1148/rg.253045117. [DOI] [PubMed] [Google Scholar]
- 15.Fisher C, Ioannou J, Sen D. Enthesitis related arthritis: 2 distinct clinical phenotypes? Pediatr Rheumatol. 1986;9(Suppl 1):P151. [Google Scholar]
- 16.Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case–control study. Arthritis Rheum. 2005;54:3573–82. doi: 10.1002/art.22181. [DOI] [PubMed] [Google Scholar]
- 17.Burgos-Vargas R, Vazquez-Mellado J, Pacheco-Tena C, Hernandez-Garduno A, Goycochea-Robles MV. A 26-week randomized, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Ann Rheum Dis. 2011;61:941–2. doi: 10.1136/ard.61.10.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tse SM, Burgos-Vargas R, Laxer RM. Anti–tumor necrosis factor α blockade in the treatment of juvenile spondyloarthropathy. Arthritis Rheum. 2006;52:2103–8. doi: 10.1002/art.21121. [DOI] [PubMed] [Google Scholar]
- 19.Batthish M, Rachlis A, Wong B, Stevens S, Anderson M, Feldman BM, et al. Intra-rater reliability of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Bath Ankylosing Spondylitis Functional Index (BASFI) in children with spondyloarthritis. Pediatr Rheumatol. 2012;10(Suppl 1):A45. [Google Scholar]