Abstract
Advances in multimodal immunotherapy have significantly reduced acute rejection rates and substantially improved 1-year graft survival following renal transplantation. However, long-term (10-year) survival rates have stagnated over the past decade. Recent studies indicate that antibody-mediated rejection (ABMR) is among the most important barriers to improving long-term outcomes. Improved understanding of the roles of acute and chronic ABMR has evolved in recent years following major progress in the technical ability to detect and quantify recipient anti-HLA antibody production. Additionally, new knowledge of the immunobiology of B cells and plasma cells that pertains to allograft rejection and tolerance has emerged. Still, questions regarding the classification of ABMR, the precision of diagnostic approaches, and the efficacy of various strategies for managing affected patients abound. This review article provides an overview of current thinking and research surrounding the pathophysiology and diagnosis of ABMR, ABMR-related outcomes, ABMR prevention and treatment, as well as possible future directions in treatment.
This review addresses the spectrum of antibody-mediated rejection after kidney transplantation, including its pathogenesis, risk factors, phenotypes, the revised Banff 2013 classification, treatment options, and outcomes. Also see meeting report by Haas et al on page 272.
Keywords: Antibody-mediated rejection, complement C4d, donor-specific antibodies, phenotype
Introduction
The widespread use of potent and specific immunosuppressive agents has significantly reduced acute cellular rejection rates and substantially improved 1-year graft survival following renal transplantation. Substantial improvement of long-term (10-year) outcomes, however, has not been realized 1–4. A recent analysis of more than 250 000 North American renal transplant recipients showed that despite modest improvements in long-term graft survival between 1989 and 2005 5, and improvements in graft half-life in the past decade for both living and deceased donor transplants 6, high attrition rates persist that stubbornly limit recent progress 5.
The ongoing therapeutic challenge is to achieve effective and safe immunosuppression and avoid unwanted toxicities to produce enduring renal allograft function 7–9. The incidence of hyperacute rejection caused by preexisting anti-HLA donor-specific antibodies (DSA) has been nearly eliminated by crossmatch and compatibility matching strategies. Similarly, the incidence of acute T cell–mediated injury has been significantly reduced with the effective multimodal application of immunosuppressive agents. However, acute and chronic antibody-mediated rejection (ABMR) are playing an increasingly critical role in kidney allograft loss and are considered among the most important barriers that limit long-term outcomes 10–14.
Although the cellular and molecular pathways that regulate ABMR are still under investigation, new knowledge of humoral immunobiology indicate that B cell and plasma cell activation results in the generation of DSA, which bind to HLA or non-HLA molecules on the endothelium 15,16. Antibody binding to endothelium and subsequent cellular activation involving complement-dependent and -independent pathways leads to the recruitment of natural killer (NK) cells, polymorphonuclear neutrophils and macrophages, which contribute to capillaritis and eventual tissue injury (Figure 1) 15–17. The morphologic nature of endothelial cell injury in acute ABMR demonstrates platelet aggregation, thrombotic microangiopathy (TMA) and neutrophilic accumulation, resulting in an early pattern of cellular necrosis and a relatively rapid decline in allograft function. Chronic ABMR results from a repetitive pattern of chronic thrombotic events and inflammatory changes, which result in cellular injury and repair. It manifests as late transplant glomerulopathy (TG) and results in a decline in renal function 18. In addition to pathology mediated directly by antibodies, recent evidence suggests that B cells and plasma cells may themselves influence rejection or tolerance 19,20. The clinical picture of ABMR has become increasingly complex, with questions abounding regarding its classification, the precision of diagnostic approaches, and the efficacy of various therapeutic strategies for safely and effectively managing affected patients 21. This article provides an overview of current progress in clinical and translational research surrounding ABMR pathophysiology and ABMR-related outcomes, prevention, treatment and future directions.
Figure 1.
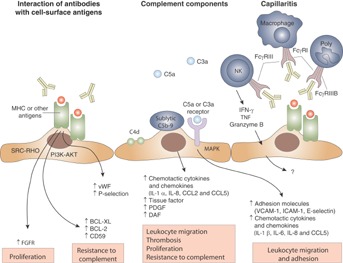
Mechanisms of donor-specific antibody-mediated endothelial injury in renal allografts. Anti-MHC antibodies may either result in direct injury to the capillary endothelium or in indirect injury via complement fixation or recruitment of inflammatory cells with Fc receptors. In cases with donor-specific antibodies that lack C4d deposition, endothelial injury and cellular recruitment could be important mediators. Poly, polymorphonuclear cell. Reproduced with permission from Farkash and Colvin 15.
Defining and diagnosing ABMR
The first description of acute ABMR identified two distinct features: neutrophils in peritubular capillaries (PTCs) and de novo antidonor HLA class I antibodies 22,23. Around the same time, C4d, a degradation product of the complement pathway that binds covalently to the endothelium, was identified as a stable marker of antidonor humoral activity 24. Subsequently, the correlation between DSA, histologic findings of microcapillary injury and diffuse (>50%) C4d deposits in the PTCs were described in acute ABMR 25. C4d and DSA were also linked to the histopathologic features of chronic ABMR 26,27. Since 2003, the Banff Working Group classification system for renal allograft biopsies has differentiated T cell–mediated rejection (TCMR) from ABMR 28,29. The most recent Banff 2013 diagnosis of ABMR, published in this issue of the journal, requires histologic evidence of acute or chronic tissue injury, evidence of current/recent antibody interaction with vascular endothelium and serologic evidence of the presence of circulating DSA 30. Importantly, C4d staining is no longer a requirement for the diagnosis of ABMR (Table 1).
Revised (Banff 2013) classification of antibody-mediated rejection (ABMR) 30
| Acute/active ABMR; all three features must be present for diagnosis1,2 |
| 1. Histologic evidence of acute tissue injury, including one or more of the following: |
| •Microvascular inflammation (g > 03 and/or ptc > 0) |
| •Intimal or transmural arteritis (v > 0)4 |
| •Acute thrombotic microangiopathy (TMA), in the absence of any other cause |
| •Acute tubular injury, in the absence of any other apparent cause |
| 2. Evidence of current/recent antibody interaction with vascular endothelium, including at least one of the following: |
| •Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by IF on frozen sections, or C4d > 0 by IHC on paraffin sections) |
| •At least moderate microvascular inflammation ([g + ptc] ≥ 2)5 |
| •Increased expression of endothelial activation and injury transcripts (ENDATs) or other gene expression markers of endothelial injury in the biopsy tissue, if thoroughly validated |
| 3. Serologic evidence of donor-specific antibodies (HLA or other antigens) |
| Chronic, active ABMR; all three features must be present for diagnosis1,6 |
| 1. Morphologic evidence of chronic tissue injury, including one or more of the following: |
| •Transplant glomerulopathy (cg > 0),7 if no evidence of chronic TMA |
| •Severe peritubular capillary basement membrane multilayering (requires electron microscopy [EM])8 |
| •Arterial intimal fibrosis of new onset, excluding other causes9 |
| 2. Evidence of current/recent antibody interaction with vascular endothelium, including at least one of the following: |
| •Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by IF on frozen sections, or C4d > 0 by IHC on paraffin sections) |
| •At least moderate microvascular inflammation ([g + ptc] ≥ 2)5 |
| •Increased expression of endothelial activation and injury transcripts (ENDATs) or other gene expression markers of endothelial injury in the biopsy tissue, if thoroughly validated |
| 3. Serologic evidence of donor-specific antibodies (HLA or other antigens) |
| C4d staining without evidence of rejection; all three features must be present for diagnosis10 |
| 1. Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by IF on frozen sections, or C4d > 0 by IHC on paraffin sections) |
| 2. g = 0, ptc = 0, cg = 0 (by light microscopy (LM) and by EM if available), v = 0; no TMA, no peritubular capillary basement membrane multilayering, no acute tubular injury (in the absence of another apparent cause for this) |
| 3. No acute cell-mediated rejection (Banff 1997 type 1A or greater) or borderline changes |
For all ABMR diagnoses, it should be specified in the report whether the lesion is C4d-positive (C4d2 or C4d3 by IF on frozen sections; C4d > 0 by IHC on paraffin sections) or without evident C4d deposition (C4d0 or C4d1 by immunofluorescence (IF) on frozen sections; C4d0 by IHC on paraffin sections).
These lesions may be clinically acute, smoldering or subclinical. Biopsies showing two of the three features, except those with donor-specific antibodies (DSA) and C4d without histologic abnormalities potentially related to ABMR or T cell–mediated rejection (TCMR) (C4d staining without evidence of rejection; see footnote 10) may be designated as “suspicious” for acute/active ABMR.
Recurrent/de novo glomerulonephritis should be excluded.
It should be noted that these arterial lesions may be indicative of ABMR, TCMR or mixed ABMR/TCMR. “v” lesions are scored in arteries having continuous media having two or more smooth muscle layers.
In the presence of acute TCMR, borderline infiltrates or evidence of infection, ptc ≥ 2 alone is not sufficient to define moderate microvascular inflammation and g must be ≥ 1.
Lesions of chronic, active ABMR can range from primarily active lesions with early transplant glomerulopathy (TG) evident only by EM (cg 1a) to those with advanced TG and other chronic changes in addition to active microvascular inflammation. In the absence of evidence of current/recent antibody interaction with the endothelium, the term active should be omitted; in such cases DSA may be present at the time of biopsy or at any previous time posttransplantation.
Includes glomerular basement membrane (GBM) duplication by EM only (cg1a) or GBM double contours by LM.
≥7 layers in one cortical peritubular capillary and ≥5 in two additional capillaries, avoiding portions cut tangentially.
While leukocytes within the fibrotic intima favor chronic rejection, these are seen with chronic TCMR as well as chronic ABMR, and are therefore helpful only if there is no history of TCMR. An elastic stain may be helpful as absence of elastic lamellae is more typical of chronic rejection and multiple elastic lamellae are most typical of arteriosclerosis, although these findings are not definitive.
The clinical significance of these findings may be quite different in grafts exposed to anti-blood-group antibodies (ABO-incompatible allografts), where they do not appear to be injurious to the graft and may represent accommodation, and anti-HLA antibodies where more clinical outcome data are needed.
C4d and the diagnosis of ABMR
C4d is a split product of C4 activation and has no known biological action. It may be activated by the classical and lectin complement pathways and serves as a footprint of antibody–antigen interactions on the surface of endothelial cells 31. Although useful, C4d has significant limitations for the diagnosis of ABMR, not least because of methodological issues (immunoperoxidase vs. immunofluorescence, frozen vs. paraffin), poor understanding of the meaning of minimal and focal staining, and its waxing and waning deposition. Staining depends on the density of the capillary network, with poor sensitivity in chronic settings, and C4d positivity has been reported in the absence of other evidence of graft injury 21. Furthermore, C4d staining may not be associated with measurable DSA in the case of non-HLA antibodies or antibodies absorbed by the allograft 31.
Overall, the sensitivity of C4d is low, and its expression depends on the density of PTCs. In this regard, a number of studies have established the concept of C4d-negative acute and chronic ABMR 32–35. Loupy et al 33 reported that C4d staining waxed and waned and was not a sensitive indicator of parenchymal disease in the first year after transplant. In this study, 55% of C4d-negative biopsies with ABMR had evidence of concomitant capillary inflammation 33. Sis et al described that 60% of kidneys with high endothelial activation and injury transcripts (ENDATs) and chronic ABMR or graft loss were C4d negative 34. Findings were confirmed by another study in which 63% of late kidney failures after biopsy were attributable to ABMR, but many were C4d negative 35. A recent microarray study from Sellarés et al 36 concluded that changes in ABMR-associated gene expression (mostly in endothelial or NK cells) correlated with the presence of capillary lesions or DSA and may predict graft failure independent of C4d staining. Together, these observations point to the low sensitivity of C4d for the diagnosis of humoral rejection and support the addition of novel biomarkers of capillary inflammation and endothelial injury, including NK cells and macrophages to the diagnosis algorithm of ABMR 33–38. This recommendation was officially acknowledged at the 11th Banff Conference on Allograft Pathology (Figure 2) 21 and was incorporated into the new Banff 2013 diagnostic criteria for ABMR 30 (Table 1).
Figure 2.
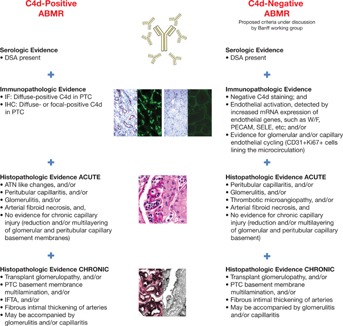
Acute and chronic definitions of ABMR based on C4d positivity. ABMR, antibody-mediated rejection; ATN, acute tubular necrosis; DSA, donor-specific antibodies; IF, immunofluorescence; IFTA, interstitial fibrosis and tubular atrophy; IHC, immunohistochemistry; PTC, peritubular capillary. Reproduced with permission from Mengel et al 21.
DSA and the diagnosis of ABMR
Terasaki et al identified HLA antibodies in the serum of patients after transplantation nearly 45 years ago 39. However, the importance of a low-strength antibody that is undetectable by cell-based methodology was not recognized until studies from the same group, three decades later, discovered a strong association between HLA antibodies detected by solid-phase assays and graft failure 40.
DSA may be directed against HLA or other endothelial cell antigens, and its presence is required for the diagnosis of acute and chronic active ABMR 21,37,41. In addition, there is growing evidence supporting the roles of preformed and de novo DSA as independent risk factors for acute and chronic ABMR and graft loss 14,41–51. A recent systematic review and meta-analysis demonstrated that the presence of DSA before transplantation was associated with a twofold greater risk of acute rejection and a 75% greater risk of graft loss 46. Despite these findings, our understanding of the biological relevance of DSA remains limited. In vitro studies suggest that anti-HLA class I alloantibodies result in endothelial cell injury and activation through both complement-dependent and complement-independent pathways 52,53. However, little is known about signal transduction in response to class II antibodies or the pathogenesis of DSA-induced renal allograft injury in actual patients. It is important to note that not all DSA fix complement or cause ABMR and, conversely, not all episodes of acute graft injury with capillary inflammation and C4d deposition are associated with DSA being detectable with standard assays. In fact, the majority of patients with DSA maintain normal kidney function for years and have long-term outcomes similar to nonsensitized patients 14,42,48.
Another important limitation is that currently available HLA antibody tests are qualitative and have not been cleared by the US Food and Drug Administration (FDA) for quantitative measurements 54. More studies are needed to identify risk stratification strategies on the basis of semiquantitative measures of DSA and calculated panel reactive antibody (PRA), subclasses of immunoglobulin G anti-HLA antibodies, and C1q complement-fixing DSA 44,55,56. Pending the results of collaborative standardization studies 57, consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation were recently published 58. These recommendations are intended to provide guidance on the use and clinical application of contemporary methods for HLA antibody detection.
ABMR classification and phenotypes
The 2011 Banff meeting report and a 2010 workshop held by the FDA both noted the confusion generated by reports on acute and chronic ABMR, and emphasized the importance of correctly defining ABMR phenotypes 21,54. In the Banff report, two principal phenotypes of acute ABMR were defined: (1) ABMR phenotype 1 in the presensitized patient, occurring early posttransplant; and (2) ABMR phenotype 2, which develops from the emergence of de novo DSA in the late posttransplant period and is thought to be mostly related to nonadherence or inadequate immunosuppression 12,59,60. However, additional characteristics—including the nature of the antibody; the significance of C4d; the severity of microcapillary injury, gene transcripts, molecular and cellular signatures; and the pathology and function of the allograft—are relevant, were included in the Banff 2013 criteria, and may subsequently affect the design of clinical trials of patients with ABMR 16,21,30,34,36 (Table 1).
Predictors of poor outcome related to antibody-mediated injury
Acute and chronic ABMR are associated with poor outcomes after kidney transplantation. Specifically, patients with acute ABMR are at greater risk for subsequent rejection, chronic ABMR and graft loss 10,14,33,42,61. Similarly, those with chronic ABMR are at increased risk for graft loss 12,13,35,60,62,63. However, not all ABMR phenotypes have poor outcomes, and many patients maintain stable graft function for years after treatment of the initial injury. We will review the independent roles of C4d, circulating antibodies, B cells and plasma cells, microcirculation injury/inflammation, subclinical ABMR and novel biomarkers to predict outcomes in patients with acute and chronic ABMR.
C4d and microvascular injury
C4d and microcirculation inflammation are independent biomarkers of subsequent rejection, chronic ABMR and graft loss in patients with acute ABMR 24,33,48,62. Loupy et al 33 demonstrated that higher increments of C4d Banff scores predict greater microvascular inflammation at both 3 months and 1 year after transplant, as well as worse TG and higher levels of class II DSA. The extent of microvascular injury was similar between biopsies with focal and diffuse C4d. However, the presence of microcirculation inflammation and class II DSA at 3 months was related to a fourfold increased risk of chronic ABMR independent of C4d 33. We recently demonstrated that focal C4d staining in postreperfusion biopsies was a significant predictor of subsequent ABMR in sensitized patients 48. Despite the important diagnostic and prognostic roles of C4d and microcirculation inflammation in acute ABMR, prospective studies are required to determine whether the treatment of isolated C4d staining or microcirculation inflammation in patients with DSA improves outcomes.
C4d and endothelial injury are also associated with poor outcomes in chronic ABMR 12,13,35,60,63–65. In support of this observation, most graft losses in the current era of immunosuppression have evidence of chronic ABMR with positive C4d staining 12,13,35. In the Deterioration in Kidney Allograft Function (DeKAF) study, patients with new-onset kidney allograft dysfunction underwent a biopsy at a mean time of 7.3 years after transplant 60. Most biopsies had some evidence of antibody-mediated injury (C4d or DSA), and the risk of subsequent graft failure was significantly increased in the presence of C4d 60. Other studies have confirmed the association of both focal and diffuse C4d staining in chronic ABMR with graft loss 64,65. Microcirculation injury defined as microcirculation inflammation (PTC and g) or microcirculation deterioration (cg and PTC multilayering) was also an important predictor of graft loss in late biopsies (>1 year after transplant), independent of C4d staining 35,63. As a result, the new Banff 2013 criteria further characterize ABMR based on current/recent antibody interactions with vascular endothelium including C4d staining, at least moderate microvascular inflammation ([g + ptc] ≥ 2), increased expression of ENDATs or gene expression of other validated markers of endothelial injury in the biopsy tissue (Table 1) 30.
In summary, there is a clear and independent association between C4d and microcirculation injury with poor outcomes in kidney transplant recipients (KTRs) with acute or chronic ABMR. Standardized risk stratification strategies are needed to better define preventive and treatment approaches for each ABMR phenotype.
Donor-specific antibodies
Preexisting 49,55 or de novo circulating antibodies 14,63 have been shown to compromise renal allograft survival. These antibodies may be directed against HLA or non-HLA molecules on endothelial cells, including major histocompatibility complex class I-related chain A antibody (MICA), and angiotensin type 1 receptor 37,41,66. In support of this observation, among recipients of HLA-identical sibling transplants, patients with no PRA had significantly higher 10-year graft survival than patients with PRA >1%, suggesting that non-HLA immunity has an important role in clinical transplantation and chronic graft loss 37.
In sensitized patients with preexisting anti-HLA-DSA, 8-year graft survival rates were significantly worse than in sensitized patients without HLA-DSA or nonsensitized patients 55. Peak HLA-DSA strength was related to the risk of acute ABMR 49,55 and graft loss 55. Conversely, in renal transplant recipients without preexisting DSA, 10-year graft survival was significantly lower for patients who developed de novo HLA-DSA 14. Histopathology of ABMR phenotype 2 could be observed in the absence of graft dysfunction. Similar findings were reported earlier, where patients with de novo DSA at the time of biopsy had worse graft survival than those with preexisting DSA, indicating that patients with ABMR phenotype 2 have worse graft outcomes than those with ABMR phenotype 1 63. De novo DSA are predominantly directed at class II donor HLA mismatches and are associated with nonadherence and cellular rejection (Figure 3) 14. Although the reason for this is unclear, it appears that class I antibodies are associated with early ABMR, whereas class II antibodies are more commonly associated with late ABMR and graft failure 14,35,48–51,63.
Figure 3.
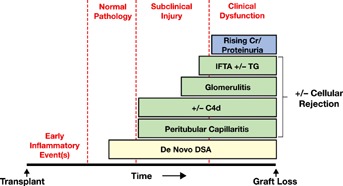
The natural history of phenotype 2 ABMR. ABMR, antibody-mediated rejection; DSA, donor-specific antibodies; IFTA, interstitial fibrosis and tubular atrophy; TG, transplant glomerulopathy. Reproduced with permission from Wiebe et al 14.
In summary, although DSA are important risk factors for graft loss, the majority of patients with DSA have stable allograft function and experience no rejection. It is therefore important to determine the pathogenic role and specificity of anti-class I and class II HLA and non-HLA-DSA and to better understand the effect of de novo DSA compared with preexisting DSA.
B cells and plasma cells
The relationship between the presence of circulating DSA and the development of antidonor B cell responses in allograft rejection and tolerance is currently under active investigation 19,20. While the absence of circulating DSA may indicate graft tolerance, it is also possible that antibodies produced as a result of a B cell response are not detectable because of binding/absorption by the graft itself 20,67. To investigate donor-directed B cell responses, a recent study 19 used donor-derived fibroblasts as targets to quantify DSA-secreting cells isolated from peripheral blood of KTRs before and after transplantation. Even in the absence of circulating DSA (with no evidence of rejection), the number of DSA-secreting cells was increased posttransplant in all patients, suggesting a greater role for B cells and plasma cells in posttransplant immune regulation than previously thought 19.
B cells may also contribute to posttransplant immune regulation through its antigen-presenting function 20. In addition to pathways used by other antigen-presenting cells (APCs), B cells can present antigen via antigen binding to the clonotypic B cell receptor 68. In turn, B cells (unlike other APCs) undergo clonal expansion, which may contribute to rejection by amplifying antidonor B cell responses 20.
Subclinical ABMR
Subclinical ABMR is defined as immunohistological evidence of ABMR in KTRs with normal renal allograft function. Evidence suggests that untreated subclinical ABMR is an important predictor of poor renal allograft outcomes 69,70. At 1-year posttransplant, those with subclinical ABMR at 3 months had more interstitial fibrosis and tubular atrophy (IFTA) and TG compared with patients who did not have subclinical ABMR at 3 months 69. Similarly, patients with C4d-negative subclinical ABMR at 3 months (defined as PTC + g > 0) had more PTC and IFTA and a lower GFR at 1 year relative to those without subclinical lesions at 3 months 69. These findings were in agreement with earlier observations that demonstrated a strong association between subclinical rejection 70 and chronic allograft nephropathy in 83 patients who received HLA-incompatible renal allografts and support the indication of protocol biopsies in sensitized recipients. Protocol biopsies can further identify subclinical TG, which is considered an important risk factor for chronic injury and graft dysfunction 69,71. As a result, it is recommended that high-risk patients (i.e. desensitized or DSA-positive/crossmatch-negative) should be monitored by protocol biopsies in the first 3 months after transplantation. Protocol biopsies may also be conducted after ABMR to determine the effectiveness of therapy and to identify prognostic indicators of outcome 58.
Novel biomarkers
New assays and molecular tests may be considered as diagnostic and prognostic tools in patients with ABMR. There is evidence that ENDATs and DSA-selective transcripts are indicators of active ABMR damage and worse graft outcomes 17,34,72. The expression of these transcripts in biopsies may provide a new tool for understanding the pathogenesis of late kidney graft loss and ABMR, as well as for predicting graft outcomes and defining ABMR even in C4d-negative biopsies in patients with antibodies 17,34. Differentially expressed microRNAs and their predicted targets identified by deep sequencing may also be candidates for further investigation to understand the mechanism and management of kidney allograft fibrosis in patients with ABMR 73. The C1q assay is another test that is designed to distinguish complement-fixing from non-complement-fixing antibodies 56. Recent studies indicate that a positive C1q assay for de novo DSA correlates with acute rejection and long-term graft loss after kidney transplantation 74–76. Other investigators have found no significant difference in graft survival between patients with or without preformed C1q-fixing DSA 77, suggesting that additional studies are needed to clarify the role of this assay in clinical transplantation. Finally, C4d-fixing luminex-binding antibodies have been reported to predict graft failure in heart transplantation, but the role of this assay in kidney transplantation is being debated 78,79. In summary, while new assays with potential diagnostic and prognostic value are being developed in the area of ABMR, these tools need to be validated by larger studies.
Preventing ABMR
More than 20 000 patients awaiting kidney transplantation in the United States are sensitized (typically owing to blood transfusion, pregnancy or previous transplants) to HLA class I and/or class II antibodies 80. Until recently, transplantation was routinely avoided in sensitized patients, at the expense of prolonging waiting time for suitably HLA-matched organs. However, with the advent of virtual crossmatch, desensitization protocols and paired kidney exchange (PKE) programs, timely kidney transplantation has become a reality for many of these high-risk patients 81,82 (Table 2). Highly sensitized patients may be able to participate in special programs such as the Eurotransplant Acceptable Mismatch Program 83, in which the HLA typing of panel donors with negative reactions is determined during screening if PRA is below 100%; alternatively, selection and crossmatching of blood donors with a single HLA mismatch to the patient’s phenotype can be undertaken 84. It has been argued that implementation of these programs may lead to similar graft survival rates to those observed in nonsensitized patients 84.
Strategies to prevent ABMR
| 1. Do not transplant highly sensitized patients |
| 2. Avoid blood transfusion |
| 3. Paired kidney exchange |
| 4. In sensitized patients, precise characterization of their alloantibodies and exact HLA typing of the donor at the time of transplantation |
| 5. Participation in special programs (such as the Eurotransplant Acceptable Mismatch Program) |
| 6. Removal of DSA (plasmapheresis, immunoadsorption) |
| 7. Direct or indirect inhibition of DSA production |
| a. Anti-B cell agents (rituximab1) |
| b. Anti-plasma cell agents (proteasome inhibitors, e.g. bortezomib1) |
| c. Rabbit anti-human thymocyte immunoglobulins (e.g. thymoglobulin)? |
| d. Costimulation blockade (e.g. belatacept)? |
| 8. Inhibition of complement cascade (eculizumab1) |
| 9. Intravenous immunoglobulin1 |
| e. Neutralizing DSA: anti-idiotypic activity |
| f. Inhibiting complement activation by binding C3b, C4b |
| g. Inhibiting activation of macrophages, neutrophils by binding FcγRs |
| h. Apoptosis of B cells (inhibits CD19 expression) |
| 10. Splenectomy |
ABMR, antibody-mediated rejection; DSA, donor-specific antibodies; FcγRs, Fc gamma.
Table 1These drugs are used off-label in solid organ transplantations.
Prevention of acute ABMR phenotype 1
Only one randomized controlled clinical trial (RCT) has been conducted to lower allosensitization prior to transplantation 85. In this study, 101 adult patients with a PRA ≥50% were enrolled in a trial sponsored by the National Institutes of Health. Patients received intravenous immunoglobulin (IVIG) 2 g/kg monthly for 4 months or an equivalent volume of placebo with additional infusions at 12 and 24 months after entry if not transplanted while on IVIG or placebo. IVIG significantly reduced PRA levels in study subjects compared with placebo, and more patients in the IVIG arm were transplanted (35% vs. 17%). Seven graft failures occurred (four IVIG, three placebo) among adherent patients with similar 2-year graft survival rates (80% IVIG, 75% placebo). The investigators concluded that IVIG is better than placebo in reducing anti-HLA antibody levels and improving transplantation rates in highly sensitized patients with end-stage renal disease. In a follow-up study by the same group, the combination of B cell depletion therapy and high-dose IVIG was shown to be effective in reducing PRA from 77 ± 18% to 44 ± 30% at the time of transplantation 86. However, recent studies have not been able to reproduce these data, specifically in patients with PRA >80% 87–89.
Two randomized clinical trials have examined the role of rabbit anti-human thymocyte globulin (rATG) as induction therapy in sensitized kidney transplants based on current or peak PRA levels 90,91. The use of rATG was associated with a significant reduction in the incidence of acute rejection and improved 1-year survival, specifically in patients who remained rejection-free, suggesting that ATG induction may be associated with better outcomes in sensitized patients 90,91. Compared with anti-IL-2 therapy (basiliximab), induction with rATG in moderately sensitized KTR was associated with reduced incidence of de novo DSA and ABMR 92. In contrast, outcomes from the CTOT02 study, including nonsensitized adults and children, found anti-IL-2 induction to be protective against the development of anti-HLA antibodies; however, no difference in allograft survival was associated with anti-HLA antibody development 93,94. Although these studies support the use of ATG and IL-2 blockade in sensitized and low-risk patients, respectively, the effects of induction with rATG or anti-IL-2 therapy on de novo DSA or long-term outcomes remain largely unknown.
Nonrandomized clinical observations suggest that a combination of plasmapheresis and low-dose IVIG combined with IL-2 blockade or rATG for induction has become the standard of care for the treatment of sensitized patients 11,46,95. Using this approach, desensitization was associated with improved patient survival compared with chronic dialysis 95. Despite these promising findings, long-term outcomes for crossmatch-positive living-donor kidney transplantation are generally inferior to nonsensitized KTRs 51,96, suggesting that better immunomodulatory strategies are required.
Alemtuzumab, a lymphocyte-depleting, CD52-specific monoclonal antibody, is increasingly used as induction therapy in renal transplantation. A recent review and meta-analysis of 10 RCTs (enrolling more than 1200 patients), as well as studies specifically in highly sensitized patients, concluded that alemtuzumab induction is associated with a comparable or lower risk of biopsy-proven acute rejection compared with rATG or IL-2 receptor antibodies 97–99. In contrast, other studies have demonstrated potential negative effects of alemtuzumab on the regulation of humoral immunity, including unexpectedly high rates of ABMR 100 and high rates of circulating alloantibody and intragraft C4d at 1-year posttransplant 100. Increased risk for ABMR with alemtuzumab may be partly mediated by dysregulation of B cell activating factor (BAFF), as an increase in BAFF mRNA expression was observed in monocytes of alemtuzumab-treated patients 101,102.
The anti-CD20 agent rituximab may also have utility as an induction agent for renal transplant recipients, although its efficacy is yet to be proven and it is not currently licensed in this setting 103. An RCT (http://ClinicalTrials.gov number NCT00565331) presented at the 2013 American Transplant Congress evaluated a single dose of rituximab as induction therapy added to standard concomitant immunosuppression (i.e. tacrolimus, mycophenolate mofetil and steroids) in patients with PRA >6% and re-transplants. The results showed that rituximab was significantly more effective than placebo at preventing biopsy-proven acute rejection within the first 6 months posttransplant, though this study did not report the effect of rituximab induction on the incidence of ABMR 104. In a retrospective study of patients receiving ABO-incompatible KTRs, rituximab induction inhibited the development of de novo DSA and reduced the rate of chronic ABMR (relative to splenectomy) in this subset of patients 105. Further induction trials of rituximab are ongoing 103, including the Rituximab Induction in Renal Transplantation (ReMIND) trial (http://ClinicalTrials.gov number NCT01095172).
Several observational studies have used bortezomib and eculizumab in their desensitization protocols. Bortezomib is a proteasome inhibitor that acts on plasma cells and is effective in removing preformed DSA when combined with plasmapheresis 106,107. It is also associated with durable reductions in DSA and stable allograft function in de novo DSA-positive renal transplant recipients 108. The efficacy of the humanized anti-C5 antibody eculizumab in the prevention of ABMR was also recently assessed in renal transplant recipients with a positive crossmatch 109. The incidence of ABMR was 8% with eculizumab compared with 41% in the control group, and the rate of TG at 1 year was also significantly lower with eculizumab 109. There is an ongoing, multicenter, international, randomized trial testing the role of eculizumab plus conventional treatment (or conventional treatment alone) that may clarify its utility (NCT00670774) 110. However, these published single-center observations have not yet been confirmed by larger studies, and none of these drugs is approved by the FDA for the prevention or treatment of ABMR. Furthermore, these protocols are associated with costs that may not be covered by insurance.
A common approach to ABMR prevention has been to avoid transplanting highly sensitized patients. However, avoiding transplant renders chronic dialysis the only option, with implications for patient health and quality of life, as well as healthcare costs. Long-term survival in posttransplant patients has been improved considerably by desensitization, and the enrollment of patients in special programs to optimize matching can lead to timely transplants with better outcomes. A recent study examined both the efficacy and cost-effectiveness of desensitization using IVIG and rituximab in 146 patients who were originally DSA-positive (PRA >80%) and transplanted with an acceptable crossmatch 111. The patient survival rate at 3 years was 96.6% in the desensitization arm compared with 79.0% for patients remaining on dialysis. Each patient treated with desensitization was estimated to save the US healthcare system $18 753. These data suggest that survival and financial gains can be achieved by a desensitization approach; however, this was a relatively small study, and the extent of the relative benefits of desensitization over dialysis will ultimately be determined by drug cost.
A growing option for the prevention of ABMR in highly sensitized patients is the use of PKE transplant programs, such as the National Kidney Registry, the Alliance for Paired Donation and the United Network for Organ Sharing Kidney Paired Donations Pilot Program 112,113 (see Table 2). Such programs enable sensitized patients with immunologically incompatible living donors to be transplanted with high-quality grafts from other living donors in similar situations who were willing to exchange organs. Although cost has been a concern for kidney exchange registries in the United States, it seems that the PKE could help participating centers avoid complex desensitization protocols while improving long-term outcomes. Furthermore, mathematical modeling predicts that an optimized matching algorithm and a national PKE program would improve outcomes and reduce healthcare costs for highly sensitized patients 114. With the rising number of highly sensitized patients in PKE programs, some centers combine desensitization and paired exchange options.
Prevention of acute ABMR phenotype 2
Nonadherence and the choice of maintenance immunosuppression may influence the development of ABMR after transplant 13,14,115. For example, calcineurin inhibitor minimization or withdrawal strategies may increase the incidence of de novo DSA and ABMR 13,14,115. Analyses from two prospective randomized clinical trials demonstrated that the conversion of cyclosporine to everolimus at 3–4.5 months after transplant was associated with significantly higher rates of de novo DSA (10.8% vs. 23%, p = 0.04) and ABMR (3% vs. 13%, p = 0.03) 116. Whereas avoiding calcineurin-based regimens may be advantageous in KTRs by reducing the potential risks of nephrotoxicity and other adverse events after transplant, the intentional or unintentional reduction of immunosuppression increases the risk of ABMR and graft loss 12,13,117. Treatment with belatacept, a selective costimulation blocker that targets CD80/CD86-CD28 interaction to prevent T cell activation, was associated with a low rate of de novo DSA over 3 years of treatment in phase III trials, although this was not an initial end point of the studies and requires confirmation 118,119. This observation is supported by experimental data demonstrating that belatacept inhibits primary T cell–dependent antibody responses and the generation of DSA in primates 120. A phase II clinical study in which conversion from a calcineurin inhibitor-based regimen to belatacept had no effect on the incidence of de novo DSA or ABMR despite higher rates of cellular rejection (7% vs. 0%) 117.
In summary, notwithstanding the advent of novel immunosuppressive agents, the ideal regimen for the prevention of ABMR phenotypes 1 and 2 in sensitized KTRs remains unknown.
Treatment of ABMR
Acute ABMR
The primary aims of therapeutic modalities for ABMR are to remove existing antibodies and inhibit their redevelopment. The management of ABMR is challenging and is associated with poorer outcomes compared with traditional anti-T cell rejection therapy for pure cell-mediated rejection 121. A recent systematic review of treatments for acute ABMR in renal allografts found 10 388 citations but only five small randomized and eight non-RCTs (Table 3) 122. Of these trials, benefit was found in five studies evaluating plasmapheresis or immunoadsorption, and small, nonrandomized controlled studies suggested benefit from rituximab or bortezomib 33,120,122,124–128,130–134. An evaluation of a small group of patients from a randomized trial of kidney allograft recipients suggested that immunoadsorption (received by five patients and compared with the outcomes of five controls) was effective in reversing severe C4d-positive ABMR 123. However, it is important to note that immunoadsorption is not practiced in the United States.
Summary of controlled trials from a systematic review assessing treatment strategies for ABMR1
| Refs. | ABMR definition | Trial design and intervention (N) | Patients with hemodialysis dependency or graft loss (intervention vs. control) |
|---|---|---|---|
| Böhmig et al (123) | Banff 1997 | Stratified RCT; 9–14 sessions of immunoadsorption (protein A) | Treatment benefit observed: |
| 0 vs. 4 at 3 weeks | |||
| ARR = 0.8 (95% CI, 0.2–0.9) | |||
| Blake et al (124) | Vascular | Stratified RCT; 5 PP treatments | No treatment benefit: |
| 4 vs. 6 at 6 months; RRR = 0.3 (95% CI, 0.001–0.8) | |||
| 10 vs. 13 at 5 years; RRR = 0.2 (95% CI, 0.001–0.5) | |||
| Bonomini et al (125) | Vascular, MP-resistant | RCT; 3–7 PP treatments | Treatment benefit observed: |
| 7 vs. 17 at 2 weeks; RRR = 0.6 (95% CI, 0.3–0.8) | |||
| Kirubakaran et al (126) | Vascular | RCT; 8 PP treatments | Trend to harm: |
| 6 vs. 3 at 1 month; RRI = 0.5 (95% CI, 0.001–0.8) | |||
| Allen et al (127) | Vascular, MP-resistant | RCT; 6 PP treatments | No treatment benefit (trend to harm at 220 days): |
| 3 vs. 4 at 6 days; RRR = 0.2 (95% CI, 0.001–0.8) | |||
| 11 vs. 9 at 220 days; RRI = 0.2 (95% CI, 0.001–0.5) | |||
| Franco et al (128) | Vascular, MP-resistant | Historical control; 6 PP treatments | Treatment benefit observed: |
| 6 vs. 13 at 3 months; OR = 0.4 (95% CI, 0.1–1.3) | |||
| Lefaucheur et al (129) | Banff 1997 | Historical control; 4 PP treatments; 2 rituximab doses | Treatment benefit observed: |
| 1 vs. 6 at 3 years; OR = 0.1 (95% CI, 0.008–0.9) | |||
| Kaposztas et al (130) | ALG-resistant | Historical control; rituximab | Treatment benefit observed: |
| 2 vs. 8 at 2 years; OR = 0.2 (95% CI, 0.04–1.09) | |||
| Vangelista et al (131) | Vascular, anti-HLA | Nonrandomized case-controlled; 4–5 PP treatments | Treatment benefit observed: |
| 1 vs. 3 | |||
| Macaluso et al (132) | ABMR (no other details) | Nonrandomized, case-controlled; 4 doses of bortezomib, 1 dose of rituximab, 5 PP treatments | Treatment benefit observed: |
| 1 vs. 10 at 3 months; OR = 0.1 (95% CI, 0.01–0.9) | |||
| Loupy et al (45) | Vascular with DSA | Nonrandomized, case-controlled; OKT3 vs. IVIG vs. PP and rituximab | Treatment benefit observed for PP and rituximab: |
| HR = 0.19 (vs. OKT3) | |||
| HR = 0.11 (vs. IVIG) | |||
| Lubetzky (133) | With DSA | Nonrandomized, case-controlled; rituximab vs. bortezomib | Treatment benefit observed for bortezomib: |
| 3 vs. 1 at 6 months; OR = 5.3 (95% CI, 0.5–59.3) | |||
| Waiser et al (134) | With DSA | Historical cohort; 4 bortezomib doses vs. 1 rituximab dose, 6 PP treatments and 30 g IVIG | No treatment benefit: |
| 2 vs. 3 (at 6 months); OR = 0.5 (95% CI, 0.06–4.0) |
ABMR, antibody-mediated rejection; ALG, antilymphocyte globulin; ARR, absolute risk reduction; CI, confidence interval; DSA, donor-specific antibodies; HR, hazard ratio; IVIG, intravenous immunoglobulin; MP, methylprednisolone; OR, odds ratio; PP, plasmapheresis; RCT, randomized controlled trial; RRI, relative risk increase; RRR, relative risk reduction; RRI, relative risk increase.
Table 1Adapted with permission from Roberts et al 122.
Ironically, there are no randomized controlled studies that support the benefits of IVIG in acute ABMR, despite its common use in this context 122. Only one randomized controlled study has found plasmapheresis to be beneficial 125; two controlled studies found no benefit 124,127 and one found potential harm 126, indicating that the role of plasmapheresis for the treatment of acute ABMR remains under debate. Uncontrolled or controlled nonrandomized studies support a role for rituximab, bortezomib, plasmapheresis and IVIG 45,128–134. However, the relative importance of these therapies is difficult to assess because treatment strategies were not standardized, doses and frequencies were not similar, and the specific drugs were combined with other agents.
One-year results were recently reported from a phase III, multicenter, randomized, placebo-controlled trial (RITUX ERAH) that examined the effect of rituximab (combined with plasmapheresis, IVIG, corticosteroids, tacrolimus and mycophenolate mofetil) on a composite measure of graft loss or absence of improvement of renal function at day 12, in patients with biopsy-proven acute ABMR. ABMR occurred after a median of 35.5 days, with no advantage of rituximab over control for the graft loss or renal function outcome 135.
Eculizumab was recently used for the treatment of multidrug-resistant ABMR 136, but there are no randomized controlled studies to confirm the efficacy of this expensive drug. In summary, efficacy data for the treatment of acute ABMR are of very low quality, and larger RCTs and dose–response studies are needed to fully evaluate therapies in this setting 122. In the absence of strong evidence to support consensus guidelines for the treatment of ABMR, the Kidney Disease: Improving Global Outcomes Transplant Work Group recommends the use of corticosteroids, plasmapheresis, IVIG, anti-CD20 antibodies and lymphocyte-depleting antibodies alone or in combination 137 (Figure 4).
Figure 4.
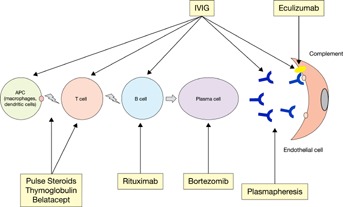
Therapeutic modalities for ABMR. ABMR, antibody-mediated rejection; APC, antigen-presenting cell; IVIG, intravenous immunoglobulins.
Chronic ABMR
Chronic ABMR is a more difficult condition to treat because irreversible tissue damage has occurred in the setting of severely compromised graft survival 138. A small-scale retrospective study of rituximab combined with standard maintenance immunosuppression (including prednisone, mycophenolate mofetil and calcineurin inhibitors) in 31 patients with chronic ABMR had encouraging results, with partial therapeutic response and an increase in median graft survival in the rituximab-treated group compared with the control group (685 days vs. 439 days, respectively). The outcomes within the rituximab group were dichotomous, with significantly different median survival time in responders compared with nonresponders and control patients, though there were no pathologic parameters that distinguished any subset of patients 139.
Clinical trials of rituximab for the treatment of chronic ABMR are ongoing or recruiting patients (NCT00476164 [RituxiCAN-C4] in the United Kingdom and NCT00307125 in the United States).
New directions and future perspectives
Despite the important role of ABMR in patient morbidity and mortality after renal transplantation, our current understanding of the pathogenesis and pathologic phenotypes of ABMR is limited. Evidence supports an important role for DSA in acute and chronic ABMR. However, not all DSA detected by current assays cause injury in the allograft and not all ABMR phenotypes cause rapid allograft failure. Similarly, C4d has significant limitations as a biomarker of ABMR. It will therefore be essential to determine risk stratification strategies for DSA, C4d and ABMR phenotypes to guide preventive and therapeutic approaches, including plasmapheresis, IVIG and anticomplement and anti-B/plasma cell therapies.
Treatment options for ABMR are being informed by growing awareness of the complex role played by B cells in acute ABMR and chronic allograft dysfunction and the underlying biological processes. B cell lineages are now known to have multiple negative effects on the alloimmune response, including antigen presentation to T cells, the production of cytokines supporting T cell activation, antibody production and tertiary lymphoid organ and lymphatic vessel formation 140. Donor-specific B cells can be detected in peripheral blood using HLA-binding tetramers 141,142, and these tetramers may also represent a potential therapeutic agent to deplete donor-specific B cells. Strategies currently used in transplantation to deplete B cells or inhibit B cell activation are rATG, alemtuzumab and rituximab. However, despite the short-term depletion of B cells, alemtuzumab is associated with altered phenotypic and functional properties of the repopulated cells 143, which may contribute to increased rates of ABMR 144,145. The maintenance immunosuppressant belatacept may provide indirect inhibition of B cells through costimulatory blockade of CD80 and CD86, as this disables the stimulation of CD28, a mediator of antibody production by B cells and B cell proliferation 146. However, belatacept is not under evaluation as a treatment for ABMR.
Limited clinical trial evidence suggests that the proteasome inhibitor bortezomib (which induces plasma cell apoptosis) may be useful in combination with plasmapheresis to reduce anti-HLA antibodies in sensitized patients and to treat ABMR following renal transplantation 138,140,147. Other investigational B cell-depleting therapies include potent anti-CD20 antibodies (e.g. ofatumumab and ocrelizumab) and an anti-CD22 antibody (epratuzumab) 138,140,148. Agents targeting the BAFF pathway, which costimulates B cell survival and expansion, are also in clinical development (e.g. atacicept and belimumab) 140,149. The inhibition of antibody effector function is another interesting area of research, and some promise has already been shown by eculizumab, an anti-C5 antibody, in the prevention and treatment of ABMR 140,149.
Many of the potential treatment options for ABMR have been imported from other areas of medicine, without appropriate clinical trials in kidney transplantation; hence, there is a need for well-designed clinical trials that use standardized and contemporary diagnostic, monitoring and therapeutic strategies for ABMR. There are challenges in organizing multicenter, prospective clinical trial study groups aimed at developing agents for DSA reduction and treatment of ABMR. There is also a bias toward developing B cell/antibody-targeting drugs for indications outside of transplantation (such as oncology or rheumatology), and the FDA has highly stringent requirements for the approval and labeling of new agents in the transplantation arena. Before novel and more effective treatments become available, the close monitoring of high-risk patients and an emphasis on adherence to well-tolerated maintenance immunosuppressants are recommended to minimize the risk of ABMR.
Acknowledgments
The authors would like to acknowledge CodonMedical (A Division of KnowledgePoint360 Group) for their assistance in conducting literature searches and coordinating the writing and editing of the manuscript, funded by Bristol-Myers Squibb.
Glossary
- ABMR
antibody-mediated rejection
- ALG
antilymphocyte globulin
- APC
antigen-presenting cell
- ARR
absolute risk reduction
- ATG
anti-human thymocyte globulin
- ATN
acute tubular necrosis
- BAFF
B cell activating factor
- CI
confidence interval
- DSA
donor-specific antibodies
- ENDATs
endothelial activation and injury transcripts
- FcγRs
Fc gamma
- FDA
US Food and Drug Administration
- HR
hazard ratio
- IF
immunofluorescence
- IFTA
interstitial fibrosis and tubular atrophy
- IHC
immunohistochemistry
- IVIG
intravenous immunoglobulin
- KTR
kidney transplant recipient
- MICA
major histocompatibility complex class I-related chain A antibody
- NK
natural killer
- OR
odds ratio
- PKE
paired kidney exchange
- Poly
polymorphonuclear cell
- PP
plasmapheresis
- PRA
panel reactive antibody
- PTC
peritubular capillary
- rATG
rabbit anti-human thymocyte globulin
- RCT
randomized controlled trial
- RRI
relative risk increase
- RRR
relative risk reduction
- TCMR
T cell–mediated rejection
- TG
transplant glomerulopathy
- TMA
thrombotic microangiopathy
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Djamali has received grant funding from the NIH, Takeda-Millennium and Bristol-Myers Squibb. Dr. Kaufman has received grant/research support from the NIH, Bristol-Myers Squibb. Dr. Ellis has been a consultant for Abbott Laboratories. Dr. Zhong has no conflicts of interest to report. Dr. Matas has received consulting fees for serving on an advisory board for Bristol-Myers Squibb. Dr. Samaniego’s institution has received grant/research support from Alexion Pharmaceuticals, NIH, Millennium Pharmaceuticals and Terasaki Foundation; she has received consulting fees from Alexion Pharmaceuticals and Sanofi; she has received payment for lectures, including service on speakers bureaus from Alexion Pharmaceuticals.
References
- 1.Coll E, Crespo M, Solé M. Lessons from cyclosporine monotherapy in renal transplantation: The impact of acute rejection on long-term allograft outcome. Transplant Proc. 2004;36:S114–S116. doi: 10.1016/j.transproceed.2004.01.116. , et al. (Suppl): [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HJ, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies. Am J Transplant. 2004;4:1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 5.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant. 2011;11:450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 6. Organ Procurement and Transplant Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR). 2011 Annual Report. Available at: http://www.srtr.org/annual_reports/. Accessed June 3, 2013.
- 7.Djamali A, Samaniego M, Muth B, et al. Medical care of kidney transplant recipients after the first posttransplant year. Clin J Am Soc Nephrol. 2006;1:623–640. doi: 10.2215/CJN.01371005. [DOI] [PubMed] [Google Scholar]
- 8.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 9.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: A therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6(5 Pt 1):876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 10.Jordan SC, Pescovitz MD. Presensitization: The problem and its management. Clin J Am Soc Nephrol. 2006;1:421–432. doi: 10.2215/CJN.01651105. [DOI] [PubMed] [Google Scholar]
- 11.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346–351. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 13.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 15.Farkash EA, Colvin RB. Diagnostic challenges in chronic antibody-mediated rejection. Nat Rev Nephrol. 2012;8:255–257. doi: 10.1038/nrneph.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010;15:42–48. doi: 10.1097/MOT.0b013e3283352a50. [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: Evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 18.Drachenberg CB, Papadimitriou JC. Endothelial injury in renal antibody-mediated allograft rejection: A schematic view based on pathogenesis. Transplantation. 2013;95:1073–1083. doi: 10.1097/TP.0b013e31827e6b45. [DOI] [PubMed] [Google Scholar]
- 19.Lynch RJ, Silva IA, Chen BJ, Punch JD, Cascalho M, Platt JL. Cryptic B cell response to renal transplantation. Am J Transplant. 2013;13:1713–1723. doi: 10.1111/ajt.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cascalho MI, Chen BJ, Kain M, Platt JL. The paradoxical functions of B cells and antibodies in transplantation. J Immunol. 2013;190:875–879. doi: 10.4049/jimmunol.1100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mengel M, Sis B, Haas M, et al. Banff 2011 meeting report: New concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS. The significance of the anti-class I antibody response: I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation. 1990;49:85–91. doi: 10.1097/00007890-199001000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Halloran PF, Schlaut J, Solez K, Srinivasa NS. The significance of the anti-class I response: II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation. 1992;53:550–555. [PubMed] [Google Scholar]
- 24.Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333–1338. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 25.Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 26.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: Identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574–582. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 27.Regele H, Bohmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–2380. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 28.Sis B, Mengel M, Haas M, et al. Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 29.Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: New diagnostic insights and standards. Am J Transplant. 2004;4:1562–1566. doi: 10.1111/j.1600-6143.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 30.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: Inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 31.Cohen D, Colvin RB, Daha MR, et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81:628–639. doi: 10.1038/ki.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas M. C4d-negative antibody-mediated rejection in renal allografts: Evidence for its existence and effect on graft survival. Clin Nephrol. 2011;75:271–278. doi: 10.5414/cnp75271. [DOI] [PubMed] [Google Scholar]
- 33.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA) Am J Transplant. 2011;11:56–65. doi: 10.1111/j.1600-6143.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 34.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 35.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 36.Sellarés J, Reeve J, Loupy A, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13:971–983. doi: 10.1111/ajt.12150. [DOI] [PubMed] [Google Scholar]
- 37.Opelz G Collaborative Transplant Study. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–1576. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 38.Benichou G, Yamada Y, Aoyama A, Madsen JC. Natural killer cells in rejection and tolerance of solid organ allografts. Curr Opin Organ Transplant. 2011;16:47–53. doi: 10.1097/MOT.0b013e32834254cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris PJ, Williams GM, Hume DM, Mickey MR, Terasaki PI. Serotyping for homotransplantation: XII. Occurrence of cytotoxic antibodies following kidney transplantation in man. Transplantation. 1968;6:392–399. [PubMed] [Google Scholar]
- 40.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 41.Zou Y, Stastny P, Süsal C, Döhler B, Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–1300. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 42.Dunn TB, Noreen H, Gillingham K, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011;11:2132–2143. doi: 10.1111/j.1600-6143.2011.03640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 44.Lefaucheur C, Antoine C, Suberbielle C, Glotz D. Mastering the risk of HLA antibodies in kidney transplantation: An algorithm based on pretransplant single-antigen flow bead techniques. Am J Transplant. 2011;11:1592–1598. doi: 10.1111/j.1600-6143.2011.03560.x. [DOI] [PubMed] [Google Scholar]
- 45.Loupy A, Lefaucheur C, Vernerey D. Outcome and therapeutic approaches in acute rejection with vascular lesions and DSAs. Am J Transplant. 2011;11:193. , et al. (Suppl 2, Abstract #546): [Google Scholar]
- 46.Mohan S, Palanisamy A, Tsapepas D, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niederhaus SV, Muth B, Lorentzen DF, et al. Luminex-based desensitization protocols: The University of Wisconsin initial experience. Transplantation. 2011;92:12–17. doi: 10.1097/TP.0b013e31821c93bb. [DOI] [PubMed] [Google Scholar]
- 48.Djamali A, Muth B, Ellis TM, et al. Increased C4d in post-reperfusion biopsies and increased donor specific antibodies at one-week post transplant are risk factors for acute rejection in mild to moderately sensitized kidney transplant recipients. Kidney Int. 2013;83:1185–1192. doi: 10.1038/ki.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh N, Djamali A, Lorentzen D, et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation. 2010;90:1079–1084. doi: 10.1097/TP.0b013e3181f6a07b. [DOI] [PubMed] [Google Scholar]
- 50.Campos EF, Tedesco-Silva H, Machado PG, Franco M, Medina-Pestana JO, Gerbase-DeLima M. Post-transplant anti-HLA class II antibodies as risk factor for late kidney allograft failure. Am J Transplant. 2006;6:2316–2320. doi: 10.1111/j.1600-6143.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 51.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76–85. doi: 10.1111/j.1600-6143.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459–2465. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela NM, Hong L, Shen XD, et al. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. Am J Transplant. 2013;13:299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archdeacon P, Chan M, Neuland C, et al. Summary of FDA antibody-mediated rejection workshop. Am J Transplant. 2011;11:896–906. doi: 10.1111/j.1600-6143.2011.03525.x. [DOI] [PubMed] [Google Scholar]
- 55.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyan DB. New approaches for detecting complement-fixing antibodies. Curr Opin Organ Transplant. 2012;17:409–415. doi: 10.1097/MOT.0b013e328355fb9b. [DOI] [PubMed] [Google Scholar]
- 57.Gebel HM, Bray RA. The evolution and clinical impact of human leukocyte antigen technology. Curr Opin Nephrol Hypertens. 2010;19:598–602. doi: 10.1097/MNH.0b013e32833dfc3f. [DOI] [PubMed] [Google Scholar]
- 58.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 59.Halloran PF, de Freitas DG, Einecke G, et al. An integrated view of molecular changes, histopathology and outcomes in kidney transplants. Am J Transplant. 2010;10:2223–2230. doi: 10.1111/j.1600-6143.2010.03268.x. [DOI] [PubMed] [Google Scholar]
- 60.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 61.Everly MJ, Everly JJ, Arend LJ, et al. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant. 2009;9:1063–1071. doi: 10.1111/j.1600-6143.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 62.Lederer SR, Kluth-Pepper B, Schneeberger H, Albert E, Land W, Feucht HE. Impact of humoral alloreactivity early after transplantation on the long-term survival of renal allografts. Kidney Int. 2001;59:334–341. doi: 10.1046/j.1523-1755.2001.00495.x. [DOI] [PubMed] [Google Scholar]
- 63.Hidalgo LG, Campbell PM, Sis B, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9:2532–2541. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 64.Poduval RD, Kadambi PV, Josephson MA, et al. Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation. 2005;79:228–235. doi: 10.1097/01.tp.0000148987.13199.10. [DOI] [PubMed] [Google Scholar]
- 65.Kedainis RL, Koch MJ, Brennan DC, Liapis H. Focal C4d+ in renal allografts is associated with the presence of donor-specific antibodies and decreased allograft survival. Am J Transplant. 2009;9:812–819. doi: 10.1111/j.1600-6143.2009.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dragun D, Müller DN, Bräsen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 67.Martin L, Guignier F, Mousson C, Rageot D, Justrabo E, Rifle G. Detection of donor-specific anti-HLA antibodies with flow cytometry in eluates and sera from renal transplant recipients with chronic allograft nephropathy. Transplantation. 2003;76:395–400. doi: 10.1097/01.TP.0000078895.24606.45. [DOI] [PubMed] [Google Scholar]
- 68.Vidard L, Kovacsovics-Bankowski M, Kraeft SK, Chen LB, Benacerraf B, Rock KL. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 1996;156:2809–2818. [PubMed] [Google Scholar]
- 69.Loupy A, Suberbielle-Boissel C, Hill GS, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9:2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 70.Haas M, Montgomery RA, Segev DL, et al. Subclinical acute antibody-mediated rejection in positive crossmatch renal allografts. Am J Transplant. 2007;7:576–585. doi: 10.1111/j.1600-6143.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 71.Gloor JM, Sethi S, Stegall MD, et al. Transplant glomerulopathy: Subclinical incidence and association with alloantibody. Am J Transplant. 2007;7:2124–2132. doi: 10.1111/j.1600-6143.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 72.Sis B, Jhangri GS, Riopel J, et al. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012;12:1168–1179. doi: 10.1111/j.1600-6143.2011.03931.x. [DOI] [PubMed] [Google Scholar]
- 73.Ben-Dov IZ, Muthukumar T, Morozov P, Mueller FB, Tuschl T, Suthanthiran M. MicroRNA sequence profiles of human kidney allografts with or without tubulointerstitial fibrosis. Transplantation. 2012;94:1086–1094. doi: 10.1097/TP.0b013e3182751efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Freitas MC, Rebellato LM, Ozawa M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95:1113–1119. doi: 10.1097/TP.0b013e3182888db6. [DOI] [PubMed] [Google Scholar]
- 75.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16:12–17. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 76.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91:342–347. doi: 10.1097/TP.0b013e318203fd26. [DOI] [PubMed] [Google Scholar]
- 77.Otten HG, Verhaar MC, Borst HP, Hene RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant. 2007;7:2809–2815. doi: 10.1111/j.1600-6143.2011.03985.x. [DOI] [PubMed] [Google Scholar]
- 78.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, luminex binding antibodies—A new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7:2809–2815. doi: 10.1111/j.1600-6143.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 79.Wahrmann M, Bartel G, Exner M, et al. Clinical relevance of preformed C4d-fixing and non-C4d-fixing HLA single antigen reactivity in renal allograft recipients. Transpl Int. 2009;22:982–989. doi: 10.1111/j.1432-2277.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 80.Blume OR, Yost SE, Kaplan B. Antibody-mediated rejection: Pathogenesis, prevention, treatment, and outcomes. J Transplant. 2012;2012:201754. doi: 10.1155/2012/201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amico P, Hönger G, Steiger J, Schaub S. Utility of the virtual crossmatch in solid organ transplantation. Curr Opin Organ Transplant. 2009;14:656–661. doi: 10.1097/MOT.0b013e328331c169. [DOI] [PubMed] [Google Scholar]
- 82.Bingaman AW, Murphey CL, Palma-Vargas J, Wright F. A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation. 2008;86:1864–1868. doi: 10.1097/TP.0b013e318191404c. [DOI] [PubMed] [Google Scholar]
- 83.Morath C, Opelz G, Zeier M, Süsal C. Prevention of antibody-mediated kidney transplant rejection. Transpl Int. 2012;25:633–645. doi: 10.1111/j.1432-2277.2012.01490.x. [DOI] [PubMed] [Google Scholar]
- 84.Claas FH, Witvliet MD, Duquesnoy RJ, Persijn GG, Doxiadis II. The acceptable mismatch program as a fast tool for highly sensitized patients awaiting a cadaveric kidney transplantation: Short waiting time and excellent graft outcome. Transplantation. 2004;78:190–193. doi: 10.1097/01.tp.0000129260.86766.67. [DOI] [PubMed] [Google Scholar]
- 85.Jordan SC, Tyan D, Stablein D, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: Report of the NIH-IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 86.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 87.Alachkar N, Lonze BE, Zachary AA, et al. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte antigen antibodies in highly sensitized patients. Transplantation. 2012;94:165–171. doi: 10.1097/TP.0b013e318253f7b6. [DOI] [PubMed] [Google Scholar]
- 88.Kozlowski T, Andreoni K. Limitations of rituximab/IVIg desensitization protocol in kidney transplantation; is this better than a tincture of time. Ann Transplant. 2011;16:19–25. doi: 10.12659/aot.881860. [DOI] [PubMed] [Google Scholar]
- 89.Marfo K, Ling M, Bao Y, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation. 2012;94:345–351. doi: 10.1097/TP.0b013e3182590d2e. [DOI] [PubMed] [Google Scholar]
- 90.Noel C, Abramowicz D, Durand D, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20:1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thibaudin D, Alamartine E, de Filippis JP, Diab N, Laurent B, Berthoux F. Advantage of antithymocyte globulin induction in sensitized kidney recipients: A randomized prospective study comparing induction with and without antithymocyte globulin. Nephrol Dial Transplant. 1998;13:711–715. doi: 10.1093/ndt/13.3.711. [DOI] [PubMed] [Google Scholar]
- 92.Mako M, Hager D, Muth B, Kaufman D, Ellis T, Djamali A. Thymoglobulin is associated with a significant reduction in de novo donor-specific antibodies and antibody mediated rejection in moderately sensitized renal transplant recipients. Am J Transplant. 2013;13:113. (Suppl 5, Abstract #265): [Google Scholar]
- 93.Chandraker A, Guleria I, Ikle D. Development of de novo anti-HLA abs in kidney transplant recipients: Final analysis of the NIH CTOT02 study. Am J Transplant. 2013;13:113. , et al. (Suppl 5, Abstract #268): [Google Scholar]
- 94.Harmon W, Guleria I, Ikle D. Children develop de novo anti-HLA antibodies (Abs) following kidney transplantation at a higher incidence than adults: An analysis of the NIH CTOT/CCTPT-02 study. Am J Transplant. 2013;13:36–337. , et al. (Suppl 5, Abstract #21): [Google Scholar]
- 95.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 96.Haririan A, Nogueira J, Kukuruga D, et al. Positive cross-match living donor kidney transplantation: Longer-term outcomes. Am J Transplant. 2009;9:536–542. doi: 10.1111/j.1600-6143.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 97.Morgan RD, O’Callaghan JM, Knight SR, Morris PJ. Alemtuzumab induction therapy in kidney transplantation: A systematic review and meta-analysis. Transplantation. 2012;93:1179–1188. doi: 10.1097/TP.0b013e318257ad41. [DOI] [PubMed] [Google Scholar]
- 98.Lü TM, Yang SL, Wu WZ, Tan JM. Alemtuzumab induction therapy in highly sensitized kidney transplant recipients. Chin Med J (Engl) 2011;124:664–668. [PubMed] [Google Scholar]
- 99.Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909–1919. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 100.Knechtle SJ, Pirsch JD, H Fechner J, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 101.Bloom D, Chang Z, Pauly K, et al. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9:1835–1845. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Todeschini M, Cortinovis M, Perico N, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol. 2013;191:2818–2828. doi: 10.4049/jimmunol.1203261. [DOI] [PubMed] [Google Scholar]
- 103.Barnett AN, Hadjianastassiou VG, Mamode N. Rituximab in renal transplantation. Transpl Int. 2013;26:563–575. doi: 10.1111/tri.12072. [DOI] [PubMed] [Google Scholar]
- 104.van den Hoogen M, Steenbergen E, Hoitsma A, Hilbrands L. Rituximab to prevent renal allograft rejection; a randomized, double-blind, placebo-controlled trial. Am J Transplant. 2013;13:112. (Suppl 5, Abstract #266.1): [Google Scholar]
- 105.Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. 2012;12:469–476. doi: 10.1111/j.1600-6143.2011.03830.x. [DOI] [PubMed] [Google Scholar]
- 106.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 107.Everly MJ, Everly JJ, Terasaki PI. Role of proteasome inhibition in sensitized transplant candidates. Chin Med J (Engl) 2011;124:771–774. [PubMed] [Google Scholar]
- 108.Everly MJ, Terasaki PI, Trivedi HL. Durability of antibody removal following proteasome inhibitor-based therapy. Transplantation. 2012;93:572–577. doi: 10.1097/TP.0b013e31824612df. [DOI] [PubMed] [Google Scholar]
- 109.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–2413. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 110. http://ClinicalTrials.gov A service of the U.S. National Institutes of Health. Available at: http://www.clinicaltrials.gov/. Accessed June 3, 2013.
- 111.Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation. 2013;95:852–858. doi: 10.1097/TP.0b013e3182802f88. [DOI] [PubMed] [Google Scholar]
- 112.Melcher ML, Leeser DB, Gritsch HA, et al. Chain transplantation: Initial experience of a large multicenter program. Am J Transplant. 2012;12:2429–2436. doi: 10.1111/j.1600-6143.2012.04156.x. [DOI] [PubMed] [Google Scholar]
- 113.Roodnat JI, Kal-van Gestel JA, Zuidema W, et al. Successful expansion of the living donor pool by alternative living donation programs. Am J Transplant. 2009;9:2150–2156. doi: 10.1111/j.1600-6143.2009.02745.x. [DOI] [PubMed] [Google Scholar]
- 114.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. JAMA. 2005;293:1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 115.Almeshari K, Pall A, Chaballout A, et al. Targeted monitoring of donor-specific HLA antibodies following renal transplantation. Clin Transplant. 2011:395–400. [PubMed] [Google Scholar]
- 116.Liefeldt L, Brakemeier S, Glander P, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant. 2012;12:1192–1198. doi: 10.1111/j.1600-6143.2011.03961.x. [DOI] [PubMed] [Google Scholar]
- 117.Rostaing L, Massari P, Garcia VD, et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: A randomized phase II study. Clin J Am Soc Nephrol. 2011;6:430–439. doi: 10.2215/CJN.05840710. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pestana JO, Grinyo JM, Vanrenterghem Y. Three-year outcomes from BENEFIT-EXT: A phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12:630–639. doi: 10.1111/j.1600-6143.2011.03914.x. , et al. [DOI] [PubMed] [Google Scholar]
- 119.Vincenti F, Larsen CP, Alberu J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. , et al. [DOI] [PubMed] [Google Scholar]
- 120.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 121.Lucas JG, Co JP, Nwaogwugwu UT, Dosani I, Sureshkumar KK. Antibody-mediated rejection in kidney transplantation: An update. Expert Opin Pharmacother. 2011;12:579–592. doi: 10.1517/14656566.2011.525219. [DOI] [PubMed] [Google Scholar]
- 122.Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients: A systematic review. Transplantation. 2012;94:775–783. doi: 10.1097/TP.0b013e31825d1587. [DOI] [PubMed] [Google Scholar]
- 123.Böhmig GA, Wahrmann M, Regele H, et al. Immunoadsorption in severe C4d-positive acute kidney allograft rejection: A randomized controlled trial. Am J Transplant. 2007;7:117–121. doi: 10.1111/j.1600-6143.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 124.Blake P, Sutton D, Cardella CJ. Plasma exchange in acute renal transplant rejection. Prog Clin Biol Res. 1990;337:249–252. [PubMed] [Google Scholar]
- 125.Bonomini V, Vangelista A, Frasca GM, Di Felice A, Liviano D’Arcangelo G. Effects of plasmapheresis in renal transplant rejection: A controlled study. Trans Am Soc Artif Intern Organs. 1985;31:698–703. [PubMed] [Google Scholar]
- 126.Kirubakaran MG, Disney AP, Norman J, Pugsley DJ, Mathew TH. A controlled trial of plasmapheresis in the treatment of renal allograft rejection. Transplantation. 1981;32:164–165. doi: 10.1097/00007890-198108000-00019. [DOI] [PubMed] [Google Scholar]
- 127.Allen NH, Dyer P, Geoghegan T, Harris K, Lee HA, Slapak M. Plasma exchange in acute renal allograft rejection: A controlled trial. Transplantation. 1983;35:425–428. doi: 10.1097/00007890-198305000-00006. [DOI] [PubMed] [Google Scholar]
- 128.Franco A, Anaya F, Niembro E, Ahijado F, Luño J, Valderrábano F. Plasma exchange in the treatment of vascular rejection: Relationship between histological changes and therapeutic response. Transplant Proc. 1987;19:3661–3663. [PubMed] [Google Scholar]
- 129.Lefaucheur C, Nochy D, Andrade J, et al. Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099–1107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 130.Kaposztas Z, Podder H, Mauiyyedi S, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 131.Vangelista A, Frasca GM, Nanni Costa A, Stefoni S, Bonomini V. Value of plasma exchange in renal transplant rejection induced by specific anti-HLA antibodies. Trans Am Soc Artif Intern Organs. 1982;28:599–603. [PubMed] [Google Scholar]
- 132.Macaluso J, Killackey M, Paramesh A, et al. Comparative study of bortezomib therapy for antibody mediated rejection. Am J Transplant. 2011;11:160. (Suppl 2, Abstract #431): [Google Scholar]
- 133.Lubetzky ML, Walker JK, Matignon M , et al. Evolving therapies for antibody mediated rejection: Is bortezomib better than rituximab [abstract SA-PO3065]. World Congress of Nephrology 2011. Vancouver, Canada; 2011.
- 134.Waiser J, Schutz M, Liefeldt L, Schonemann C, Neumayer H-H, Budde K. Treatment of antibody-mediated renal allograft rejection with bortezomib or rituximab. Am J Transplant. 2010;10:466. doi: 10.1093/ndt/gfr465. (Suppl 4, Abstract #1504): [DOI] [PubMed] [Google Scholar]
- 135.Sautenet B, Blancho G, Buchler M, et al. One year results of the effects of rituximab on acute humoral rejection in renal transplantation: RITUX ERAH, a multicenter randomized placebo controlled trial. Am J Transplant. 2013;13:112. doi: 10.1097/TP.0000000000000958. , et al. (Suppl 5, Abstract #266): [DOI] [PubMed] [Google Scholar]
- 136.Locke JE, Magro CM, Singer AL, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231–235. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 137.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. (Suppl 3): [DOI] [PubMed] [Google Scholar]
- 138.Fehr T, Gaspert A. Antibody-mediated kidney allograft rejection: Therapeutic options and their experimental rationale. Transpl Int. 2012;25:623–632. doi: 10.1111/j.1432-2277.2012.01453.x. [DOI] [PubMed] [Google Scholar]
- 139.Smith RN, Malik F, Goes N, et al. Partial therapeutic response to rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transpl Immunol. 2012;27:107–113. doi: 10.1016/j.trim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clatworthy MR. Targeting B cells and antibody in transplantation. Am J Transplant. 2011;11:1359–1367. doi: 10.1111/j.1600-6143.2011.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sato Y, Sahara H, Tsukahara T, et al. Improved generation of HLA class I/peptide tetramers. J Immunol Methods. 2002;271:177–184. doi: 10.1016/s0022-1759(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 142.Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13:2280–2292. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heidt S, Hester J, Shankar S, Friend PJ, Wood KJ. B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naïve B cells. Am J Transplant. 2012;12:1784–1792. doi: 10.1111/j.1600-6143.2012.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Willicombe M, Roufosse C, Brookes P, et al. Antibody-mediated rejection after alemtuzumab induction: Incidence, risk factors, and predictors of poor outcome. Transplantation. 2011;92:176–182. doi: 10.1097/TP.0b013e318222c9c6. [DOI] [PubMed] [Google Scholar]
- 145.Knechtle SJ, Pascual J, Bloom DD, et al. Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: Clinical results and immune monitoring. Am J Transplant. 2009;9:1087–1098. doi: 10.1111/j.1600-6143.2009.02581.x. [DOI] [PubMed] [Google Scholar]
- 146.Martin ST, Tichy EM, Gabardi S. Belatacept: A novel biologic for maintenance immunosuppression after renal transplantation. Pharmacotherapy. 2011;31:394–407. doi: 10.1592/phco.31.4.394. [DOI] [PubMed] [Google Scholar]
- 147.Lemy A, Toungouz M, Abramowicz D. Bortezomib: A new player in pre- and post-transplant desensitization. Nephrol Dial Transplant. 2010;25:3480–3489. doi: 10.1093/ndt/gfq502. [DOI] [PubMed] [Google Scholar]
- 148.Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: A review. J Transplant. 2012;2012:193724. doi: 10.1155/2012/193724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Webber A. Update on treatment options for antibody-mediated rejection [oral presentation]. Presented at UCSF Transplant Symposium, San Francisco, CA; 2012, September 27–28. Available at: http://www.ucsfcme.com/2012/slides/MMC13001/12.%20Allison%20Webber.pdf. Accessed July 31, 2013.


