Treatment induced neuropathy of diabetes is an iatrogenic neuropathy that develops in the setting of a rapid improvement in glycaemic control. Through retrospective review of case records, Gibbons and Freeman reveal that symptom severity and the risk of developing the disorder correlate strongly with the magnitude of glycaemic change.
See Low and Singer (10.1093/brain/awu327) for a scientific commentary on this article.
The neural substrate of Gilles de la Tourette syndrome is unknown. Worbe et al. use probabilistic tractography to demonstrate widespread structural abnormalities in cortico-striato-pallido-thalamic white matter pathways—likely arising from abnormal brain development—in patients with this syndrome.
Keywords: diabetic neuropathy, painful neuropathy, autonomic neuropathy, insulin neuritis
Abstract
Treatment-induced neuropathy in diabetes (also referred to as insulin neuritis) is considered a rare iatrogenic small fibre neuropathy caused by an abrupt improvement in glycaemic control in the setting of chronic hyperglycaemia. The prevalence and risk factors of this disorder are not known. In a retrospective review of all individuals referred to a tertiary care diabetic neuropathy clinic over 5 years, we define the proportion of individuals that present with and the risk factors for development of treatment-induced neuropathy in diabetes. Nine hundred and fifty-four individuals were evaluated for a possible diabetic neuropathy. Treatment-induced neuropathy in diabetes was defined as the acute onset of neuropathic pain and/or autonomic dysfunction within 8 weeks of a large improvement in glycaemic control—specified as a decrease in glycosylated haemoglobin A1C (HbA1c) of ≥2% points over 3 months. Detailed structured neurologic examinations, glucose control logs, pain scores, autonomic symptoms and other microvascular complications were measured every 3–6 months for the duration of follow-up. Of 954 patients evaluated for diabetic neuropathy, 104/954 subjects (10.9%) met criteria for treatment-induced neuropathy in diabetes with an acute increase in neuropathic or autonomic symptoms or signs coinciding with a substantial decrease in HbA1c. Individuals with a decrease in HbA1c had a much greater risk of developing a painful or autonomic neuropathy than those individuals with no change in HbA1c (P < 0.001), but also had a higher risk of developing retinopathy (P < 0.001) and microalbuminuria (P < 0.001). There was a strong correlation between the magnitude of decrease in HbA1c, the severity of neuropathic pain (R = 0.84, P < 0.001), the degree of parasympathetic dysfunction (R = −0.52, P < 0.01) and impairment of sympathetic adrenergic function as measured by fall in blood pressure on tilt-table testing (R = −0.63, P < 0.001). With a decrease in HbA1c of 2–3% points over 3 months there was a 20% absolute risk of developing treatment-induced neuropathy in diabetes, with a decrease in HbA1c of >4% points over 3 months the absolute risk of developing treatment-induced neuropathy in diabetes exceeded 80%. Treatment-induced neuropathy of diabetes is an underestimated iatrogenic disorder associated with diffuse microvascular complications. Rapid glycaemic change in patients with uncontrolled diabetes increases the risk of this complication.
Introduction
Treatment-induced neuropathy in diabetes (TIND) is a painful, autonomic neuropathy that develops in the setting of rapid improvements in glycaemic control in individuals with a long history of hyperglycaemia (Gibbons and Freeman, 2010). It may occur in individuals with type 1 or type 2 diabetes (Ellenberg, 1974; Archer et al., 1983; Tesfaye et al., 1996; Vital et al., 1997) treated with insulin, oral hypoglycaemic medications and, in rare cases, severe dietary restriction (Gibbons and Freeman, 2010). Also referred to as ‘insulin neuritis’, this disorder is considered a rare iatrogenic complication of diabetes (Ellenberg, 1974; Archer et al., 1983; Tesfaye et al., 1996; Vital et al., 1997; Dabby et al., 2009).
Individuals with TIND typically present with pain and autonomic dysfunction in association with an improvement in glycaemic control. The pain is usually burning or shooting in a length-dependent, distal or diffuse, proximal pattern and is frequently accompanied by allodynia and hyperalgesia. Autonomic symptoms appear at the same time, or shortly after the onset of neuropathic pain. The neuropathy is predominantly small fibre, affecting autonomic and somatosensory fibres, with little or no involvement of large myelinated nerve fibres on examination or nerve conduction studies (Dabby et al., 2009; Gibbons and Freeman, 2010). Autonomic testing shows mild-to-moderate sympathetic and parasympathetic dysfunction. Some patients present with prominent manifestations of autonomic dysfunction including orthostatic hypotension and syncope (Gibbons and Freeman, 2010).
The relative frequency of TIND is unknown. We conducted a retrospective review of all patients seen in a tertiary referral diabetic neuropathy clinic between January 2008 and December 2012 to characterize the proportion of individuals with neuropathy that present with TIND and the associated microvascular complications. We also determined the risk factors associated with development of TIND and the predictors of disease severity.
Materials and methods
This study was approved by the institutional review board of Beth Israel Deaconess Medical Centre.
Study design
This is a retrospective review of all individuals seen for evaluation of diabetic neuropathy over a 5-year period (1 January 2008 to 31 December 2012) in a tertiary referral clinic. Subjects were followed for their diabetes at Joslin Diabetes Centre and were referred to the Joslin Diabetes Centre or the Beth Israel Deaconess Medical Centre Neuropathy Clinic for evaluation of neuropathy.
All individuals with diabetes were evaluated for a possible diagnosis of TIND—defined as the acute onset of neuropathic pain and/or autonomic dysfunction within 8 weeks of a documented improvement in glycaemic control. The following three criteria were used to make a diagnosis of TIND: (i) a decrease in glycosylated haemoglobin (HbA1c) of ≥2% points per 3 months (e.g. an HbA1c decrease from 10% to 8% over 3 months, or 14% to 10% over 6 months would both qualify); (ii) the acute onset of neuropathic pain (>3 point increase in an 11 point Likert scale) and/or autonomic dysfunction developing over 2 weeks and of sufficient severity to cause subjects to seek medical attention; and (iii) the neuropathic pain and/or autonomic symptoms began within 8 weeks of the documented decrease in HbA1C. Patients were questioned in detail, and daily glucose logs reviewed, to determine the exact date(s) of the change in glucose control.
Prior to development of TIND, all subjects were followed at Joslin Diabetes Centre for routine diabetes care and had physical examinations, pain assessment and HbA1c values measured every 3–6 months by an endocrinologist. With development of symptoms, subjects had a neurologic evaluation that included a detailed, structured examination, complete blood count, erythrocyte sedimentation rate, thyroid function tests, serum B12, comprehensive metabolic panel and serum and urine protein electrophoresis. Yearly (or more frequent) retinal examinations and spot urine tests for microalbuminuria were carried out as part of routine care.
Physical examination
Detailed neurologic examinations were performed every 3–6 months for the duration of follow-up. The physical examination was quantified using the neuropathy impairment score in the lower limb (NIS-LL) (Dyck et al., 1997; Bril, 1999). Allodynia and hyperalgesia were monitored at each visit and reported as present or absent in a length-dependent or diffuse fashion. Allodynia was assessed in two ways: (i) dynamic mechanical allodynia: the presence of pain evoked by stroking areas of reported pain with a light brush; and (ii) cold allodynia: the presence of pain evoked by the application of a cold tuning fork on areas of reported pain. The presence of either mechanical or cold allodynia was considered a positive result. Hyperalgesia was defined as an increase in pain perception to pinprick compared to a non-painful area. Patients with suspected TIND also underwent electromyography and nerve conduction studies to determine the neurophysiologic characteristics of the underlying neuropathy.
Pain scores
Subjects rated their pain on an 11-point Likert scale at every neurologic examination (where 0 = no pain and 10 = maximal pain). The location, exacerbating factors and character of pain were recorded at each visit. Subjects were treated with medication to reduce neuropathic pain, including anticonvulsants, antidepressants and opioids, typically in combination. Pain scores were measured while ON medication.
Autonomic questionnaires
Subjects completed the Boston Autonomic Symptom Questionnaire that addressed symptoms related to cardiovascular, gastrointestinal, genito-urinary, vasomotor and sudomotor dysfunction at the time of evaluation. Autonomic symptoms were scored on an 11-point scale (where 0 = no symptoms and 10 = most severe symptoms) as previously described (Gibbons and Freeman, 2010; Gibbons et al., 2013). A symptom was considered present if score was >2, as previously described (Gibbons and Freeman, 2010).
Autonomic testing
Seventy-three subjects underwent cardiovascular parasympathetic function testing (the heart rate response to deep respiration and the Valsalva manoeuvre) and cardiovascular sympathetic function (the blood pressure response to a Valsalva manoeuvre and tilt-table testing to 60° for 45 min). Patients had continuous ECG monitoring, continuous beat-to-beat blood pressure recordings, and manual blood pressure measurements every minute during tilt-table testing.
Statistics
Data are presented as mean ± standard deviation. Pearson correlations were used to describe relationships between tests. Chi-square and Fisher’s exact test were used to describe differences in responses between groups. Descriptive statistics, interquartile differences, odds ratios, relative risk and absolute risk were used to describe factors involved in, or complications associated with TIND. A P-value < 0.05 was considered significant. Bonferroni corrections were made for multiple comparisons. All analysis performed using SPSS 17 (SPSS, IBM Inc).
Results
Patient characteristics
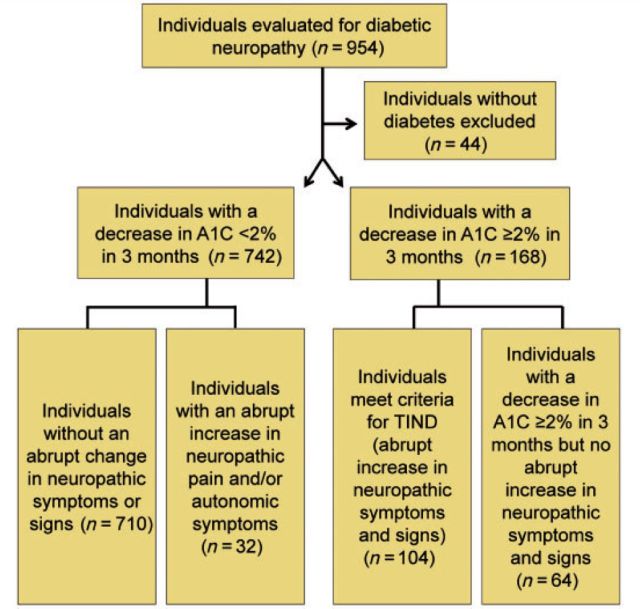
A total of 954 patients were seen in the diabetic neuropathy clinic over 5 years, of which 910 had diabetes. A decrease in HbA1c of ≥2% over 3 months was present in 168 individuals. Of these, 104/168 met criteria for TIND, i.e. the acute onset of neuropathic or autonomic symptoms or signs coinciding with the change in glucose control. Of the individuals with diabetes, 742/910 did not have a decrease in HbA1c ≥ 2% over 3 months. Among individuals with a change in HbA1c of <2% over 3 months, only 4.3% (32/742) reported an abrupt increase in neuropathic pain or autonomic symptoms (P < 0.001, X2 versus those with a decrease in HbA1c). These results are summarized in Fig. 1.
Figure 1.
Study participants. All patients without diabetes were excluded from analysis. The remaining subjects were divided into those with large decreases in glycosylated haemoglobin over 3 months (HbA1c decrease ≥2% over 3 months) and those lesser changes to HbA1c. These groups were further subdivided into those that had an acute increase in neuropathic or autonomic symptoms, and those that did not.
Demographics
General demographic information for each of the groups of subjects is shown in Table 1. Most commonly used medications are noted in Table 2. No other potential causes of neuropathy were found in the individuals with TIND. Four individuals with type 1 diabetes and three individuals with type 2 diabetes smoked tobacco. In all subjects with TIND, the change in glucose control that resulted in a decrease in HbA1c of ≥2% points could be tracked to a specific day or week via the daily glucose logs. The majority of individuals (95 of 104) had an abrupt, volitional change in their glycaemic control for specific reasons such as fear of complications, a significant other becoming involved in their medical care, a new treatment plan by physician, or change in lifestyle priorities. All of the individuals with a history of eating disorders had normal laboratory studies (general chemistries, B12, folate, complete blood counts) and no evidence of ongoing nutritional deficiency.
Table 1.
Demographic information
| TIND–type 1 DM (n = 76) | TIND–type 2 DM (n = 28) | type 1 DM without TIND (n = 71) | type 2 DM without TIND (n = 703) | Pain without TIND type 1 DM (n = 8) | Pain without TIND type 2 DM (n = 24) | |
|---|---|---|---|---|---|---|
| Gender | 60 F, 16 M | 13 F, 15 M | 36 F, 35 M | 337 F, 366 M | 5 F, 3 M | 11 F, 13 M |
| Age (years) | 25.0 ± 6.7 | 50.9 ± 6.8 | 27.4 ± 7.2 | 52.1 ± 6.1 | 27.1 ± 6.1 | 53.1 ± 7.6 |
| Duration of diabetes (years) | 9.6 ± 3.8 | 5.4 ± 2.8 | 10.2 ± 5.6 | 5.5 ± 3.3 | 10.0 ± 5.8 | 6.2 ± 4.4 |
| HbA1c 3 months prior to evaluation (%) | 14.6 ± 3.3* | 13.3 ± 1.2† | 7.3 ± 1.7 | 7.6 ± 2.2 | 9.9 ± 1.3 | 10.4 ± 1.7 |
| HbA1c at the time of initial neurologic evaluation (%) | 7.5 ± 0.8‡ | 8.5 ± 0.7‡ | 7.4 ± 1.6 | 7.5 ± 2.3 | 8.1 ± 1.2 | 8.8 ± 1.3 |
| Pain scores 3 months prior to evaluation (0–10 scale, 10 max) | 1.4 ± 0.7* | 1.3 ± 0.6† | 3.2 ± 2.1 | 3.1 ± 2.8 | 3.2 ± 2.1 | 3.5 ± 1.9 |
| Pain score at the time of initial neurologic evaluation (0–10 scale, 10 max) | 8.9 ± 1.7*‡ | 7.6 ± 2.3†‡ | 3.3 ± 2.4 | 3.1 ± 2.9 | 4.4 ± 2.4 | 5.1 ± 2.3 |
| NIS-LL (0–88 scale, 88 max) | 4.7 ± 1.3 | 9.6 ± 4.3 | 5.8 ± 2.4 | 10.2 ± 5.3 | 5.6 ± 4.3 | 9.4 ± 5.5 |
| Retinopathy 1 year after TIND (%) | 72 (95%)* | 22 (79%)† | 14 (18%) | 196 (27%) | 2 (25%) | 9 (38%) |
| Microalbuminuria 1 year after TIND (%) | 66 (87%)* | 21 (75%)† | 9 (11%) | 131 (18%) | 2(25%) | 7 (29%) |
*P < 0.01 versus type 1 diabetes without TIND.
†P < 0.01 versus type 2 diabetes without TIND.
‡P < 0.01 versus initial (pre-TIND) scores.
DM = diabetes mellitus; NIS-LL = neuropathy impairment score in the lower limb.
Table 2.
Frequency of medications used by individuals with TIND
| Medication | Type 1 DM (n = 76) | Type 2 DM (n = 28) | Type 1 DM without TIND (n = 79) | Type 2 DM without TIND (n = 727) |
|---|---|---|---|---|
| Insulin | 100% | 21% | 100% | 28% |
| Oral antihyperglycaemic | 0% | 78% | 0% | 87% |
| Ace inhibitor | 68% | 86% | 67% | 81% |
| ß-blocker | 8% | 24% | 9% | 27% |
| Calcium channel blocker | 5% | 17% | 7% | 15% |
| Glucagon like peptide 1 agonist | 0% | 24% | 0% | 28% |
| Diuretic | 3% | 21% | 4% | 25% |
| HMG-CoA reductase inhibitor | 22% | 83% | 23% | 84% |
| Anti-epileptic | 70%* | 76%† | 22% | 32% |
| Tricyclic antidepressant | 61%* | 29%† | 7% | 11% |
| Serotonin norepinephrine reuptake inhibitor | 13% | 34%† | 5% | 18% |
| Opioid | 46%* | 54%† | 4% | 4% |
*P < 0.01 versus type 1 diabetes without TIND.
†P < 0.01 versus type 2 diabetes without TIND.
DM = diabetes mellitus.
Neuropathic pain
Neuropathic pain was a universal finding in individuals with TIND. A summary of the pain scores at the time of presentation is shown in Table 1. None of the individuals with TIND reported significant neuropathic pain prior to the onset of glycaemic change (all had pain scores 0–2/10 before a decrease in HbA1C). Typically, individuals with TIND reported the onset of severe burning pain (pain scores 4–10/10) within 2–6 weeks of the improvement in glucose control. Burning pain was present in all subjects with TIND. Paraesthesias were present in 93/104 subjects and shooting pain in 88/104 subjects. Hyperalgesia and allodynia were common in the distribution of the pain. There was a strong correlation between the change in HbA1c and the severity of neuropathic pain (R = 0.84, P < 0.001). The association between pain severity, pain distribution and change in HbA1c is highlighted in Fig. 2. Individuals with TIND all reported ongoing sleep disturbances typically described as difficulty with sleep initiation and sleep duration secondary to neuropathic pain. These individuals reported no record of sleep problems prior to the development of TIND.
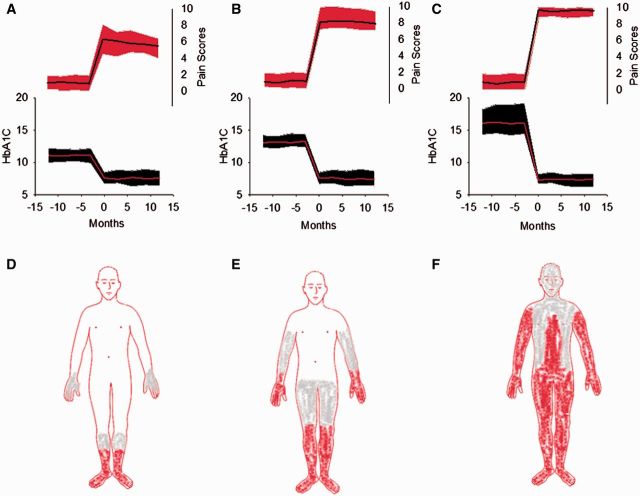
Figure 2.
Complications and risks associated with TIND. (A–F) The 104 individuals with TIND are grouped by change in glycosylated HbA1c scores. (A) The 27 individuals with a decrease in HbA1c of 2–3.9% are shown. (B) The 52 individuals with a decrease in HbA1c of 4–7% are shown. (C) The 25 individuals with a decrease in AbA1c of >7% are shown. (A–C) The lower portion of the graph (left y-axis) reveals the glycosylated haemoglobin (HbA1c) scores over time. The mean value is shown in red and the standard deviation in black. The upper portion of the graph (right y-axis) reveals neuropathic pain scores during the same time frame. The mean value is shown in black and the standard deviation in red. The representative distribution of neuropathic is shown in D–F, with the area in red representing pain common to all individuals, and the area in grey common to many individuals. (D) The least widespread pain distribution in the individuals with the smallest change in HbA1C (corresponding to A). (E) The pain distribution in the individuals with moderate decreases in HbA1C (B). (F) The group with the largest decrease in HbA1C (C) has widespread neuropathic pain.
Physical examination
The summary of the examination scores for all groups is shown in Table 1. All 76 individuals with type 1 diabetes and TIND had normal neuropathy impairment score in the lower limb motor examination subscores, as did 22 of 28 with type 2 diabetes. The six individuals with motor involvement had distal weakness in the toe extensors and/or toe flexors. Some subjects had diminished or absent ankle reflexes (18 with type 2 diabetes and four with type 1 diabetes). Sensory loss (if present) consisted of reduced or absent pinprick sensation at the great toe and/or reduced vibratory detection. Physical examination findings, by neuropathy impairment score in the lower limb score, did not significantly differ between those with and without TIND. The extent of cutaneous distribution of allodynia and hyperalgesia was much greater in individuals with TIND and correlated with the magnitude of change in HbA1c (Fig. 2D–F).
Seventy-four individuals with TIND had EMG and nerve conduction studies performed during their evaluation. The results were normal in 56/74 individuals. The remaining subjects, predominantly those with type 2 diabetes, had a mild sensory, axonal neuropathy.
Risk of developing TIND
A diagnosis of TIND occurred in 104/168 individuals with a decrease in HbA1c of ≥2% over 3 months. The absolute risk of developing TIND in relationship to the decrease in HbA1c is shown in Fig. 3. The individual data points for those 168 individuals with an HbA1c decrease of ≥2% over 3 months is shown in Fig. 3B.
Figure 3.
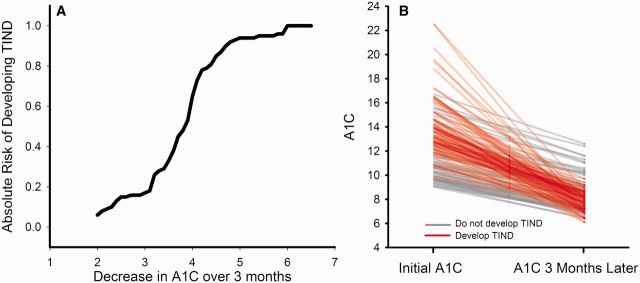
Risk of developing TIND. (A) A survival curve plotting the total number of patients (n = 168) with a decrease in HbA1c of ≥2% over 3 months. The absolute risk of developing TIND is plotted against the change in HbA1c over a 3-month period of time. (B) Individual data lines for all 168 individuals with a change in HbA1c ≥2% over 3 months. Individuals that develop TIND are shown with red lines and those that do not develop TIND are shown with grey lines.
Autonomic symptoms
Ninety-eight individuals with TIND completed autonomic symptom questionnaires. The risk of developing each autonomic symptom related to the magnitude of decrease in HbA1c is shown in Fig. 4. Erectile dysfunction was noted in 28/31 males with TIND, compared to 135/417 males without TIND (P < 0.001, X2).
Figure 4.
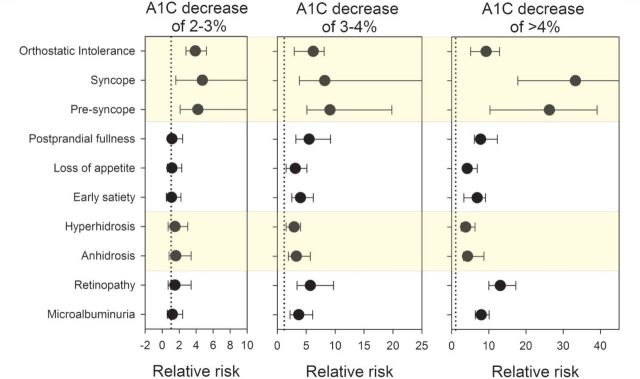
Relative risk of complications by change in HbA1c. The relative risk of developing complications (shown in black circles) with 95% confidence intervals is shown for changes in HbA1c of 2–3%, 3–4% or >4% over 3 months. The relative risk of developing each complication is reported against the rate exhibited by the 742 individuals without large glycaemic change. The dotted lines display a relative risk of 1 (i.e. the confidence intervals that cross the dotted line do not have a statistically significant increase in risk of that complication).
Autonomic dysfunction in TIND
Seventy-three individuals completed autonomic testing within 2–5 months of the onset of neuropathic pain. The results of autonomic testing are shown in Table 3. The results for both groups, in all tests, were abnormal compared to age-related normative values. There were strong correlations between the magnitude of decrease in HbA1c over 3 months and worsening autonomic function. A greater change in HbA1c resulted in worsening parasympathetic function as determined by the expiratory to inspiratory ratio (R = −0.52, P < 0.01) and the Valsalva ratio (R = −0.55, P < 0.01). Greater sympathetic adrenergic dysfunction also correlated with a greater change in HbA1c over 3 months as determined by the fall in systolic blood pressure during tilt-table test (R = −0.63, P < 0.001), the fall in blood pressure during phase 2 of the Valsalva manoeuvre (R = 0.49, P < 0.001), and the diminished phase 4 blood pressure overshoot during the Valsalva manoeuvre (R = −0.59, P < 0.001). As noted in Table 3, the individuals with type 1 diabetes had greater autonomic dysfunction than those with type 2 diabetes across all tests. The slopes of the regression lines describing the correlation between the change in HbA1c and a particular autonomic test did not differ by the type of diabetes, or by the type of treatment used to control glucose.
Table 3.
Autonomic function
| Test | Type 1 diabetes (n = 58) | Type 2 diabetes (n = 15) | P-value |
|---|---|---|---|
| Heart rate variability with deep breathing | 7.1 ± 2.7 | 10.1 ± 2.3 | <0.05 |
| Expiratory to inspiratory ratio | 1.11 ± 0.05 | 1.17 ± 0.05 | <0.05 |
| Valsalva ratio | 1.28 ± 0.14 | 1.36 ± 0.11 | NS |
| Phase 2 drop in Valsalva blood pressure (mmHg) | 35 ± 19 | 31 ± 16 | NS |
| Phase 4 Valsalva blood pressure overshoot (mmHg) | 7 ± 6 | 14 ± 9 | <0.05 |
| Baseline blood pressure (mmHg) | 114/75 ± 9/5 | 135/84 ± 11/8 | <0.01 |
| Baseline heart rate (bpm) | 92 ± 11 | 83 ± 9 | <0.01 |
| Tilt blood pressure (mmHg) | 94/63 ± 7/6 | 117/76 ± 12/7 | <0.01 |
| Tilt heart rate (bpm) | 108 ± 13 | 92 ± 8 | <0.01 |
NS = not significant.
Concomitant diseases
In individuals with type 1 diabetes and TIND, there was a high incidence of diabetic anorexia (intentionally withholding insulin to cause weight loss) (Steel et al., 1987; Young et al., 1988; Takii et al., 2008). Fifty-two of 60 females and 10 of 16 males with type 1 diabetes had a history of diabetic anorexia or were receiving ongoing treatment in an eating disorders clinic.
None of the 28 patients with type 2 diabetes and TIND had a history of eating disorders. However, six individuals reported substantial weight loss (>10 lbs) due to the implementation of severe dietary restriction to improve glycaemic control. Sixteen patients with type 2 diabetes used oral hypoglycaemic medications while six used insulin and oral hypoglycaemic medications for glycaemic control. Individuals with type 2 diabetes had hypertension (24/28) and hyperlipidaemia (25/28).
Associated microvascular disease
In patients with TIND there was a high incidence of associated microvascular disease (Table 1). Most patients with TIND had rapid progression of retinopathy that developed in conjunction with the onset of neuropathic pain as previously reported (Gibbons and Freeman, 2010). Prior to development of TIND, 65/104 individuals had no retinopathy, 35/104 had non-proliferative retinopathy, whereas 4/104 had proliferative retinopathy. Twelve months after the development of TIND, 10/104 individuals had no retinopathy, 54/104 had non-proliferative retinopathy and 40/104 had proliferative retinopathy (P < 0.001, Fisher’s exact test). Prior to development of TIND, 18/104 had evidence of microalbuminuria, while 12 months after the development of TIND, 87/104 had evidence of microalbuminuria (P < 0.001, X2).
Discussion
TIND is a small fibre and autonomic neuropathy that appears after rapid improvements in glucose control. In this manuscript, we demonstrate that: (i) there is an unexpectedly high proportion of individuals with TIND in a tertiary referral diabetic clinic; (ii) the risk of developing TIND is associated with the magnitude and rate of change in HbA1c; (iii) neuropathic pain and autonomic dysfunction severity correlate with the magnitude of change in HbA1c; (iv) patients with Type 1 diabetes and a history of eating disorders are at high risk for developing TIND; and (v) TIND can occur with use of insulin or oral hypoglycaemic agents.
TIND was present in 10.9% of all individuals seen in a tertiary referral diabetic neuropathy clinic over a 5-year period. The frequency is considerably higher than expected given the number of cases published since the initial report of Caravati (1933). There are several possible explanations for the high numbers of identified individuals. The Diabetes Control and Complications Trial, published >30 year ago, and many subsequent publications have emphasized the benefits of rigorous glycaemic control in preventing the microvascular complication of diabetes in Type 1 (DCCT Research Group, 1988; Group, 1993; Martin et al., 2006; Albers et al., 2010) and Type 2 (Davies et al., 2006; Rubino et al., 2007; Ziegler et al., 2009) diabetes. These reports have played an important role in the substantial reduction in HbA1c in the diabetic population in the years since these reports; over the period 1988–94, 40% of the USA diabetic population had a HbA1c ≥8.0% compared to 23% over the 2003–2006 period (http://www.cdc.gov/diabetes/statistics/a1c/a1c_dist.htm). The subsequent incorporation of HbA1c measures in physician quality measurement programmes and the growing role played by physician performance measures in physician ratings by payers, social media and other stake holders has reinforced the emphasis on lowering HbA1c values. Further, it is likely that some previously reported cases of acute painful neuropathy and acute autonomic neuropathy in diabetic subjects may have been unrecognized cases of TIND (Ellenberg, 1958, 1974; Archer et al., 1983; Castellanos et al., 1996; Vital et al., 1997; Leow and Wyckoff, 2005). Finally, we suspect that in many cases the relationship between a change in glycaemic control and the development of an acute neuropathy in diabetes was not considered.
TIND differs from the most prevalent generalized neuropathy of diabetes, the distal sensory-motor polyneuropathy, in several respects. The neuropathic pain has an acute onset, appearing within 8 weeks of glycaemic change, in contrast with the more insidious onset in the distal sensory-motor polyneuropathy (Veves et al., 2008; Tesfaye et al., 2011). The pain in TIND is more severe, and poorly responsive to interventions including opioids, whereas most patients with distal sensory-motor polyneuropathy respond to non-opioid interventions (Gibbons and Freeman, 2010). Although the distribution of the pain is length-dependent in individuals with TIND, it is frequently far more extensive than in distal sensory-motor polyneuropathy and the associated allodynia and hyperalgesia are much more prevalent (Freeman et al., 2014). Autonomic symptoms and signs are common, prominent and appear acutely, in contrast to the relatively lower prevalence, gradual onset and slow progression in distal sensory-motor polyneuropathy (Tesfaye et al., 2010). Finally, both the pain and autonomic features may be reversible in some patients (Hilton et al., 1983; Dabby et al., 2009; Gibbons and Freeman, 2010).
Our data indicate that the severity of TIND is associated with the magnitude of the change of HbA1c, however, it is also clear that the rate of change is important (e.g. a 4% point fall in the HbA1c will have a greater impact if occurring over 3 months than over 6 months). The pathogenic mechanisms whereby this change in glucose results in nerve damage and/or dysfunction are not known. Proposed mechanisms include endoneurial ischaemia due to epineurial arterio-venous shunts (Tesfaye et al., 1996), apoptosis due to glucose deprivation (Honma et al., 2003), microvascular neuronal damage due to recurrent hypoglycaemia (Ohshima and Nukada, 2002), and ectopic firing of regenerating axon sprouts, but these possibilities are unproven. The association of TIND with prior weight loss and diabetic anorexia may provide some insight into a potential mechanism. TIND has features in common with the acute painful and autonomic neuropathy following bariatric surgery. This neuropathy has been attributed to vitamin deficiencies, malnutrition and inflammatory injury (Thaisetthawatkul et al., 2004; Koffman et al., 2006; Juhasz-Pocsine et al., 2007). A potential role for inflammation as a cause of TIND is supported by studies showing an increase in pro-inflammatory cytokines provoked by experimental hypoglycaemia (Dotson et al., 2008; Razavi et al., 2009). This is reinforced by the association of hyperalgesia with prior exposure to hypoglycaemia (Gibbons et al., 2012). However, there are also reports of the development of TIND without associated hypoglycaemia (Dabby et al., 2009). Additional mechanistic studies are necessary to determine the underlying pathophysiology.
Other microvascular complications are commonly seen in patients with TIND (Gibbons and Freeman, 2010). The simultaneous development of TIND, retinopathy and nephropathy in our cohort suggests a common systemic mechanism likely resulting in microvascular disease. Prior reports of ‘early worsening retinopathy’ associate a greater risk of retinopathy development with every percentage point decrease in the glycosylated haemoglobin, a result that parallels the neuropathy development in TIND (Group, 1998). Furthermore, a link between hypoglycaemia, production in pro-inflammatory cytokines, and the development of retinopathy has been proposed (Chantelau et al., 2010).
Our data demonstrate that the magnitude of the change in HbA1c, and not the particular treatment that causes the change (insulin, oral hypoglycaemic medication or diet control), is the major risk factor for TIND. We did not have sufficient numbers of subjects at each starting HbA1c value to determine if a decrease in HbA1c conveyed the same risk at all starting points (i.e. it was not clear if a change in HbA1c from 11% to 7% carried the same risk as decrease from 15% to 11%). Further study and greater numbers of subjects are required to answer that question. However, our data do provide some guidance for prevention. There is a significant increase in incidence of TIND when the decrease in HbA1c is >2% points over 3 months. This suggests that limiting treatment goals to an HbA1c decrease of <2% per 3 months is a reasonable cut-off. Patients with a higher baseline HbA1c, a history of diabetic anorexia or weight loss may be at high risk for TIND and particular care is warranted with intensive glycaemic management of these patients.
The appropriate management of glycaemia in individuals with TIND is not known. Initial reports suggested that pain and sensory symptoms of TIND disappeared with cessation of insulin therapy (Caravati, 1933) and deliberate re-establishment of poor metabolic control has been proposed to treat the early worsening of diabetic retinopathy, a rare complication of rigorous glycaemic control (Chantelau and Meyer-Schwickerath, 2003) and a disorder that has features in common with TIND. However, at present, there is insufficient evidence to suggest ‘permissive hyperglycaemia’ (Chantelau and Meyer-Schwickerath, 2003) as a management strategy for TIND. Prospective studies of glycaemic management in TIND are warranted. The symptoms of TIND, pain and autonomic dysfunction can be treated symptomatically. Although initially refractory to therapeutic interventions (particularly the pain), both improve over time (Gibbons and Freeman, 2010).
There are a number of limitations to our study. All subjects were referred to a tertiary care neuropathy clinic, and may not provide an estimate of the proportion of people with TIND or the risk of TIND in diabetic individuals in a primary care centre or the general population. Also, our definition of TIND—the onset of pain or autonomic dysfunction within 8 weeks of a decrease in the HbA1c of ≥2%—may be conservative and underestimate the prevalence of this disorder.
Treatment-induced neuropathy is an iatrogenic cause of an acute, painful autonomic neuropathy in patients with poor glycaemic control. Although the underlying mechanism is not yet known, there is a clear relationship between a rapid rate of glycaemic control and the development of microvascular complications. Prospective therapeutic and mechanistic studies are necessary define the pathophysiology and appropriate treatment of the disorder.
Funding
This research was supported by NIH K23 NS050209 (CHG).
Glossary
Abbreviation
- TIND
treatment-induced neuropathy in diabetes
References
- Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer AG, Watkins PJ, Thomas PK, Sharma AK, Payan J. The natural history of acute painful neuropathy in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1983;46:491–9. doi: 10.1136/jnnp.46.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):8–13. doi: 10.1159/000052074. [DOI] [PubMed] [Google Scholar]
- Caravati CM. Insulin neuritis: a case report. Va Med Mon. 1933;59:745–6. [Google Scholar]
- Castellanos F, Mascias J, Zabala JA, Ricart C, Cabello A, Garcia-Merino A. Acute painful diabetic neuropathy following severe weight loss. Muscle Nerve. 1996;19:463–7. doi: 10.1002/(SICI)1097-4598(199604)19:4<463::AID-MUS6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Chantelau E, Meyer-Schwickerath R. Reversion of ‘early worsening' of diabetic retinopathy by deliberate restoration of poor metabolic control. Ophthalmologica. 2003;217:373–7. doi: 10.1159/000071355. [DOI] [PubMed] [Google Scholar]
- Chantelau E, Meyer-Schwickerath R, Klabe K. Downregulation of serum IGF-1 for treatment of early worsening of diabetic retinopathy: a long-term follow-up of two cases. Ophthalmologica. 2010;224:243–6. doi: 10.1159/000260231. [DOI] [PubMed] [Google Scholar]
- Dabby R, Sadeh M, Lampl Y, Gilad R, Watemberg N. Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients. Biomed Pharmacother. 2009;63:707–9. doi: 10.1016/j.biopha.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–22. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- DCCT Research Group. Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes. 1988;37:476–81. [PubMed] [Google Scholar]
- Dotson S, Freeman R, Failing HJ, Adler GK. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care. 2008;31:1222–3. doi: 10.2337/dc07-2243. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Melton LJ, O'Brien PC, Service FJ. Approaches to improve epidemiological studies of diabetic neuropathy: insights from the Rochester Diabetic Neuropathy Study. Diabetes. 1997;46 (Suppl 2):S5–S8. doi: 10.2337/diab.46.2.s5. [DOI] [PubMed] [Google Scholar]
- Ellenberg M. Diabetic neuropathy precipitating after institution of diabetic control. Am J Med Sci. 1958;236:466. [PubMed] [Google Scholar]
- Ellenberg M. Diabetic neuropathic cachexia. Diabetes. 1974;23:418–23. doi: 10.2337/diab.23.5.418. [DOI] [PubMed] [Google Scholar]
- Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–76. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Adler GK, Bonyhay I, Freeman R. Experimental hypoglycemia is a human model of stress-induced hyperalgesia. Pain. 2012;153:2204–9. doi: 10.1016/j.pain.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One. 2013;8:e84716. doi: 10.1371/journal.pone.0084716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67:534–41. doi: 10.1002/ana.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67:534–41. doi: 10.1002/ana.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group DR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Group DR. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- Hilton P, Spathis GS, Stanton SL. Transient autonomic and sensory neuropathy in newly diagnosed insulin dependent diabetes mellitus. Br Med J. 1983;286:686. doi: 10.1136/bmj.286.6366.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma H, Podratz JL, Windebank AJ. Acute glucose deprivation leads to apoptosis in a cell model of acute diabetic neuropathy. J Peripher Nerv Syst. 2003;8:65–74. doi: 10.1046/j.1529-8027.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- Juhasz-Pocsine K, Rudnicki SA, Archer RL, Harik SI. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology. 2007;68:1843–50. doi: 10.1212/01.wnl.0000262768.40174.33. [DOI] [PubMed] [Google Scholar]
- Koffman BM, Greenfield LJ, Ali II, Pirzada NA. Neurologic complications after surgery for obesity. Muscle Nerve. 2006;33:166–76. doi: 10.1002/mus.20394. [DOI] [PubMed] [Google Scholar]
- Leow MK, Wyckoff JA. Acute dysautonomia: a rare manifestation of diabetic autonomic neuropathy masquerading as pheochromocytoma. J Peripher Nerv Syst. 2005;10:382–3. doi: 10.1111/j.1085-9489.2005.00051.x. [DOI] [PubMed] [Google Scholar]
- Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–4. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima J, Nukada H. Hypoglycaemic neuropathy: microvascular changes due to recurrent hypoglycaemic episodes in rat sciatic nerve. Brain Res. 2002;947:84–9. doi: 10.1016/s0006-8993(02)02910-4. [DOI] [PubMed] [Google Scholar]
- Razavi NL, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–8. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Rubino A, Rousculp MD, Davis K, Wang J, Bastyr EJ, Tesfaye S. Diagnosis of diabetic peripheral neuropathy among patients with type 1 and type 2 diabetes in France, Italy, Spain, and the United Kingdom. Prim Care Diabetes. 2007;1:129–34. doi: 10.1016/j.pcd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Steel JM, Young RJ, Lloyd GG, Clarke BF. Clinically apparent eating disorders in young diabetic women: associations with painful neuropathy and other complications. Br Med J. 1987;294:859–62. doi: 10.1136/bmj.294.6576.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii M, Uchigata Y, Tokunaga S, Amemiya N, Kinukawa N, Nozaki T, et al. The duration of severe insulin omission is the factor most closely associated with the microvascular complications of Type 1 diabetic females with clinical eating disorders. Int J Eat Disord. 2008;41:259–64. doi: 10.1002/eat.20498. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Malik R, Harris N, Jakubowski JJ, Mody C, Rennie IG, et al. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis) Diabetologia. 1996;39:329–35. doi: 10.1007/BF00418349. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Vileikyte L, Rayman G, Sindrup S, Perkins B, Baconja M, et al. doi: 10.1002/dmrr.1225. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011 Jun 21. doi: 10.1002/dmrr.1225. [Epub ahead of print] PubMed PMID: 21695762. [DOI] [PubMed] [Google Scholar]
- Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63:1462–70. doi: 10.1212/01.wnl.0000142038.43946.06. [DOI] [PubMed] [Google Scholar]
- Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–74. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Vital C, Vital A, Dupon M, Gin H, Rouanet-Larriviere M, Lacut JY. Acute painful diabetic neuropathy: two patients with recent insulin- dependent diabetes mellitus. J Peripher Nerv Syst. 1997;2:151–4. [PubMed] [Google Scholar]
- Young RJ, Ewing DJ, Clarke BF. Chronic and remitting painful diabetic polyneuropathy. Correlations with clinical features and subsequent changes in neurophysiology. Diabetes Care. 1988;11:34–40. doi: 10.2337/diacare.11.1.34. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10:393–400. doi: 10.1111/j.1526-4637.2008.00555.x. [DOI] [PubMed] [Google Scholar]