Senatorov et al. reveal a bilateral reduction in anterior insula volume, and bilateral increase in amygdala volume, in alcohol-dependent subjects compared to healthy controls. Post-mortem histological studies suggest that the lower anterior insula volumes may reflect a 60% reduction in von Economo neurons in subjects with a history of alcoholism.
Keywords: insula, amygdala, von Economo neurons, alcoholism, MRI
Abstract
The insula, a structure involved in higher order representation of interoceptive states, has recently been implicated in drug craving and social stress. Here, we performed brain magnetic resonance imaging to measure volumes of the insula and amygdala, a structure with reciprocal insular connections, in 26 alcohol-dependent patients and 24 healthy volunteers (aged 22–56 years, nine females in each group). We used an established morphometry method to quantify total and regional insular volumes. Volumetric measurements of the amygdala were obtained using a model-based segmentation/registration tool. In alcohol-dependent patients, anterior insula volumes were bilaterally reduced compared to healthy volunteers (left by 10%, right by 11%, normalized to total brain volumes). Furthermore, alcohol-dependent patients, compared with healthy volunteers, had bilaterally increased amygdala volumes. The left amygdala was increased by 28% and the right by 29%, normalized to total brain volumes. Post-mortem studies of the anterior insula showed that the reduced anterior insular volume may be associated with a population of von Economo neurons, which were 60% diminished in subjects with a history of alcoholism (n = 6) as compared to subjects without a history of alcoholism (n = 6) (aged 32–56 years, all males). The pattern of neuroanatomical change observed in our alcohol-dependent patients might result in a loss of top-down control of amygdala function, potentially contributing to impaired social cognition as well as an inability to control negatively reinforced alcohol seeking and use.
Introduction
In humans and other primates, the insular cortex is a key element of circuitry that represents higher order interoceptive states, making this structure an important substrate of subjective feelings, emotions and self-awareness (Craig, 2009). Within the insula, the posterior subdivision receives higher order visceral afferents, while the anterior subdivision is thought to encode their emotional salience (Dupont et al., 2003). As predicted by the ‘somatic marker theory’ (Bechara et al., 2000), this circuitry is also engaged by complex external stimuli that are emotionally salient, such as social exclusion stress (Eisenberger et al., 2003). Social stress is a potent trigger of craving and relapse in people with addictive disorders (Sinha et al., 2011), pointing to a potential link between insula function and addiction. Direct support for this link has been provided by several observations. For instance, structural integrity of the insula was found to be necessary for maintaining addiction to cigarette smoking (Naqvi et al., 2007), while functional brain imaging studies have consistently found a correlation between insula activity and conscious cravings for several drug categories, including alcohol (Naqvi and Bechara, 2010).
Reductions in insular surface or grey matter volume have been reported in patients with several psychiatric disorders, including schizophrenia (Takahashi et al., 2005), fragile X syndrome (Cohen et al., 2011), and Williams syndrome (Cohen et al., 2010), while an increase in anterior insula volume was reported in obsessive-compulsive disorder (Nishida et al., 2011). Little is known, however, about potential structural pathology of the insular cortex in alcoholism. One study reported altered shape of the insula in alcoholic patients (Jung et al., 2007), and several others, including our own previous report, found that alcoholism is associated with decreased volume (Makris et al., 2008) and cortical thickness (Momenan et al., 2012) of the right insula and decreased grey matter volume of bilateral insula (Demirakca et al., 2011). Here, we sought to further investigate these findings by carrying out a volumetric analysis of the insular cortex, with a specific interest in the anterior insula, in recently detoxified alcohol-dependent patients (ADPs). We were additionally interested in the amygdala, a subcortical structure that has extensive structural (Augustine, 1996; Nieuwenhuys, 2012) and functional connections with the insula (Stein et al., 2007; Roy et al., 2009; Robinson et al., 2010; Cauda et al., 2011). An inverse relationship between changes in insula and amygdala volumes has been reported in certain pathological conditions such as Williams syndrome (Cohen et al., 2010), while enlargement of the amygdala has also been demonstrated in major depressive disorder (Frodl et al., 2003; Hamilton et al., 2008) and chronic social stress (Davidson and McEwen, 2012). We compared ADPs with healthy volunteers and hypothesized that alcohol dependence is associated with: (i) a decreased volume of the anterior insula, normalized to total brain volume; (ii) an increased volume of the amygdala, normalized to total brain volume; and (iii) an increased ratio of the volumes of the amygdala and insula.
Materials and methods
MRI acquisition and processing
All images were acquired using T1-weighted MP-RAGE pulse sequence with matrix 256 × 256 × 124 on 1.5 T General Electric MRI scanner (General Electric). Imaging parameters were: echo time = 12 ms; repetition time = 100 ms; field of view = 24 cm. Voxel dimensions were 0.9375 × 0.9375 × 2.0 mm3. A 12-channel RF coil was used.
The total brain volume measurements, including total grey matter volumes and white matter volumes, were performed by deleting non-brain tissue from an image of the whole head using the Brain Extraction Tool and an in-house intensity-based segmentation algorithm (Momenan et al., 1997). The Brain Extraction Tool is documented and freely available for download from FSL [FMRIB (Functional Magnetic Resonance Imaging of the Brain) Software Library] (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/).
An established manual morphometric method was used to quantify total and regional insular volumes (Pressler et al., 2005; Takahashi et al., 2005; Cohen et al., 2010; Nishida et al., 2011). Briefly, brain images were standardized by realigning their orientation in three dimensions using five points in the mid-sagittal plane, including the superior and posterior edges of the anterior commissure, the inferior edge of the posterior commissure, and two other mid-sagittal points chosen at will. These images were reconstructed into contiguous coronal, axial and sagittal slices with a 1-mm thickness. The insular cortex was manually delineated on consecutive slices using axial and sagittal images to adjust the border of insula in three dimensions. To ensure the blind, an individual not directly involved in the project randomly coded the images. The tracing was performed by two independent raters with good knowledge of brain anatomy using interactive pen display Cintiq 21UX (Wacom Co., Ltd). Interrater reliability was established by calculating the intraclass correlation coefficient of insula volumes (ICC = 0.83).
The sulcus centralis serves as an approximate boundary between the anterior agranular and the posterior granular areas of the insula (Nieuwenhuys, 2012). When brain images are aligned as above, the sulcus centralis lies roughly along the coronal plane via the anterior commissure that corresponds to 0 mm in anterior-posterior direction (Fig. 1B). We designated all voxels located within or anterior to this plane (y ≥ 0 mm) as belonging to the anterior insula, and all voxels located posterior to this plane (y < 0 mm), as belonging to the posterior insula, as shown in Fig. 1B.
Figure 1.
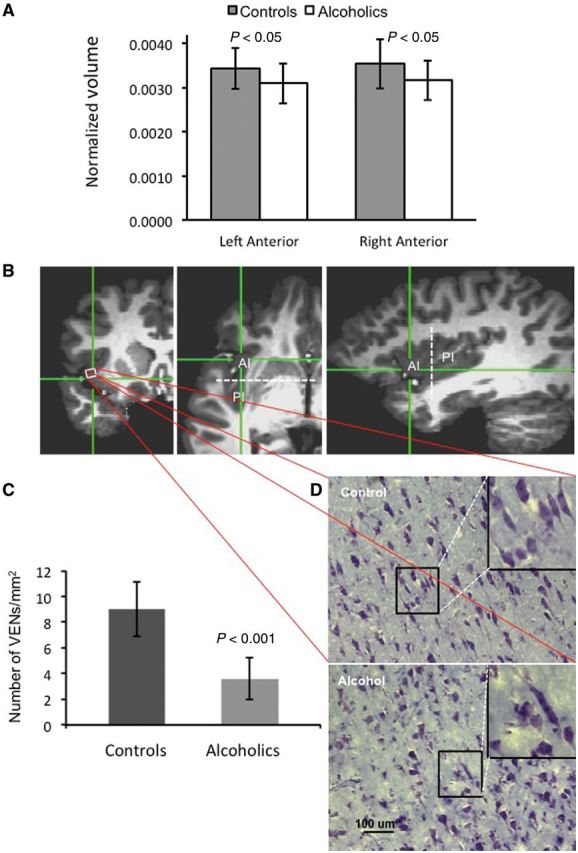
Volumetric measurements of the anterior insula in ADPs versus healthy volunteers. (A) Normalized volumes of the left and right, anterior and posterior insular subdivisions in ADPs versus healthy volunteers. Note the statistically significant bilateral decrease in the anterior insula of ADPs. (B) Magnetic resonance images in three different planes to show the location of tissue samples taken for histological investigation. Hair-crosses show approximate location of histological sections shown in (C). Dotted white line indicates a coronal plane through the anterior commissure with y = 0, which represents the estimated border between anterior and posterior insular divisions. (C) Quantitative analysis of VENs and all Nissl stained cells in ADPs and healthy volunteers. Note the reduction in the number of VENs in ADPs versus healthy volunteers. (D) Representative images of Nissl staining through the anterior insula. Note the paucity of VENs in ADPs as compared with healthy volunteers. Insets show enlarged view of VENs.
Amygdala measurements were performed using FIRST (FMRIB’s Integrated Registration and Segmentation Tool), a model-based segmentation/registration tool (Patenaude et al., 2011). The shape/appearance models used in FIRST are constructed from manually segmented images provided by the Centre for Morphometric Analysis (Massachusetts General Hospital, Boston, MA). The manual labels are parameterized as surface meshes and modelled as a point distribution model. Deformable surfaces are used to automatically parameterize the volumetric labels in terms of meshes; the deformable surfaces are constrained to preserve vertex correspondence across the training data. Furthermore, normalized intensities along the surface normals are sampled and modelled. The shape and appearance model is based on multivariate Gaussian assumptions. Shape is then expressed as a mean with modes of variation (principal components). Based on these learned models, FIRST searches through linear combinations of shape modes of variation for the most probable shape instance given the observed intensities in each participant’s T1 image. While FIRST is an automated method to obtain volume measurements, the program uses a highly sophisticated algorithm that has several tests (including segmentation and statistical procedures) that attempt to correct for and minimize type I and type II errors. In addition, this software provides outlines of segmentation results, slice-by-slice (grey matter, white matter, CSF boundaries), for visual inspection. This feature was used to ascertain the segmentation integrity to the extent that an automated algorithm can be verified.
Histological analysis of von Economo neurons
Anterior insula samples of post-mortem human brains were cryoprotected with 30% sucrose and frozen. Five consecutive sets containing 16 to 20-µm thick coronal sections from each insula were cut on cryostat and mounted on gelatinized slides. Every eighth section was stained with Cresyl violet. Five stained sections from each right anterior insula of subjects with a history of alcoholism (n = 6) and controls (n = 6) were used for quantitative analysis. The cell counting was performed by an experienced morphologist with good knowledge of brain histology, blinded to the diagnostic status of the slices. To ensure the blind, an individual not directly involved in the project randomly coded the sections. von Economo neurons (VENs), also known as ‘spindle cells’, were recognized using characteristic morphology (Butti et al., 2013) and counted in each microscopic field of 0.54 mm2 using a ×20 microscope objective. Results for VENs were depicted as counts per mm2 after counting the total number of VENs found in 20 examined microscope fields totalling 10.8mm2 (four fields per section × five sections per case). Quantitative analysis of the total number of Nissl stained cells was performed using a Leica DM6000CS light microscope (Leica Microsystem Inc) at ×20 magnification and images were captured by an attached digital camera (Q Imaging). Densitometry counts were obtained using BIOQUANT software (BIOQUANT Image Analysis Corporation) and data were reported as total number of cells divided per square millimetre of surface.
Participants
MRI studies were performed on 26 ADPs (nine females) who had completed detoxification at the NIH Clinical Research Centre, Bethesda, MD. Twenty-four healthy volunteers (nine females) were also recruited (see Table 1 for more demographic information). Each participant received a physical and neurological examination, performed by an attending physician, and a clinical MRI, read by a radiologist. Other laboratory results and instruments, including standard blood work and an ECG (electrocardiogram), were administered as part of the screening procedure to determine each individual’s eligibility. The participants in this study were deemed as ‘healthy’ by the attending physician and were void of any neurological disorders. The extensive screening process also included the administration of the Structured Clinical Interview for the DSM (Diagnostic and Statistical Manual of Mental Disorders)—SCID. SCIDs were performed for preliminary assessment by trained research assistants. All SCID interviews were reviewed and approved and any suspected clinical conditions were followed up and diagnosed by an attending physician. Individuals with current or past primary diagnoses of schizophrenia, bipolar disorder or psychotic disorders were excluded. Participants with mood, anxiety or drug use disorders were excluded if they were on any psychotropic medications. For a summary of participants with comorbid substance use and mood or anxiety disorders, see Table 2. Four participants in the healthy volunteer group had past alcohol dependence (diagnosed by DSM-IV; see below for our justification regarding including these individuals in the study).
Table 1.
Demographic and alcohol consumption data
| Diagnosis | Controls, mean ± SD | ADPs, mean ± SD |
|---|---|---|
| Total number | 24 | 26 |
| Number of females | 9 | 9 |
| Number of males | 15 | 17 |
| Age (years) | 31.5 ± 8.9 | 34.2 ± 7.6, t = 2.016, P = 0.247 |
| Education (years) | 15.5 ± 4.2 | 13.4 ± 1.8, t = 2.012, P = 0.026 |
| Years of alcohol intoxicationa | N/A | 13.0 ± 7.2 |
| Age of first intoxicationa | N/A | 21.9 ± 5.9 |
| Times treated for alcohol abusea | N/A | 4.1 ± 4.4 |
| Alcohol Dependence Scale | N/A | 22.8 ± 6.1 |
| Penn Alcohol Craving Scaleb | N/A | 9.3 ± 7.7 |
| Depression Scorec | N/A | 7.4 ± 5.3 |
| Anxiety Scorec | N/A | 7.6 ± 4.2 |
| Days of sobriety before scan | N/A | 22.6 ± 2.9 |
aObtained from the Addiction Severity Index.
bAdministered at Day 19 of treatment.
cScores obtained from the Comprehensive Psychopathological Rating Scale for depression and anxiety symptoms; administered at Day 23 of treatment.
N/A = not available.
Table 2.
Comorbid disorders in participants
| Diagnosis (lifetime unless indicated) | Controls (n = 24) | ADPs (n = 26) |
|---|---|---|
| Alcohol dependence (current, past) | 0, 4 | 26, 0a |
| Alcohol abuse (current, past) | 0, 3 | 0a |
| Any other substance disorder (current, past) | 0, 3 | 10, 16 |
| Any anxiety disorder (current, past) | 0, 0 | 14, 6 |
| Panic disorder | 0 | 1 |
| Social phobia | 0 | 5 |
| Agoraphobia | 0 | 1 |
| Generalized anxiety disorder | 0 | 4 |
| Post-traumatic stress disorder | 0 | 10 |
| Any mood disorder (current, past) | 0, 1 | 3, 5 |
| Major depressive disorder | 0 | 6 |
| Dysthymic disorder | 0 | 1 |
| Smoking (current only) | 4 | 20 |
| Any mood, anxiety, or other substance use disorder (excluding alcohol and smoking) | 4 | 23 |
aA diagnosis of past alcohol dependence and current alcohol abuse are not considered if an individual meets alcohol dependence criteria.
All ADPs were assessed using the Alcohol Dependence Scale for alcohol dependence severity (Skinner and Allen, 1982), the Penn Alcohol Craving Scale (Flannery et al. 1999) for craving, the Addiction Severity Index (McLellan et al. 1980) for lifetime alcohol-related problems and the Comprehensive Psychopathological Rating Scale (Asberg et al., 1978) for depression and anxiety symptoms during inpatient detoxification and treatment. Average age, years of education, drinking measures (from the Addiction Severity Index), the Alcohol Dependence Scale, the Penn Alcohol Craving Scale (obtained on Day 19 of treatment, closest to the average scan date), the Comprehensive Psychopathological Rating Scale for depression and anxiety symptoms (obtained on Day 23 of treatment), and the days of sobriety and days of admission before the MRI scan are summarized in Table 1. On average, ADPs were sober for 22.6 days (with the range from 20 to 30 days) before the MRI scan.
For the post-mortem histology study, we obtained human tissue from the NICHD (Eunice Kennedy Shriver National Institute of Child Health and Human Development) Brain and Tissue Bank at the University of Maryland, Baltimore, MD. The post-mortem brain database was screened by NICHD Brain and Tissue Bank personnel for suitable subjects who were without additional comorbidities, based on the available medical histories of the subjects. Brain samples of 12 males (six alcoholics and six controls; aged 32–56 years) who had died from cardiovascular diseases, were included in the study. All insula samples were from the right hemisphere, fixed in 10% formalin 8–20 h post-mortem and sectioned coronally at 5–10 mm interval (http://medschool.umaryland.edu/btbank/method2.asp).
Statistical analysis
Statistical analysis was carried out using the software package JMP-SAS 10 (SAS Institute Inc). For between-group (ADP versus healthy volunteer) comparisons of brain structure volumes, we used an analysis of covariance (ANCOVA) with age and education as covariates (Makris et al., 2008). To ensure that the assumptions underlying the use of ANCOVA were met, we performed the following tests: the box plot and the residual-by-predicted plot indicated an absence of outliers; the Shapiro-Wilk’s test indicated normal distribution for both the data and residuals. The data in both groups demonstrated homoscedasticity using a two-sided F-test. We also examined the regression slopes for equivalency and found that the slopes were parallel. The F-test did not show a significant effect on the slopes; a high leverage and influence analysis was performed using Hat and Cook’s D Influence statistics. We used 2(P + 1) / n and 4 / n, respectively, as cut-off values. We found one observation with very high influence; finally, the magnitude of multicollinearity was analysed by considering the size of the variance inflation rate. All values were less than the cut-off value of 10. A two-tailed alpha of 0.05 was chosen as the threshold for statistical significance for all statistical tests. Degrees of freedom for ANCOVA were calculated based on equation n − 1 − K, where n is the number of observation (participants) and K is the number of covariates. To account for multiple comparisons, the Holm–Bonferroni method was used.
We used total brain volume (as opposed to intracranial volume) to normalize our volumetric measurements. While intracranial volume is a widely used normalizing factor to detect overall brain shrinkage, it will not be a sufficient normalization factor to determine whether the observed effects are global and similar in entire brain or local to a particular region (i.e. the insula). Normalization by intracranial volume will not reveal selective differences in effect of alcohol unless the volumes of all regions of the entire brain are segmented and compared. As we had a priori hypothesized that alcohol has a selective effect on insular regions in contrast to the rest of brain, we chose total brain volume as the more appropriate normalization factor. For calculating the amygdala/insula ratio for each brain, we divided the total volume of the amygdala by the total volume of the insula. Pearson correlation (bivariate analysis) was used to examine the relationship between the volumes of the insula and the amygdala. We used a two-way ANOVA to evaluate statistical interactions (e.g. region × diagnosis) between the volumes of the insula and the amygdala between the two groups.
There were very few alcoholic non-smokers; therefore, we were unable to compare the effects of cigarette smoking on healthy volunteers and ADPs. To study the effect of smoking on structural brain changes in ADPs, we probe the relationship between the number of pack-years and the volumes of the insula and amygdalae by using multiple regression analysis (ANCOVA) with age and years of lifetime alcohol intoxication as covariates.
Student’s t-test with Holm–Bonferroni correction was used for the statistical analysis of the correlation histological data. To check for similarity, we used an equivalence test (two one-sided t-tests), and provided confidence intervals.
Results
Alcohol-dependent patients with comorbid drug use
Seventeen of 26 ADPs met criteria for a lifetime substance use disorder other than alcohol (Table 2). Each ADP, however, cited alcohol as their primary drug of choice. We examined the years of heavy drinking for alcoholics with and without a history of other comorbid substance use disorders, and found that they were not statistically significant with mean values of 14.1 years [95% confidence interval (CI): 3.9, 15.3] and 9.6 years (95% CI: 10.3–18.0).
Intracranial and total brain volume
We did not find a significant difference between the intracranial volume and the total brain volume in healthy volunteers as compared with ADPs (Table 3).
Table 3.
Absolute and normalized volumes of different head compartments and brain structures
| Diagnosis | Healthy volunteers (n = 24), mean ± SD | ADPs (n = 26), mean ± SD |
|---|---|---|
| Total brain (cm3) | 1169 ± 110 | 1134 ± 148, F = 0.592, P = 0.4461 |
| Intracranial volume (cm3) | 1378 ± 85 | 1322 ± 209, F = 1.200, P = 0.279 |
| Left anterior insula (mm3) | 4007 ± 544 | 3512 ± 655, F = 6.586, P = 0.014 |
| Normalized to total brain | 0.00351 ± 0.00038 | 0.00311 ± 0.00046, F = 6.242, P = 0.016 |
| Normalized to ICV | 0.00293 ± 0.00033 | 0.00267 ± 0.00038, F = 5.020, P = 0,030 |
| Right anterior insula (mm3) | 4162 ± 720 | 3579 ± 621, F = 6.772, P = 0.012 |
| Normalized to total brain | 0.00358 ± 0.00055 | 0.00316 ± 0.00044, F = 5.817, P = 0.020 |
| Normalized to ICV | 0.00298 ± 0.00042 | 0.00272 ± 0.00034, F = 5.351, P = 0.025 |
| Left amygdala (mm3) | 1183 ± 231 | 1522 ± 283, F = 15.301, P = 0.0003 |
| Normalized to total brain | 0.00105 ± 0.00023 | 0.00134 ± 0.00017, F = 16.581, P = 0.0002 |
| Normalized to ICV | 0.00106 ± 0.00018 | 0.00134 ± 0.00018, F = 16.581, P = 0.0002 |
| Right amygdala (mm3) | 1104 ± 273 | 1424 ± 315, F = 11.287, P = 0.001 |
| Normalized to total brain | 0.00099 ± 0.00025 | 0.00125 ± 0.00020, 13.031, P = 0.001 |
| Normalized to ICV | 0.00082 ± 0.00019 | 0.00108 ± 0.00017, 18.021, P = 0.0001 |
ICV = intracranial volume.
Insular cortex measurements
Because there are significant functional and structural differences between the anterior and posterior insula, we performed separate image analysis of these subdivisions. We found significant decreases in the volumes of both left (12%) and right (13%) anterior insula in ADPs as compared with healthy volunteers (Table 3). When we normalized the measurements to the total brain volume, decreases in the left and right anterior insula were 10 and 11%, respectively (Fig. 1A). The differences in both the absolute and normalized volumes of the posterior insula between ADPs and healthy volunteers were not statistically significant. Absolute volumes for the left posterior insula were 3404 mm3 (95% CI: 3135–3673) in ADPs and 3522 mm3 (95% CI: 3310–3736) in healthy volunteers. Absolute volumes for the right posterior insula were 3132 mm3 (95% CI: 2868–3396) in ADPs and 3426 mm3 (95% CI: 3169–3683) in healthy volunteers. Normalized volumes for the left posterior insula were 0.0030 (95% CI: 0.0028–0.0033) in ADPs and 0.0031 (95% CI: 0.0028–0.0033) in healthy volunteers. Normalized volumes for the right posterior insula were 0.0028 (95% CI: 0.0025–0.0030) in ADPs and 0.0030 (95% CI: 0.0027–0.0032) in healthy volunteers. Additionally, an equivalence test showed that the posterior insula volume means were practically equivalent for ADPs and healthy volunteers.
Four healthy volunteers had past alcohol dependence (diagnosed by DSM-IV). In our primary analysis, we excluded these participants and found a significant difference in insula and amygdala volumes between healthy volunteers and ADPs. However, previous studies have shown that a highly significant portion of insula volume is recovered after 3 months of abstinence from alcohol (Demirakca et al., 2011). Therefore, we included these four healthy volunteers in the reanalysis to increase the power of the outcome. Finally, because alcohol may affect males and females differently, we used a two-way ANOVA to assess for differential effects of gender on the anterior insula volume in ADPs versus healthy volunteers and did not find a gender × group interaction (P = 0.76).
Histological analysis of von Economo neurons
To further investigate the underlying cellular mechanisms of the decreased anterior insula volume in ADPs, we performed a histological analysis of post-mortem brain tissues with a specific focus on populations of VENs in the anterior insula. We found a 60% reduction in the population of VENs in the anterior insula of subjects with a history of alcoholism compared to the control subjects (Fig. 2C). We did not find a difference in the total number of Nissl stained cells between alcoholics and controls (628 ± 117 and 625 ± 117/mm2).
Figure 2.
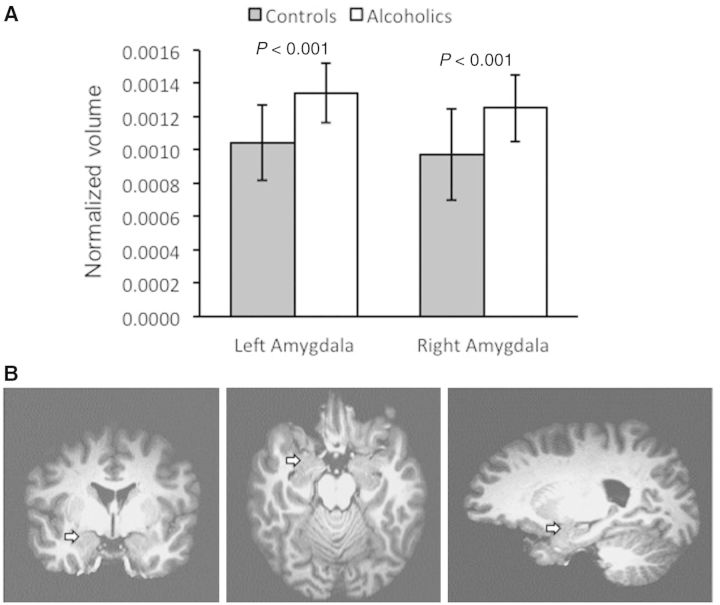
Volumetric measurements of the amygdala in ADPs versus healthy volunteers. (A) Normalized volumes of the left and right amygdala in ADPs versus healthy volunteers. Note the statistically significant bilateral increase in the amygdala of ADPs. (B) Magnetic resonance images in three different planes to show the location of the amygdala (arrows).
Amygdala measurements
The insula has direct reciprocal connections with the amygdala (Augustine, 1996; Nieuwenhuys, 2012). Therefore, we hypothesized increased volumes of the amygdala in ADPs. We used FIRST software (Patenaude et al., 2011) to obtain subcortical volumes (the complete list of these structures and their corresponding volumes for each group is provided in Supplementary Table 1). Our volumetric studies indicated an increase in both absolute (Table 3) and normalized volumes of the amygdala in ADPs as compared with healthy volunteers. To account for any differences in brain size, we used normalized data. The right and left amygdala were enlarged by 29 and 28%, respectively (Fig. 2A).
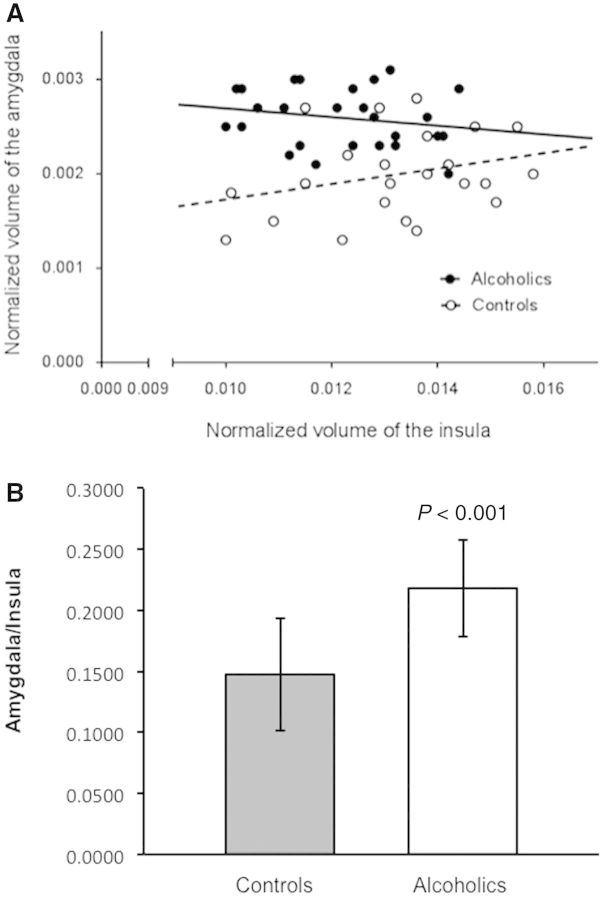
To determine the potential relationship between reduction of the insular cortex and the enlargement of the amygdala, we measured correlations between the total volumes of the insular cortex and the amygdala. We found only a weak positive correlation between the total volumes of the insular cortex and amygdala in healthy volunteers and a weak negative correlation between the total volumes of the insular cortex and amygdala volumes in ADPs (Fig. 3A). While the overall relationship between the amygdala and insula was not significant, there was a trend-level effect of insula × group interaction on the amygdala (P = 0.05), and amygdala × group interaction on the insula (P = 0.06). In addition, we found a 48% increase in the amygdala/insula ratio in ADPs as compared with healthy volunteers (Fig. 3B).
Figure 3.
Analysis of the relationship between normalized volumes of the amygdala and insula in ADPs versus healthy volunteers. (A) Regression plot amygdala versus insula. Note a weak positive correlation between the volumes of the insular cortex volume and the amygdala in healthy volunteers and a weak negative correlation between the insular cortex and the amygdala in ADPs. There was a trend-level effect of insula × group interaction on the amygdala (P = 0.05), and amygdala × group on the insula (P = 0.06). (B) Increase in the amygdala/insula ratio in ADPs as compared with healthy volunteers.
Effects of major depressive disorder on volumetric abnormalities in alcohol-dependent patients
Enlarged amygdala volumes have been reported in some patients with major depressive disorder (Frodl et al., 2003; Hamilton et al., 2008). While none of the healthy volunteers had suffered from major depressive disorder, six ADPs had a history of this disorder. We used this subpopulation to examine whether the volumetric changes in ADPs could be explained by comorbid depression. Therefore, we measured the anterior insula volumes in ADPs with (n = 6) and without (n = 19) a history of major depressive disorder and found them similar, with mean values of 0.0063 (95% CI: 0.0058–0.0068) versus 0.0066 (95% CI: 0.0062–0.0070). We also measured the amygdala volumes in ADPs with (n = 6) or without (n = 19) a history of major depressive disorder and found them similar as well, with mean values of 0.0026 (95% CI: 0.0024–0.0027) versus 0.0026 (95% CI: 0.0025–0.0028).
Effects of smoking on the volumes of the insula and amygdala in alcohol-dependent patients
Comorbid cigarette smoking could potentially account for some of the variance associated with grey matter changes in ADPs (Gazdzinski et al., 2005). We assessed the exposure-effect relationship between the number of pack-years of cigarette smoking and the insula and amygdala volumes in ADPs using age and alcohol exposure (years of lifetime intoxication) as covariates. We did not find a significant effect of smoking on the volumes of either the anterior insula (P = 0.75, F = 0.105) or amygdala (P = 0.232, F = 2.992).
Discussion
We report three main findings, namely alcohol dependence was associated with (i) a bilateral decrease in anterior insula volume; (ii) a bilateral enlargement of the amygdala, a subcortical structure with direct reciprocal connections to the insular cortex; and (iii) an increase in the amygdala/insula volume ratio. While some early CT and MRI morphometric studies reported a general loss of grey and white matter and an increase in CSF volumes in ADPs, subsequent studies suggested that tissue loss is greatest in the frontal white matter in the alcoholic brain (Kril et al., 1997) and there is an inverse relationship between age and grey/white matter volumes (Hommer, 2003). Because total brain volumes were largely unaffected in the current ADP population, our finding of bilaterally reduced insula volumes is unlikely to represent non-specific brain tissue loss in alcoholism. This conclusion is further supported by the observation that the anterior insula volumes were bilaterally reduced in our ADPs, while no group differences were found in posterior insula volumes. Altered insula shape and reduced left-right asymmetry (Jung et al., 2007), as well as decreased cortical thickness in the area of the right insula (Momenan et al., 2012) have previously been reported in alcoholism, but little else is known about potential structural pathology of the insular cortex in this condition.
At this time, we cannot differentiate whether the decreased anterior insula volumes are due to the neurotoxic effects of alcohol or, conversely, if these changes signify a risk factor, predisposing individuals to alcoholism. Decreased anterior insula volumes in ADPs might reflect a lower total number of neurons; however, it is also possible that this subregion harbours a cell population that is selectively impacted in alcoholism. A population fitting this description is the VENs, large bipolar neurons with an atypical spindle- or corkscrew-shaped soma and thick basal and apical dendrites. VENs are found in layer Vb of the anterior insular as well as the anterior cingulate cortex of humans and other mammalian species with large brains and complex social behaviour (Butti et al., 2013). Indeed, our histological studies showed a significant reduction in the number of VENs in the anterior insula of ADPs compared to healthy volunteers. Although we cannot exclude that some other cell populations are affected in ADPs, we did not find a significant difference in the total number of Nissl stained cells in the anterior insula of ADPs compared to healthy volunteers. Previously, VENs have been implicated in several neuropsychiatric disorders whose symptomatology include deficient emotional regulation and social cognition, e.g. behavioural variant frontotemporal dementia (Kim et al., 2012), autism (Santos et al., 2011), early-onset schizophrenia (Brune et al., 2010) and agenesis of the corpus callosum (Kaufman et al., 2008).
In addition to the bilateral reduction of anterior insula volumes in ADPs, we found bilateral enlargement of amygdala volumes. The amygdala has extensive reciprocal connections with the anteriobasal subdivision of the insula (Augustine, 1996; Nieuwenhuys, 2012), and shows functional connectivity with the insula (Robinson et al., 2010), including during resting state (Roy et al., 2009; Cauda et al., 2011) and emotional processing (Stein et al., 2007). The amygdala enlargement observed in our study is unlikely to be driven by comorbid depression. Although enlargement of the amygdala has previously been reported in some cohorts of patients with major depressive disorder (Frodl et al., 2003; Hamilton et al., 2008), we found similar amygdala volumes in ADPs with and without major depressive disorder.
Further neuropathological studies will be required to determine whether the increased amygdala volumes we reported in ADPs are predominantly of neuronal, glial or vascular origin. The primate amygdala does not harbour neuronal progenitors, excluding the possibility that neurogenesis is a contributing mechanism. However, a prolonged history of alcohol dependence has many similarities with chronic stress (Heilig and Koob, 2007), and it has previously been shown that chronic social stress can result in increased amygdala volume due to sprouting of new dendrites (Davidson and McEwen, 2012). There is certain disagreement between the studies of the amygdala volume in ADPs. Wrase et al. (2008) reported reduced volumes of the amygdala, hippocampus and ventral striatum volumes in ADPs, while Makris et al. (2008) reported that only left, but not right amygdala, was reduced in ADPs. These discrepancies may be due to differences in methodology, such as variation in anatomical definition, correction for brain volume, positional correction, or manual versus automatic segmentation. In addition, both studies reporting decreased amygdala volumes included much older participants. Thus, it is possible that structural changes in the amygdala go through two stages in the alcoholic brain. Initially, there is an increase in the volume of the amygdala due to collateral sprouting, the process in which outgrowths develop from the shafts of existing (or injured) axons or dendrites. However, alcohol-induced degeneration of the amygdala, perhaps due to the loss of cortical fibres and the atrophy of amygdala neurons, could later result in diminished amygdala size.
An inverse relationship between insula and amygdala volumes, similar to that found in our study, has been reported in William’s syndrome, a genetic disorder characterized by hyperaffiliative behaviour with atypical expressive language (Cohen et al., 2010). Authors suggested that a functional imbalance between the amygdala and the insula may result from the aberrant anatomy of the insular cortex in William’s syndrome. Our data suggest that a similar imbalance may also exist in the alcoholic brain. However, the precise functional correlates of the present insula and amygdala findings remain to be established. In healthy volunteers, infusion of alcohol resulting in moderate blood alcohol levels (breath alcohol concentration of 0.08 g%, or 80 mg/dl) has been reported to impair or eliminate the amygdala blood oxygenation level-dependent response, resulting in a decreased ability to discriminate between fearful and neutral faces (Gilman et al., 2008). This finding, which was related to abnormally high amygdala activation in response to normal faces, was hypothesized to reflect impairment of social cognition under the influence of alcohol. Alcohol intake resulting in similar blood alcohol levels was subsequently shown to selectively inhibit insula activity during an emotional face matching task (Padula et al., 2011). If the anterior insula contributes to conscious representations of emotional salience associated with threatening stimuli, then acute inhibition of its activity by alcohol could contribute to impaired social cognition observed during intoxication. A loss of anterior insular cortex in chronic alcoholism could then lead to a persistence of this impairment into abstinence. A challenge for future research is to reconcile these findings with the recent observation of increased, rather than decreased, insula activation in alcoholics during exposure to a social exclusion stressor (Maurage et al., 2012), although this difference may be related to the complexity of the stressor.
We conclude that alcohol dependence is associated with a bilateral decrease in the volume of the anterior insula, and with a significant reduction in the number of VENs. In addition, we observed an enlargement in the amygdala of ADPs, and an increase in the amygdala and insula volume ratio. We hypothesize that the changes in the volumes of the insula and the amygdala might be related based on the following arguments: (i) anatomically, there are extensive reciprocal connections between the insular cortex and the amygdala; (ii) functional connectivity between the insular cortex and the amygdala has been shown in multiple studies of the human brain; and (iii) there are commonalities in the functions and properties of the insula and the amygdala (Moraga-Amaro and Stehberg, 2012). The reduction in anterior insula volume could result from alcohol-induced chronic injury to the VENs, while the enlargement of the amygdala may be caused by collateral sprouting secondary to neurodegenerative changes in the insula. We cannot at this point differentiate whether volumetric changes are due to the neurotoxic effects of alcohol on VENs, reflect a predisposition to alcohol consumption in subjects with smaller numbers of VENs, smaller insula, and larger amygdalae, or both. These structural changes in the brains of alcoholics may result in a functional imbalance, which could lead to impairments of emotional processing and social cognition.
Limitations
The present study has several methodological limitations. First, we included female patients in similar numbers in both groups. While including both genders is a common practice in MRI studies examining the effects of alcohol on the brain (Gilman et al., 2008; Eijk et al., 2013) there may be a differential effect of gender on the patient group. Alcohol-dependent females may have greater reductions in grey matter volumes compared to alcohol-dependent males, despite significantly fewer years of heavy drinking (Hommer et al., 2001). However, this topic remains controversial (for a review, see Hommer, 2003). A recent voxel-based morphometry study reported no significant gender × diagnosis interactions for global and regional (including insula) brain volumes, or voxel-based morphometry results (Demirakca et al., 2011). Similarly, our analysis did not find differential effects of gender on the insula volume in ADPs versus healthy volunteers.
We were not able to report detailed background information for the subjects included in the VEN histological analysis. Samples provided by NICHD Brain and Tissue Bank most often originate from hospital autopsies performed solely for post-mortem diagnosis, and the acquisition of donors with alcoholic and/or psychiatric background presents particular challenges. Such subjects are often single, unemployed, isolated from their families, and have very limited access to medical care. These individuals are considered high risk for going missing or becoming homeless. The incomplete retrospective characterization of such cases is a well-known and accepted limitation in studies examining human brain tissue of subjects with mental illnesses (Dedova et al., 2009).
Although 1×1 mm in plane resolution of our images is consistent with many volumetric studies, higher resolution scans (e.g. collecting 1 mm isotropic voxels) would have provided more precise measurements; however, the significant between-group differences in this study are unlikely to be altered as a result of differences in the slice thickness, as our previous studies have used similar volumetric measurements. Finally, it is possible that some unaccounted or underestimated confounding factors between groups, e.g. smoking and anxiety rates, may have affected our results in indeterminable ways.
Acknowledgements
The authors have no disclosures. The authors wish to thank Rick Reynolds of Scientific and Statistical Computing Core, National Institutes of Mental Health, NIH and Michael Kerich of Section on Brain Electrophysiology and Imaging, LCTS, NIAAA, NIH for great deal of technical support in processing our data.
Glossary
- ADP
alcohol-dependent patient
- VEN
von Economo neuron
Funding
This research was supported by the Intramural Research Program of the NIH, NIAAA.
Supplementary material
Supplementary material is available at Brain online.
References
- Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl. 1978;271:5–27. doi: 10.1111/j.1600-0447.1978.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Brune M, Schobel A, Karau R, Benali A, Faustmann PM, Juckel G, et al. Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathol. 2010;119:771–8. doi: 10.1007/s00401-010-0673-2. [DOI] [PubMed] [Google Scholar]
- Butti C, Santos M, Uppal N, Hof PR. Von Economo neurons: clinical and evolutionary perspectives. Cortex. 2013;49:312–26. doi: 10.1016/j.cortex.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Mock JR, Nichols T, Zadina J, Corey DM, Lemen L, et al. Morphometry of human insular cortex and insular volume reduction in Williams syndrome. J Psychiatr Res. 2010;44:81–9. doi: 10.1016/j.jpsychires.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Nichols T, Brignone L, Hall SS, Reiss AL. Insular volume reduction in fragile X syndrome. Int J Dev Neurosci. 2011;29:489–94. doi: 10.1016/j.ijdevneu.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–95. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedova I, Harding A, Sheedy D, Garrick T, Sundqvist N, Hunt C, et al. The importance of brain banks for molecular neuropathological research: the New South Wales tissue resource centre experience. Int J Mol Sci. 2009;10:366–84. doi: 10.3390/ijms10010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kämmerer N, Welzel-Marquez H, Hermann D, Heinz A, et al. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–85. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat. 2003;25:113–19. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res. 2013;37:67–74. doi: 10.1111/j.1530-0277.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–95. [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–44. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–95. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–91. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–5. [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Jung YC, Jang DP, Namkoong K, Ku J, Kim JJ, Park S, et al. Shape deformation of the insula in alcoholics: reduction of left-right asymmetry. Neuroreport. 2007;18:1787–91. doi: 10.1097/WNR.0b013e3282f193b4. [DOI] [PubMed] [Google Scholar]
- Kaufman JA, Paul LK, Manaye KF, Granstedt AE, Hof PR, Hakeem, et al. Selective reduction of Von Economo neuron number in agenesis of the corpus callosum. Acta Neuropathol. 2008;116:479–89. doi: 10.1007/s00401-008-0434-7. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Sidhu M, Gaus SE, Huang EJ, Hof PR, Miller BL, et al. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex. 2012;22:251–9. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, et al. Disrupted regulation of social exclusion in alcohol-dependence: an FMRI study. Neuropsychopharmacology. 2012;37:2067–75. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved diagnostic instrument for substance abuse patients: the addiction severity index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Momenan R, Hommer D, Rawlings R, Ruttimann U, Kerich M, Rio D. Intensity-adaptive segmentation of single-echo T1-weighted magnetic resonance images. Hum Brain Mapp. 1997;5:194–205. doi: 10.1002/(SICI)1097-0193(1997)5:3<194::AID-HBM4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, Hommer DW. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012;204:101–11. doi: 10.1016/j.pscychresns.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Moraga-Amaro R, Stehberg J. The insular cortex and the amygdala: shared functions and interactions. In: Ferry B, editor. The amygdala: a discrete multitasking manager. 2012. ISBN: 978-953-51-0908-2, InTech, DOI: 10.5772/48495. [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123–63. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- Nishida S, Narumoto J, Sakai Y, Matsuoka T, Nakamae T, Yamada K, et al. Anterior insular volume is larger in patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:997–1001. doi: 10.1016/j.pnpbp.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Padula CB, Simmons AN, Matthews SC, Robinson SK, Tapert SF, Schuckit MA, et al. Alcohol attenuates activation in the bilateral anterior insula during an emotional processing task: a pilot study. Alcohol Alcohol. 2011;46:547–52. doi: 10.1093/alcalc/agr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler M, Nopoulos P, Ho BC, Andreasen NC. Insular cortex abnormalities in schizophrenia: relationship to symptoms and typical neuroleptic exposure. Biol Psychiatry. 2005;57:394–8. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 2010;31:173–84. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, et al. Von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res. 2011;1380:206–17. doi: 10.1016/j.brainres.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Hagino H, Tanino R, Kawasaki Y, et al. Volumetric MRI study of the short and long insular cortices in schizophrenia spectrum disorders. Psychiatry Res. 2005;138:209–20. doi: 10.1016/j.pscychresns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–84. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]