See Berg (doi:10.1093/brain/awu320) for a scientific commentary on this article.
In a long-term follow-up study of children who underwent temporal lobe surgery for treatment of epilepsy, Skirrow et al. identify no significant pre-to-post-surgery memory losses, but instead robust improvements in memory functions supported by the unoperated temporal lobe. The integrity of remaining temporal lobe structures places constraints on long-term memory outcomes.
Keywords: drug-resistant epilepsy, epilepsy surgery, children, memory, hippocampus
Abstract
The temporal lobes play a prominent role in declarative memory function, including episodic memory (memory for events) and semantic memory (memory for facts and concepts). Surgical resection for medication-resistant and well-localized temporal lobe epilepsy has good prognosis for seizure freedom, but is linked to memory difficulties in adults, especially when the removal is on the left side. Children may benefit most from surgery, because brain plasticity may facilitate post-surgical reorganization, and seizure cessation may promote cognitive development. However, the long-term impact of this intervention in children is not known. We examined memory function in 53 children (25 males, 28 females) who were evaluated for epilepsy surgery: 42 underwent unilateral temporal lobe resections (25 left, 17 right, mean age at surgery 13.8 years), 11 were treated only pharmacologically. Average follow-up was 9 years (range 5–15). Post-surgical change in visual and verbal episodic memory, and semantic memory at follow-up were examined. Pre- and post-surgical T1-weighted MRI brain scans were analysed to extract hippocampal and resection volumes, and evaluate post-surgical temporal lobe integrity. Language lateralization indices were derived from functional magnetic resonance imaging. There were no significant pre- to postoperative decrements in memory associated with surgery. In contrast, gains in verbal episodic memory were seen after right temporal lobe surgery, and visual episodic memory improved after left temporal lobe surgery, indicating a functional release in the unoperated temporal lobe after seizure reduction or cessation. Pre- to post-surgical change in memory function was not associated with any indices of brain structure derived from MRI. However, better verbal memory at follow-up was linked to greater post-surgical residual hippocampal volumes, most robustly in left surgical participants. Better semantic memory at follow-up was associated with smaller resection volumes and greater temporal pole integrity after left temporal surgery. Results were independent of post-surgical intellectual function and language lateralization. Our findings indicate post-surgical, hemisphere-dependent material-specific improvement in memory functions in the intact temporal lobe. However, outcome was linked to the anatomical integrity of the temporal lobe memory system, indicating that compensatory mechanisms are constrained by the amount of tissue which remains in the operated temporal lobe. Careful tailoring of resections for children undergoing epilepsy surgery may enhance long-term memory outcome.
Introduction
Chronic, medication-resistant epilepsy during childhood is linked to cognitive compromise (Rantanen et al., 2011; Berg et al., 2012), with repercussions across the lifespan (Helmstaedter et al., 2003; Helmstaedter and Elger, 2009; Berg et al., 2012). Temporal lobe surgery can be offered as treatment for focal temporal lobe epilepsy, yielding freedom from seizures in many children and adults (62–63% at ≥5 years post-surgery; Tellez-Zenteno et al., 2005).
The temporal lobes underpin the declarative memory system (Squire and Zola-Morgan, 1991; Tulving and Markowitsch, 1998), including episodic memory (memory unique to individual events), and semantic memory (established knowledge about concepts, facts, objects and word meanings) (Tulving, 1972; Baddeley, 2001). In children with temporal lobe epilepsy, a dissociation between episodic and semantic memory for verbal material was recently reported (Smith and Lah, 2011), although the neural substrates for this dissociation were not indicated. Previous research has documented a partial division of labour for memory within the temporal lobe: the hippocampus and medial temporal structures are emphasized in supporting episodic memory, whereas anterior ventral and lateral temporal cortices are implicated in semantic memory (Vargha-Khadem et al., 1997; Mishkin et al., 1998; Patterson et al., 2007; Binder and Desai, 2011).
Anterior temporal lobe surgery frequently involves resection of portions of the hippocampus and anterior temporal neocortex (Clusmann, 2008). Many adults experience verbal memory decline (Sherman et al., 2011), and naming and semantic memory deficits (Hermann et al., 1994; Lambon Ralph et al., 2012) after left temporal surgery. Right temporal surgery is more frequently linked to non-verbal memory impairments (Vaz, 2004). The effects may be protracted: one study reports ongoing verbal memory decline up to 9 years after left temporal surgery (Rausch et al., 2003), and two others report short-term decline followed by stabilization or recovery after 6–10 years (Alpherts et al., 2006; Andersson-Roswall et al., 2010, 2012). Post-surgical memory outcome shows high interindividual variability (Sherman et al., 2011). In adults, memory decline has been linked to higher preoperative memory function (Rausch et al., 2003), older age at seizure onset (Andersson-Roswall et al., 2010), more extensive resections (Graydon et al., 2001; Helmstaedter et al., 2002), less hippocampal pathology presurgically (Hermann et al., 1992; Trenerry et al., 1993), smaller residual hippocampal volumes post-surgery (Baxendale et al., 2000) and ipsilesional memory and language dominance (Stroup et al., 2003; Binder et al., 2008).
The effects of temporal lobe surgery in childhood are less extensively studied. Vulnerability of verbal memory function to left temporal lobe resection has been reported in some studies (Szabo et al., 1998; Dlugos et al., 1999; Jambaque et al., 2007; Meekes et al., 2013), but not others (Helmstaedter and Elger, 1998; Williams et al., 1998; Mabbott and Smith, 2003; Beaton et al., 2012; Gonzalez et al., 2012; Oitment et al., 2013). Heightened brain plasticity in childhood may facilitate cognitive reorganization and limit any detrimental effects of early surgical intervention (Helmstaedter and Elger, 1998; Gleissner et al., 2005). Transient verbal memory deficits have been reported as emerging shortly after left temporal surgery, and resolving after 1 year (Gleissner et al., 2002, 2005). However, post-surgical follow-up is generally limited to 1–2 years and more extensive follow-up may be required to establish the full developmental impact of surgery. Two recent studies provide an initial insight into long-term outcome after epilepsy surgery in children (Viggedal et al., 2012; Smith et al., 2014), but report mixed outcome in heterogeneous cohorts (including temporal and extra-temporal resections) with limited presurgical data. The possibility of ‘growing into a deficit’ cannot be ruled out (Gleissner et al., 2005), and this outcome deserves careful consideration given the diminishing neocortical plasticity with entry into adulthood (Helmstaedter and Elger, 1998, 2009). Furthermore, it is not yet known if the extent of resection to both the hippocampus and the temporal neocortex is as functionally relevant to memory outcome in children as it is for adults.
We used a combination of longitudinal evaluations (with pre- to post-surgical assessments with identical tests) as well as additional cross-sectional comparisons at long-term follow-up (using age-appropriate measures in adulthood) to investigate predictors of long-term declarative memory outcome after temporal lobe surgery in childhood. We report on memory outcome in a sample in whom we previously reported long-term improvements in intellectual function (Skirrow et al., 2011). Here we examined associations between memory function and anatomical integrity of components of the temporal lobe memory system. Based on the evidence from adult studies, we examined the association of (i) visual and verbal memory in relation to side of surgery; (ii) episodic memory outcome with post-surgical mesial temporal and hippocampal integrity; and (iii) semantic memory outcome with anterior ventral and lateral temporal integrity.
Materials and methods
Participants
Fifty-three patients with medication-resistant epilepsy who underwent investigations for epilepsy surgery between 1992 and 2002 were recruited from Great Ormond Street Hospital NHS Trust. Inclusion criteria were medication-resistant lesional temporal lobe epilepsy (having tried at least two anti-epileptic medications), minimum follow-up period of 5 years and minimum age of 16 years at follow-up. See Skirrow et al. (2011) for further recruitment details.
Forty-two children underwent temporal lobe surgery for either hippocampal sclerosis or dysembryoplastic neuroepithelial tumours (DNT). Surgery was performed by one neurosurgeon (W.H.). Cases with dual pathology were excluded from this study. Eleven children were treated only pharmacologically over the follow-up period and served as a non-surgical control group. All participants had focal unilateral temporal lobe abnormalities visible on MRI with corroborating findings from either EEG or functional imaging investigations, or both. In most non-surgical cases, surgery was not offered due to discordance of EEG or SPECT/PET changes with MRI findings (five right-sided, six left-sided lesions). The control group may have therefore presented with a more complex seizure disorder or less focal seizure onsets than the surgical group in addition to differences in pathologic diagnosis. Participant demographics are provided in Table 1.
Table 1.
Demographical and longitudinal data
| Temporal lobe surgery group |
Non-surgical control (n = 11) |
||||||
|---|---|---|---|---|---|---|---|
| Left (n = 25) | Right (n = 17) | ||||||
| Gender (M/F) | 11/14 | 10/7 | 4/7 | ||||
| Lesion type (HS/DNT) | 17/8 | 9/8 | - | ||||
| Baseline data available | Wechsler IQ scales | 24 | 15 | 11 | |||
| WMS-R | 23 | 11 | 8 | ||||
| CAVLT | 14 | 11 | 7 | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age at (years) | Epilepsy onset | 3.6 | 3.8 | 4.6 | 4.4 | 3.7 | 3.2 |
| Surgery | 13.0 | 3.2 | 13.8 | 2.7 | - | - | |
| Final assessment | 23.2 | 3.8 | 21.9 | 3.8 | 20.8 | 3.1 | |
| FSIQ | Baseline | 81.1 | 21.3 | 84.3 | 17.7 | 77.1 | 18.3 |
| Final assessment | 90.2 | 20.2 | 86.1 | 20.7 | 78.5 | 16.5 | |
| Resection volume (cm3) | 15.2 | 5.1 | 16.3 | 8.6 | - | - | |
HS = hippocampal sclerosis; resection volume = post-surgical resection cavity volume.
Preoperative Wechsler IQ scales: n = 2 WPPSI-R UK, n = 3 WISC-R UK, n = 41 WISC-III UK, n = 2 WAIS-R UK, n = 2 WAIS-III UK.
CAVLT: pre-operative CAVLT in n = 4 and CAVLT-II in n = 28.
Detailed information on co-occurring developmental disorders in study participants was not available preoperatively. However, at baseline 16 individuals (eight left and four right surgical, four non-surgical controls) and at follow-up nine individuals (four left surgical, three right surgical, and two non-surgical) had full-scale IQ (FSIQ) scores <70. A UK statement of disability was held by 16 individuals before surgery (five non-surgical controls, four left and seven right surgery cases) and at follow-up by 17 individuals (six non-surgical controls, four left and seven right surgery cases).
Neuropsychological assessment
Baseline measures were obtained before surgery. In patients with repeat surgery (n = 2), baseline assessments preceded the first surgery. In the non-surgical control group, baseline denotes the first assessment at our centre.
Intellectual function
The Wechsler Intelligence scales provided FSIQ, and verbal and performance IQ (PIQ). Scales administered (WISC-R, WISC-III, WAIS-R and WAIS-III) depended on the age of the participant and date of assessment. Intercorrelations between different test versions were high (R = 0.89–0.91). Baseline data were available in 94% of participants (missing because of young age at assessment n = 1, intellectual disability n = 2).
Verbal semantic memory function
IQ-derived semantic memory function was assessed, as in previous research in patients with developmental amnesia (Vargha-Khadem et al., 1997), using the mean of scaled scores on vocabulary, comprehension and information subtests from Wechsler Intelligence Scales.
The British Picture Vocabulary Scale, Second Edition (BPVS II; Dunn et al., 1999) was used to examine receptive vocabulary at follow-up. Trials involved selecting one of four images which represented a spoken word. Parameters of interest included age-equivalence and raw scores.
Category fluency was assessed at follow-up only in a subset of participants (16 right-sided and 16 left-sided surgery, seven non-surgical controls). Participants generated as many words as possible in 1 min for each of the following categories: animals, fruit, birds, water animals, dogs, household items, vehicles, and boats. Correct responses were summed to provide a total category fluency index.
Verbal and visual memory function
The Wechsler Memory Scale-Revised (WMS-R; Wechsler and Stone, 1945) measured immediate and delayed recall of: (i) two prose passages (story recall), with simpler versions for children age ≤12 (n = 14) or those over the age of 12 but with lower FSIQ levels (range 45–78) at baseline (n = 5); and (ii) geometric designs (design recall). Baseline data were missing in 21% of the sample (due to young age n = 1, intellectual disability n = 2, introduction of a new memory measure n = 2, not known n = 6). Additional WMS-R data were available from 23 individuals for at least one interim assessment between baseline and follow-up. To maintain comparability across test versions, percentage recalled are reported for each subtest.
The Children’s Auditory Verbal Learning Test (CAVLT-II; Talley, 1993) measured acquisition and retention of a list of 16 concrete nouns. A target list was presented and recalled over five learning trials, followed by an interference trial, and delayed recall of the target list. Parameters of interest include learning capacity (total words recalled in learning trials), and loss after delay (words recalled in Trial 5 − number recalled after delay). Similar measures have been reported as sensitive to left temporal lobe dysfunction in children (Gleissner et al., 2002; Helmstaedter et al., 2003).
The Doors and People Test (Baddeley et al., 1994) was completed at follow-up in a subset of participants (17 right-sided and 17 left-sided surgery, nine non-surgical controls), providing measures of verbal and visual recall (recall of names in response to photographs of people, and learning and recall of simple geometric designs) and verbal and visual recognition (selecting printed names of people amongst distractors, and selecting pictures of doors amongst distractors). Percentage recalled is reported.
Structural MRI acquisition and analysis
Presurgical volume T1-weighted MRI brain scans (available for 33 surgical participants (78%) were acquired on a 1.5 T Siemens Vision System using a 3D magnetization-prepared rapid gradient-echo sequence (repetition time 10 ms; echo time 4 ms; flip angle 12°; voxel size: 1.0 × 1.0 × 1.25 mm). At follow-up, all participants (surgical participants and non-surgical controls) underwent MRI scans using a 1.5 T Siemens Avanto System. Three-dimensional volume T1-weighted scans were acquired using a 3DFLASH sequence (repetition time 11 ms; echo time 5 ms; flip angle =15°, field of view = 256 mm, matrix size 256 × 256, voxel size 1.0 × 1.0 × 1.0 mm).
Intracranial volume
Each MRI image was segmented into white matter, grey matter and CSF using the VBM toolbox (methods described in Skirrow et al., 2011), and volumes were summed to yield an index of intracranial volume.
Examination of temporal lobe structures
Manual tracing of brain volumes of interest was carried out in the software package MRIcron (C. Rorden, http://www.mccauslandcenter.sc.edu/mricro/mricron/), with reference to the contralesional hemisphere. Measurements included presurgical DNT lesion volumes, post-surgical resection cavity volumes (resection volume), and pre- and post-surgical hippocampal volumes. Hippocampal volumes were measured by manual tracing on coronal images. Measurements included the uncus, and were delineated by anatomical boundaries: the white matter tract of the subiculum, choroid plexus, the lateral ventricle and the alveus as borders, which were not included. To ascertain reliability of hippocampal volumes, ratings for 28 hippocampi were repeated independently by a trained research assistant, showing excellent reliability (Cronbach’s alpha = 0.992).
A rating system of temporal lobe integrity devised by Graydon et al. (2001) was applied to code post-surgical tissue integrity in the temporal lobe. Using the intact brain hemisphere as reference, MRI images were tilted until the sylvian fissure appeared horizontal from a sagittal view. Structural limits of temporal lobe were then defined using coronal markers in the intact hemisphere (Supplementary Fig. 1): the temporal pole was defined as extending from the most anterior part of the temporal lobe to the slice preceding the frontotemporal junction; the remaining temporal lobe structures were defined as extending from the frontotemporal junction up to the coronal slice abutting the splenium of the corpus callosum. Tissue integrity was coded independently within five temporal lobe structures: the superior, middle and inferior temporal gyri, temporal pole and mesial region, including the parahippocampal gyrus and hippocampus. Tissue integrity was coded slice by slice moving from anterior to posterior: 1 = total resection, 2 = partial resection (at least one-third of tissue missing), 3 = predominantly intact (less than one-third of tissue missing), and 4 = fully intact. To control for interindividual differences in the length of the temporal lobes, ratings for superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus and mesial region were summed in anterior, middle and posterior thirds and structural integrity was expressed as the proportion of the maximal possible rating.
Functional MRI to determine language lateralization
Language lateralization was examined at follow-up using functional MRI. A reliable and well-validated semantic retrieval task was used (Liegeois et al., 2002). Stimuli were presented in a block design over two test runs, each with 10 active task (covert verb generation) and rest (listening to amplitude-modulated white noise) phases. Functional data were acquired using whole brain echo-planar pulse sequence (repetition time =2570 ms, echo time = 50 ms, flip angle = 90°, field of view = 192 × 192, slice thickness = 3 mm, 1 mm interslice gap, slices = 30, matrix size = 64 × 64, voxel size = 3 × 3 ×4 mm3).
Analysis was performed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Data processing included coregistration and realignment, spatial normalization and computation of the first-level contrast (verb generation versus rest) after covariation with movement regressors. Using methods described previously (Croft et al., 2013), a region of interest was placed over an extended Broca’s region bilaterally (Brodmann’s areas 44, 45 and 47 and precentral and middle frontal gyri), and threshold independent lateralization indices were calculated for each participant (Wilke and Lidzba, 2007). Consistent with cut-off values frequently used in language functional MRI studies (Binder et al., 1996; Pahs et al., 2013), participants were grouped into typical (lateralization indices ≥ +0.2) and atypical language lateralization (bilateral: lateralization indices between +0.2 and −0.2; and right lateralized: lateralization indices ≤ −0.2).
Ethics
The Great Ormond Street Hospital Ethics Committee approved the study. Written informed consent was obtained from all participants in accordance with the standards of the Declaration of Helsinki.
Statistical analysis
Postoperative change in memory function and brain volumes were obtained by subtracting baseline from follow-up values: positive values index increases and negative values index decrements. Analyses were carried out in SPSS version 20.0 and SAS. Normality of data was assessed with the Shapiro-Wilk statistic, followed by parametric and non-parametric tests, as appropriate. Cross-sectional comparisons were carried out with independent samples t-tests, Mann-Whitney U-tests, analysis of covariance (ANCOVA), Kruskal Wallis, chi-square and Fisher’s exact tests, as required.
Memory outcome independent of general intellectual functioning was investigated: (i) analyses of pre- to post-surgical memory change covaried for concurrent change in FSIQ; (ii) analysis of memory outcome covaried for FSIQ at follow-up; and (iii) analysis of IQ-derived semantic memory covaried for performance IQ (because of subtest overlap with FSIQ). The influence of language lateralization on memory outcome was investigated by repeating analyses after covarying for lateralization indices.
Repeated measures ANCOVAs investigated effects of time-at-assessment (baseline and follow-up), group (right-sided and left-sided surgery and non-surgical control), and different recall intervals (immediate and delayed), where available. Greenhouse-Geisser adjustments to degrees of freedom, rounded up to the nearest whole digit, are reported where necessary. Post hoc comparisons were completed with one-way ANOVA of memory change across groups followed by Tukey’s HSD and paired samples t-tests. Analyses were repeated excluding the non-surgical control group, to verify that any failure to detect group effects were not due to the smaller sample size of the non-surgical control group. As results for different recall intervals (immediate versus delayed) were equivalent for the above analyses, immediate and delayed recall for WMS-R subtests were then averaged to yield story recall and design recall composites for subsequent analyses.
Brain structural correlates of memory outcome were investigated with bivariate and partial correlations, as appropriate. Analyses of brain structural measures which did not adjust for head size (resection volume and hippocampal volumes) covaried for intracranial volume. Ratings of temporal lobe integrity were already expressed as the proportion of the maximal possible rating, and correlational analyses of these measures did not therefore covary for intracranial volume. Analysis of mesial temporal integrity covaried for ipsilesional hippocampal volumes, to investigate impact of mesial temporal integrity beyond the hippocampus. Significant correlations between memory function and brain structural measures were re-examined after covarying first for FSIQ and then additionally for language lateralization indices. Because of the hypothesis-driven nature of the analysis, correction of multiple testing was not carried out.
Stepwise linear regression analysis was used to explore clinical predictors of memory outcome after surgery (excluding brain volumetric predictors): including age at onset of epilepsy, duration of epilepsy, lesion type (DNT or hippocampal sclerosis), current seizure status and medicated versus medication-free. The influence of FSIQ was regressed out for all outcome variables, with the exception of IQ-derived semantic memory, which regressed out performance IQ instead. Diagnostic analyses included examination of influential points, normality of residuals, and multicollinearity.
The non-surgical control sample served to provide reference data for the surgical group for longitudinal and cross-sectional analyses, but were not included in analyses specifically pertaining to post-surgical outcome, including analysis of brain correlates of cognitive function and in regression analysis of clinical predictors of post-surgical outcome.
Results
Clinical measures
At baseline, groups did not differ for duration of epilepsy [F(2,41) = 0.05, P = 0.95], age at onset (Kruskal-Wallis test, P = 0.71) or age at testing (Kruskal-Wallis test, P = 0.15). Before surgery 22 patients experienced daily seizures, 23 weekly seizures and 8 monthly. Daily seizures were more common in left temporal lobe surgery candidates than the other two groups (56% versus 29% in right surgical candidates and 27% non-surgical controls), although differences between groups were non-significant (χ2 = 5.46, P = 0.24). Baseline differences between groups were not present for any of the outcome variables reported or for FSIQ (all P > 0.08). Right and left surgical participants did not differ for age at surgery (t = 1.26, P = 0.21).
Long-term clinical outcome has been reported elsewhere (Skirrow et al., 2011). At follow-up six surgical participants (14%) continued to experience regular seizures and 18 (43%) remained on medication. In the non-surgical control group, seven (64%) participants continued to experience regular seizures, and eight (73%) remained on medication. Seizure freedom (defined as experiencing no seizures in the preceding year) was seen in 86% of surgical participants and 46% of controls, with duration of seizure freedom ranging from 1 to 13 years (mean 7.8 years). Although seizure freedom was significantly more frequent in the surgical group (Fisher’s exact test, P = 0.002), duration of seizure freedom for those who were seizure-free did not differ (t = 0.45, P = 0.66)
Resection volume and temporal lobe integrity
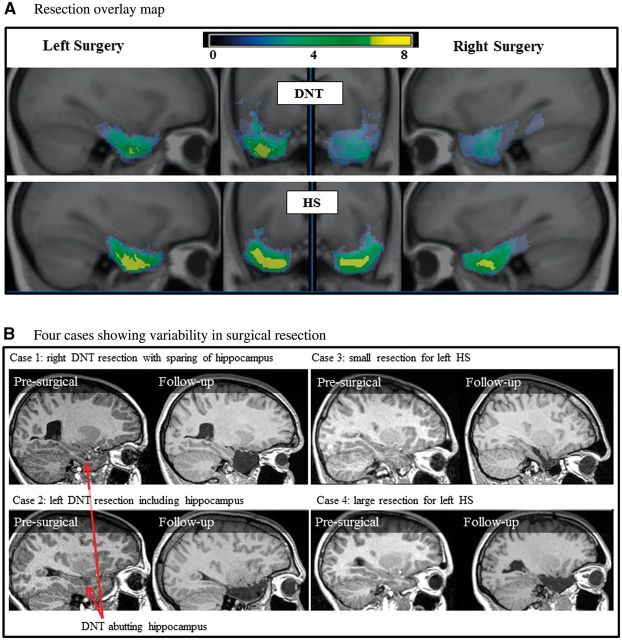
Surgical resections were tailored to the visible individual pathology of participants, and were therefore variable (see resection overlay in Fig. 1A and example cases in Fig. 1B). Mean (and range) of resection volumes for the participant groups were: hippocampal sclerosis: 16.5 cm3 (8.9–28.1); DNT: 14.3 cm3 (1–34.1); mean (and range) of ipsilesional hippocampal volumes for participant groups: hippocampal sclerosis: 0.55 cm3 (0.2–1.1); DNT: 1.69 cm3 (0.5–3.6). Resection volume did not differ by lesion type (DNT or hippocampal sclerosis) or side of surgery (P = 0.09 and P = 0.60, respectively). Ipsilesional hippocampal volumes were significantly smaller in patients with hippocampal sclerosis (pre-surgically: t = 2.68, P = 0.004, post-surgically: t = 4.10, P = 0.001). In seven individuals with DNT lesions the hippocampus was spared (two left, five right). Preoperative FSIQ did not correlate with resection volume (r = −0.25, P = 0.12). Age at surgery did not correlate with total resection volume (r = −0.02, P = 0.89), but did correlate with hippocampal resection volume (r = 0.43, P = 0.01), with less extensive hippocampal resections in older children.
Figure 1.
Variability in surgical resection. (A) Overlay map of resections for surgical patients with DNT and hippocampal sclerosis (HS). Highest overlap of tissue removal is indicated in yellow. (B) MRI scans from four individual surgical participants preoperatively and at follow-up showing variability in resections even for the same lesion type (DNT or hippocampal sclerosis). Post-surgical resection volume and remaining ipsilesional hippocampal volumes for presented cases are as follows: Case 1: resection volume = 20.7 cm3, hippocampus = 2.4 cm3; Case 2: resection volume = 19.8 cm3, hippocampus = 0.6 cm3; Case 3: resection volume = 9.0 cm3, hippocampus = 1.1 cm3; Case 4: resection volume = 18.3 cm3, hippocampus = 0.7 cm3.
Measures of post-surgical temporal lobe integrity revealed that posterior and middle temporal regions (excluding the mesial region), were infrequently subject to surgical resection. Anterior temporal regions, including the temporal pole, and the middle mesial region, were therefore selected for further correlational analyses.
Language lateralization
Atypical lateralization was seen in 9/50 participants (two right-sided and four left-sided surgery and three non-surgical controls) and was not related to presence of intellectual dysfunction or underlying pathology. Groups did not differ significantly with respect to lateralization indices (Kruskal-Wallis test P = 0.37).
Longitudinal change in memory function
IQ-derived semantic memory function
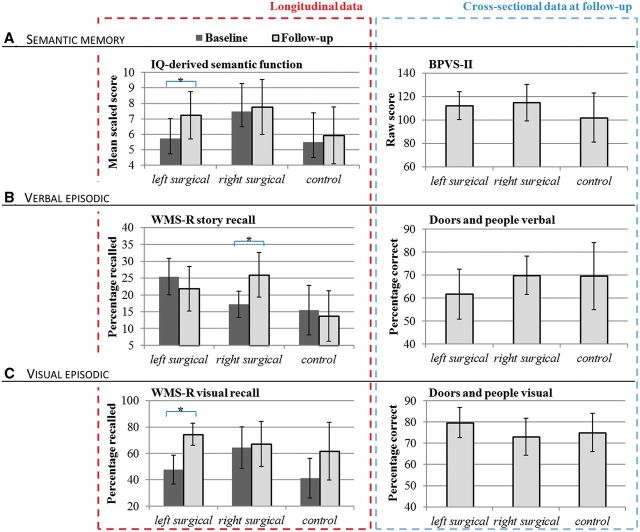
Analysis of IQ-derived semantic memory function (2 × 2 repeated measures ANCOVA) revealed no significant main effect of group [F(2,44) = 1.06, P = 0.36]. However, a significant interaction between group and time-at-assessment was seen [F(2,44) = 3.63, P = 0.04], driven by a significant improvement in the left surgical group which was not present in the other groups (post hoc group comparison P = 0.04). Results are presented graphically in Fig. 2A.
Figure 2.
Memory function. Memory function from longitudinal (left) and cross-sectional analyses (right), presenting means and 95% confidence intervals (error bars) in left surgical, right surgical and non-surgical control groups. (A) Semantic memory function: IQ-derived semantic memory and BPVS-II. (B) Verbal episodic memory from WMS-R and The Doors and People Test. (C) Visual memory from WMS-R and The Doors and People Test. *Significant pre- to post-surgical changes (P < 0.05).
Wechsler memory scale
No main effect of group was seen for story recall [F(2,38) = 1.59, P = 0.22]. An interaction between time-at-assessment and group [F(2,38) = 3.38, P = 0.04] was present, driven by post-surgical improvements in the right surgical group, which were not seen in the left surgical participants (post hoc group comparison P = 0.04). Results are presented graphically in Fig. 2B and C.
No main group effect was seen for equivalent analyses of design recall [F(2,37) = 0.10, P = 0.38]. An interaction between group and time-at-assessment was present [F(2,37) = 4.64, P = 0.02], driven by improvements in the left surgical participants which were absent in the right surgical group (post hoc group comparison P = 0.02) (Fig. 2C). Changes in control participants were non-significant in comparison to changes in either surgical group.
As there were no significant group differences or interactions for immediate and delayed recall of WMS-R test measures, average story recall and design recall were used for further analyses, to reduce the number of comparisons. Correlational analyses revealed that the greater improvements in both visual and verbal memory measures were seen for individuals who initially had lower scores (correlation of pre-surgical scores with post-surgical change: visual memory: r = −0.55, P < 001; verbal memory: r = −0.59, P < 0.001), in line with our earlier findings showing more improvement of intellectual function in individual with lower IQs pre-surgically (Skirrow et al., 2011). However, as all analyses controlled for change in IQ, these changes cannot be accounted for by post-surgical changes in intellectual function.
It was important to ascertain that changes from child to adult story recall measures during the course of follow-up did not account for post-surgical changes in performance. This was examined with multilevel models, which incorporated all available longitudinal data, and controlled for changes in test versions across assessments. Findings remained unchanged (Supplementary material).
Children’s Auditory Verbal Learning Test
Repeated measures ANCOVA revealed no significant effect of group [loss after delay: F(1,25) = 0.37, P = 0.69; learning capacity F(1,27) = 0.74, P = 0.49], nor any main effects or interactions (minimum P = 0.18).
Group differences at follow-up
Semantic memory
Receptive vocabulary did not significantly differ between groups [F(3,52) = 2.57, P = 0.09]. All groups had receptive vocabulary below their chronological age (mean age = 22.8 years, mean BPVS age equivalence = 11.7 years). As the BPVS is not normed for adults, it is not possible to identify whether impairments were beyond or in-line with intellectual function. However, only nine individuals (17%; three left-sided and four right-sided surgery, two non-surgical controls) reached test ceiling (age equivalence = 17), and all others obtained scores below this level and below their chronological age.
Category fluency did not differ between groups [F(3,38) = 0.90, P = 0.41]. Scores for the animal category were lower in patients compared to published norms for this age range (reference range: 19.9–21.5; right-sided surgery: 17.4; left-sided surgery: 17.6; non-surgical control: 16.9; Tombaugh et al., 1999). Results for both semantic memory measures were unchanged after covarying for lateralization indices. Results are presented graphically in Fig. 2A.
Visual and verbal memory
Repeated measures ANCOVA of Doors and People test results revealed a significant group by task interaction [F(2,39) = 3.32, P = 0.047], with left surgical participants showing a significantly better visual than verbal memory score (post hoc paired samples t-test: t = 4.25, P = 0.001). Findings remained unchanged after covarying for language functional MRI lateralization indices. Results are presented graphically in Fig. 2B and C.
Brain correlates of post-surgical memory change
No significant correlations were seen between change in memory function pre- to post-surgically with quantitative measures of brain volume change (quantification of the post-surgical integrity of the temporal lobe, resection volume and pre- to post-surgical change in hippocampal volume).
Brain correlates of memory outcome at follow-up
Resection volume, residual hippocampal volume and integrity of the temporal pole were significantly correlated with a number of memory measures at long-term follow-up (Table 2).
Table 2.
Correlations of ipsilesional temporal lobe structural measures and memory outcome at follow-up
| Left temporal lobe surgery, ipsilesional |
Right temporal lobe surgery, ipsilesional |
|||||
|---|---|---|---|---|---|---|
| Memory measure | Volume |
Temporal pole integrity | Volume |
Temporal pole integrity | ||
| Resection | Hippocampus | Resection | Hippocampus | |||
| Verbal memory | ||||||
| WMS-R Story | −0.39 | 0.50*,a,b | 0.13 | −0.27 | 0.47 | 0.20 |
| D&P Verbal memory | −0.24 | 0.56*,a,b | 0.41 | −0.37 | 0.52*,a | 0.41 |
| CAVLT Learning | −0.48* | 0.36 | 0.60**,a,b | −0.43 | 0.34 | 0.12 |
| CAVLT loss after delay | −0.01 | 0.04 | −0.17 | −0.49 | 0.17 | 0.10 |
| Visual memory | ||||||
| WMS-R Design | −0.41* | 0.34 | 0.41* | −0.32 | 0.21 | 0.15 |
| D&P Visual memory | −0.18 | 0.51*,a | 0.27 | −0.53*,a | 0.12 | 0.10 |
| Semantic memory | ||||||
| IQ-derived semantic memory | −0.34 | 0.19 | 0.29 | −0.36 | 0.33 | 0.24 |
| Category fluency | −0.67**,a,b | 0.57*,a | 0.53* | −0.39 | 0.46 | 0.39 |
| BPVS | −0.47* | 0.29 | 0.50*,a,b | −0.28 | 0.41 | 0.22 |
*P < 0.05, **P < 0.01.
aSignificant after controlling for FSIQ.
bSignificant after controlling for language lateralization index. Although findings are frequently significant in the left but not right surgical sample, differences between correlation coefficients are not significant between groups (test for significance between two correlation coefficients, minimum P = 0.09).
Bold correlation values are significant at p < 0.05.
Verbal and visual memory
Story recall was associated with residual hippocampal volume after left temporal surgery. Correlation coefficients between WMS-R story recall and hippocampal volume were of a similar magnitude in the right surgical group, but fell short of significance (P = 0.07). Doors and People Test verbal memory correlated with residual hippocampal volume after both right and left surgery. Visual episodic memory was correlated with resection volume bilaterally, and ipsi-lesional hippocampal volume and temporal pole integrity after left temporal surgery.
List learning and retention as measured by the CAVLT was associated with resection volume and the neocortical integrity of the temporal pole in left surgical participants.
Semantic memory
The IQ-derived semantic memory index did not correlate with any structural measures of the temporal lobe at follow-up. In contrast, receptive vocabulary (BPVS) was correlated with integrity of the temporal pole and with resection volume in the left temporal lobe, without associations with ipsi-lesional hippocampal volume. Category fluency was strongly associated with resection volume and temporal pole integrity, but also with remaining ipsilesional hippocampal volume after left temporal lobe surgery.
Controlling for pre-surgical lesions and memory function
Correlations with resection volumes were repeated in analyses controlling for preoperative DNT lesion volumes. These yielded unchanged results, excepting WMS-R design, which showed trending significance in the left surgical group (P = 0.08), and Doors and People Test visual memory in the right group (P = 0.06). After controlling for preoperative WMS-R, correlations between verbal memory and ipsilesional hippocampal volume at follow-up remained significant in left surgical participants (r = 0.50, P = 0.02).
Clinical predictors of post-surgical memory outcome
Linear regression analysis was used to determine clinical predictors [age at seizure onset, duration, current seizure status, lesion type (DNT or hippocampal sclerosis), current anti-epileptic medication] of FSIQ-independent memory outcome in the surgical cohort. Shorter duration of epilepsy was associated with better verbal memory function on the Doors and People Test [F(1,32) = 5.30 P = 0.03, R2 = 0.14]. List-learning on the CAVLT [F(1,41) = 15.73, P < 0.001, R2 = 0.26] and WMS-R story recall [F(1,41) = 5.13, P < 0.03, R2 = 0.09] were positively associated with post-surgical seizure freedom. Visual memory, IQ-derived semantic memory and category fluency were not predicted by any clinical factor beyond the contribution of FSIQ or PIQ.
Discussion
Here we report the first study investigating brain correlates of long-term memory outcome after childhood temporal lobe surgery. We did not find deficits in memory function in surgical participants in comparison to a non-surgical control group with a history of medication resistant epilepsy. However, after surgery we noted improvements in memory functions typically subserved by the contralesional temporal lobe: visual memory gains after left-sided resections, and verbal memory gains after right-sided resections. The IQ-derived semantic memory index showed improvement after left temporal lobe surgery. However, it is worth noting that similar improvements post-surgically were not seen for list learning (CAVLT), indicating that some aspects of memory change after surgery whereas others do not.
Post-surgical cognitive improvement has been interpreted as the release of reserve capacities which were suppressed or irritated by epilepsy (Helmstaedter et al., 2003). The current study showed a release of memory function (design and story recall specifically) in the un-operated temporal lobe, in line with previous reports in children and adults (Kuehn et al., 2002; Baxendale et al., 2008).
Indices of brain structure were not associated with post-surgical change in memory function but were linked to long-term memory outcome at follow-up, most clearly after left temporal surgery: smaller resections were associated with better semantic, verbal and visual memory outcome. Greater integrity of the temporal pole was associated with better semantic memory and list learning, and larger residual hippocampal volume was correlated with better verbal episodic memory. Findings were independent of intellectual function, and functional MRI indices of language lateralization.
Although at follow-up we report no significant memory loss compared to preoperative levels, some evidence of short-term decline and medium-term recovery of IQ-derived semantic memory was seen in a subgroup of participants who underwent repeat IQ assessment between 9 months and 2 years after surgery (Supplementary material). Analysis of this subgroup indicates short-term loss in both left and right surgical participants, which resolve at long-term follow-up, replicating previous research (Gleissner et al., 2005).
The impact of ongoing seizures, age at onset and duration of epilepsy
The high rate of seizure freedom in our study may have rendered it less sensitive to the effects of continuing seizures on memory outcome. Nevertheless, our results indicate that better memory outcome was linked to postoperative seizure freedom and shorter duration of seizures. We found a less profound impact of the effect of age at seizure onset than in previous reports (Cormack et al., 2007; Rantanen et al., 2011; Berg et al., 2012). The vast majority of our participants had early seizure onset (28% within the first year of life, and 77% within the first 5 years of life), which may have reduced the likelihood of finding such an association. Recent findings in adults indicate the deleterious effects of ongoing seizures, with progressive mesial temporal atrophy with continuing seizures (Bernhardt et al., 2013). Larger samples would be required to tease apart the effects of age at onset and duration of epilepsy on memory outcome.
Resection volume and memory outcome
Resection volume was correlated with a variety of memory functions, even after controlling for presurgical lesion volumes. This is in agreement with research in adults showing better cognitive outcome after less extensive resections (Helmstaedter et al., 1996, 2002; Graydon et al., 2001; Morino et al., 2006). Findings indicate that individual tailoring of resections to already non-functioning tissue may optimize memory outcome.
More extensive resections have been reported as associated with better seizure control, albeit inconsistently (Ozkara et al., 2008; Dunlea et al., 2010). In our sample, long-term seizure freedom was high, and those with continuing seizures (five hippocampal sclerosis, one DNT, none of whom underwent repeat surgery) had larger resection volumes, which may have been due to more extensive pathology in these patients preoperatively.
Memory and the anatomical integrity of the temporal lobes
Smith and Lah (2011) reported dissociable episodic and semantic memory impairments in children with temporal lobe epilepsy. Our results indicate that resections in different temporal lobe structures are correlated with memory domains, which are tapped into by different cognitive tests.
The importance of the hippocampi in episodic memory across development has been shown in children with bilateral hippocampal insult (Vargha-Khadem et al., 1997). Our study shows that this extends to a certain degree to children with the unilateral temporal lobe lesions. However, we must emphasize that our data do not speak to previously reported distinctions between recognition and recall (Adlam et al., 2009) as some of our tests of verbal and visual memory combined scores across both processes. An association between verbal memory measures and residual hippocampal volume was seen, most clearly after left temporal surgery. Our findings suggest some bilaterality of the effect of hippocampal volumes on verbal episodic memory, in line with previous research in adults (Baxendale et al., 2000; Saling, 2009), as correlation coefficients were of a similar magnitude in the right surgical group, but fell short of significance. Bilateral organization could be accounted for by compensatory processes in response to seizures during development (Gleissner et al., 2002), also reflected in increased incidence of atypical language representation (Janszky et al., 2003).
Substantial clinical and neuroscience research in adults (Patterson et al., 2007; Lambon Ralph et al., 2009; Hirni et al., 2013), and a recent report in adults with temporal lobe surgery (Lambon Ralph et al., 2012) have shown associations between temporal pole integrity and semantic memory function. Our study is the first to show a similar relationship in children with temporal lobe surgery. Performance on measures of receptive vocabulary (BPVS), list learning (CAVLT) and category fluency were all correlated with the post-surgical integrity of the temporal pole. By contrast, the IQ-derived semantic memory index did not correlate with any neuroanatomical measures of the temporal lobe. We have previously reported correlations between IQ change and change in whole brain grey matter volume in the same sample (Skirrow et al., 2011). This may indicate that the IQ-derived semantic memory index may be related to more distributed cortical functions, unlike the semantic operations probed by the BPVS and category fluency.
Hippocampal volumes were correlated with both episodic and some semantic memory measures. The association of hippocampal volume with category fluency measures is not surprising as semantic memory processes may facilitate episodic memory function in complex cognitive tasks (Saling, 2009; Hirni et al., 2013). A factor analytic study in children with temporal lobe epilepsy has found both category fluency and story recall to load onto episodic and semantic memory factors (Smith and Lah, 2011), indicating that these particular measures may rely on a combination of semantic and episodic retrieval processes (i.e. recall of household items can be facilitated by a mental ‘walk through’ of a person’s home). However, in our study, story recall and verbal episodic measures probed by the Doors and People Test were correlated only with hippocampal volumes.
The developmental context
Our findings link brain structure indices to memory outcome at follow-up rather than change in memory function from pre- to post-surgery. This may reflect constraints on long-term compensation and recovery processes in childhood and adolescence. Our findings in a sample of children differ with respect to previous reports in adults, which emphasize post-surgical loss of function in association with volumetric brain changes (Baxendale et al., 2000; Graydon et al., 2001). We summarize our findings and interpretation in Fig. 3.
Figure 3.
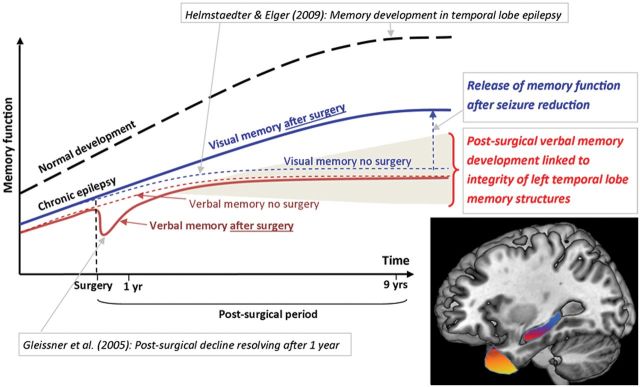
A diagrammatic overview of memory change before and after left temporal lobe surgery in children. Interpretation of our findings in the context of previous research: in dashed lines we present a schema of our findings in the non-surgical sample, and in solid lines findings from the surgical sample. Normal development is indicated with a dashed black line. Previous research from Helmstaedter and Elger (2009) showed an earlier and lower developmental peak of memory function for individuals with temporal lobe epilepsy, compared with their healthy peers. Gleissner et al. (2005) showed a short-lived decline in memory function at 3 months after temporal lobe surgery, resolving after just 1 year in children. Our findings indicate no differences in verbal memory outcome between children who undergo left temporal lobe surgery and those that do not, but indicate significant post-surgical improvements in visual memory function. Shading: Representation of the variability in the postoperative developmental trajectory of memory which may be optimized by tailoring of resections within the temporal lobe structures critical to declarative memory (brain image: showing in yellow the left temporal pole subserving semantic memory, and in red the left hippocampus subserving verbal episodic memory).
We propose that just as with language functions, which are often less strongly lateralized after early-onset focal epilepsy, developmental plasticity and compensatory processes are active during the course of chronic childhood epilepsy, resulting in a greater bilaterality of memory function than noted in adults. This is supported by previous evidence showing that the verbal memory deficits of adults and adolescents with left rather than right temporal lobe epilepsy are not detectable in younger children (Helmstaedter and Elger, 2009; Cormack et al., 2013).
Previous research has shown that patients with temporal lobe epilepsy reach the peak of memory function (the developmental crossover from memory progression during childhood and adolescence into adult age-related decline) at a much early stage than their peers (at 16–17 years versus 23–24 years; Helmstaedter and Elger 2009). The aim of early surgical intervention is to optimize the developmental trajectory of children and adolescents with epilepsy, and it is possible that successful surgical intervention, cessation of seizures and reduction of pharmacotherapy may enhance cognitive development. We found enhanced development of memory functions typically subserved by the un-operated temporal lobe, which may reflect the release of cognitive functions from the detrimental effects of epileptic activity and seizures.
In our study, declarative memory outcome was linked to the volume of tissue remaining in the operated temporal lobe. This indicates that although post-surgical recovery processes are in operation in children, they are likely to be limited by the functional tissue which remains within the ipsilesional hippocampus and temporal neocortex, and which continue to underpin memory function (Bonelli et al., 2013). Our findings are in line with results in other developmental cohorts: a study of neonatal hypoxia revealed that memory outcome in mid-childhood was related to the level of bilateral atrophy in the hippocampi sustained neonatally (Cooper et al., 2013); another study in children with temporal lobe epilepsy suggested that verbal semantic memory may be related to the extension of lesions into anterior temporal regions (Cormack et al., 2013). These findings, along with our current ones, indicate that tissue loss within these critical regions is likely to place a constraint on the long-term postoperative development of memory functions.
Limitations and future directions
In this study we did not evaluate potential preoperative interindividual differences in the extent of temporal lobe pathology which may have influenced variability in resections carried out. Advanced pre- and intraoperative imaging may help in better delineating the boundaries between functional and non-functional (lesional) tissue, thus further minimizing the impact of resection on memory outcome.
Our sample was relatively large in comparison with previous studies of paediatric temporal lobe surgery in children. However, it is important to stress that the sample is small in relation to the number of comparisons carried out. Although our analyses were targeted in relation to the exploration of specific hypotheses, the findings do require replication. Furthermore, a non-epilepsy control group would be a useful addition to future research, particularly with regards to measures that are not age-normed into adulthood (e.g. CAVLT, BPVS), to provide an indication of whether post-surgical improvements are in line with normal developmental trajectories or are accelerated post-surgically.
Post-surgical language lateralization did not significantly influence the pattern of results seen at follow-up. However, we were unable to evaluate the relationship between reorganizational capacity and memory outcome, as preoperative functional MRI was not in use during pre-surgical assessments at the time of this study. Preoperative language organization is likely to have influenced memory outcome (Binder et al., 2008), but cannot be assessed with language functional MRI at follow-up, as post-surgical reorganization may occur after seizure cessation (Helmstaedter et al., 2006). Finally, the anterior temporal regions have been identified to play an important role in language development in children with focal epilepsy (de Koning et al., 2009; Croft et al., 2014). The prospective assessment of both memory and language development after temporal lobe surgery should be incorporated in future research.
Conclusions and implications
We report post-surgical improvements in memory functions subserved by the un-operated temporal lobe after extensive post-surgical follow-up. However, our findings indicate that although cognitive development and recovery may be facilitated by seizure cessation after temporal lobe surgery, they are likely to be simultaneously constrained by surgical resections in brain regions that are critical to declarative memory function. Surgical intervention with tailored resections may optimize the life-long development of memory: first by altering the trajectory of early memory decline in temporal lobe epilepsy, and second, by as far as possible, leaving intact the critical brain structures which underpin the post-surgical recovery of declarative memory. Our results call for a controlled evaluation of different surgical approaches to investigate the optimization of outcome in the context of the required compromises between seizure control and memory function.
Funding
Research presented in this paper was funded by Epilepsy Research UK, Great Ormond Street Children’s Charity (UK) and the Volkswagenstiftung (Germany). This work was undertaken at GOSH/UCL Institute of Child Health who received a proportion of funding from the UK Department of Health's NIHR Biomedical Research Centres funding scheme.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank everyone who took part for their help and support over the many years of this study. We thank Elisa Wrona for assistance in volumetry of the hippocampus.
Glossary
Abbreviations
- BPVS
British Picture Vocabulary Scale
- CAVLT
Children’s Auditory Verbal Learning Test
- DNT
dysembryoplastic neuroepithelial tumour
- FSIQ
Full-scale Intelligence Quotient
- WMS-R
Wechsler Memory Scale-Revised
References
- Adlam A, Malloy M, Miskin M, Vargha-Khadem F. Dissociation between recognition and recal in developmental amnesia. Neuropsychologia. 2009;47:2207–10. doi: 10.1016/j.neuropsychologia.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpherts WCJ, Vermeulen J, van Rijen PC, da Silva FHL, van Veelen CWM. Verbal memory decline after temporal epilepsy surgery? A 6-year multiple assessments follow-up study. Neurology. 2006;67:626–31. doi: 10.1212/01.wnl.0000230139.45304.eb. [DOI] [PubMed] [Google Scholar]
- Andersson-Roswall L, Engman E, Samuelsson H, Malmgren K. Cognitive outcome 10 years after temporal lobe epilepsy surgery: a prospective controlled study. Neurology. 2010;74:1977–85. doi: 10.1212/WNL.0b013e3181e39684. [DOI] [PubMed] [Google Scholar]
- Andersson-Roswall L, Malmgren K, Engman E, Samuelsson H. Verbal memory decline is less frequent at 10 years than at 2 years after temporal lobe surgery for epilepsy. Epilepsy Behav. 2012;24:462–7. doi: 10.1016/j.yebeh.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The concept of episodic memory. Phils T Roy Soc B. 2001;356:1345–50. doi: 10.1098/rstb.2001.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Emslie H, Nimmo-Smith I. Doors and people: a test of visual and verbal recall and recognition. Bury St. Edmunds, England: Thames Valley Test Co.; 1994. [Google Scholar]
- Baxendale S, Thompson PJ, Duncan JS. Improvements in memory function following anterior temporal lobe resection for epilepsy. Neurology. 2008;71:1319–25. doi: 10.1212/01.wnl.0000319699.04265.fd. [DOI] [PubMed] [Google Scholar]
- Baxendale SA, Thompson PJ, Kitchen ND. Postoperative hippocampal remnant shrinkage and memory decline - A dynamic process. Neurology. 2000;55:243–9. doi: 10.1212/wnl.55.2.243. [DOI] [PubMed] [Google Scholar]
- Beaton AE, Durnford A, Heffer-Rahn PE, Kirkham F, Griffin A, Gray WL. Transsylvian selective amygdalohippocampectomy in children with hippocampal sclerosis: seizure, intellectual and memory outcome. Seizure. 2012;21:699–705. doi: 10.1016/j.seizure.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes a prospective cohort study. Neurology. 2012;79:1384–91. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Kim H, Bernasconi N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology. 2013;81:1840–7. doi: 10.1212/01.wnl.0000436069.20513.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–36. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–94. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–84. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Thompson PJ, Yogarajah M, Powell RH, Samson RS, McEvoy AW, Symms MR, Koepp MJ, Duncan JS. Memory reorganization following anterior temporal lobe resection: a longitudinal functional MRI study. Brain. 2013;136:1889–900. doi: 10.1093/brain/awt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusmann H. Predictors, procedures, and perspective for temporal lobe epilepsy surgery. Semin Ultrasound Ct. 2008;29:60–70. doi: 10.1053/j.sult.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Cormack F, Cross JH, Isaacs E, Harkness W, Wright I, Vargha-Khadem F, Baldeweg T. The developmenbt of intellectual abilities in pediatric temporal lobe epilepsy. Epilepsia. 2007;48:201–4. doi: 10.1111/j.1528-1167.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- Cormack F, Vargha-Khadem F, Wood SJ, Cross JH, Baldeweg T. Memory in paediatric temporal lobe epilepsy: effects of lesion type and side. Epilepsy Res. 2013;98:355–9. doi: 10.1016/j.eplepsyres.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Gadian DG, Jentschke S, Goldman A, Munoz M, Pitts G, et al. Neonatal hypoxia, hippocampal atrophy, and memory impairment: evidence of a causal sequence. Cereb Cortex. 2013 doi: 10.1093/cercor/bht332. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft LJ, Rankin PM, Liegeois F, Banks T, Cross JH, Vargha-Khadem F, et al. To speak, or not to speak? The feasibility of imaging overt speech in children with epilepsy. Epilepsy Res. 2013;107:195–9. doi: 10.1016/j.eplepsyres.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Croft LJ, Baldeweg T, Sepeta L, Zimmaro L, Berl MM, Gaillard WD. Vulnerability of the ventral language network in children with focal epilepsy. Brain. 2014;137:2245–57. doi: 10.1093/brain/awu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning T, Versnel H, Jennekens-Schinkel A, van Schooneveld MM, Dejonckere PH, van Rijen PC, et al. Dutch Collaborative Epilepsy Surgery Programme (DuCESP). Language development before and after temporal surgery in children with intractable epilepsy. Epilepsia. 2009;50:2408–19. doi: 10.1111/j.1528-1167.2009.02264.x. [DOI] [PubMed] [Google Scholar]
- Dlugos DJ, Moss EM, Duhaime AC, Brooks-Kayal AR. Language-related cognitive declines after left temporal lobectomy in children. Pediatr Neurol. 1999;21:444–9. doi: 10.1016/s0887-8994(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Dunlea O, Doherty CP, Farrell M, Fitzsimons M, O'Brien D, Murphy K, et al. The Irish epilepsy surgery experience: long-term follow-up. Seizure. 2010;19:247–52. doi: 10.1016/j.seizure.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM, Whetton C, Burley J. The British Picture Vocabulary Scale. Windsor: NFER-Nelson; 1999. [Google Scholar]
- Gleissner U, Sassen R, Lendt M, Clusmann H, Elger CE, Helmstaedter C. Pre- and postoperative verbal memory in pediatric patients with temporal lobe epilepsy. Epilepsy Res. 2002;51:287–96. doi: 10.1016/s0920-1211(02)00158-4. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Sassen R, Schramm J, Elger CE, Helmstaedter C. Greater functional recovery after temporal lobe epilepsy surgery in children. Brain. 2005;128:2822–9. doi: 10.1093/brain/awh597. [DOI] [PubMed] [Google Scholar]
- Gonzalez LM, Mahdavi N, Anderson VA, Harvey AS. Changes in memory function in children and young adults with temporal lobe epilepsy: a follow-up study. Epilepsy Behav. 2012;23:213–9. doi: 10.1016/j.yebeh.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Graydon FJ, Nunn JA, Polkey CE, Morris RG. Neuropsychological outcome and the extent of resection in the unilateral temporal lobectomy. Epilepsy Behav. 2001;2:140–51. doi: 10.1006/ebeh.2001.0163. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE. Functional plasticity after left anterior temporal lobectomy: reconstitution and compensation of verbal memory functions. Epilepsia. 1998;39:399–406. doi: 10.1111/j.1528-1157.1998.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–30. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE, Hufnagel A, Zentner J, Schramm J. Different effects of left anterior temporal lobectomy, selective amygdalohippocampectomy, and temporal cortical lesionectomy on verbal learning, memory, and recognition. J Epilepsy. 1996;9:39–45. [Google Scholar]
- Helmstaedter C, Fritz NE, Perez PAG, Elger CE, Weber B. Shift-back of right into left hemisphere language dominance after control of epileptic seizures: Evidence for epilepsy driven functional cerebral organization. Epilepsy Res. 2006;70:257–62. doi: 10.1016/j.eplepsyres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–32. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89–94. doi: 10.1002/ana.10260. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G, Berry AD, 3rd, Dohan FC., Jr Pathological status of the mesial temporal lobe predicts memory outcome from left anterior temporal lobectomy. Neurosurgery. 1992;31:652–6. doi: 10.1227/00006123-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G, Clement L. Dysnomia after left anterior temporal lobectomy without functional mapping: frequency and correlates. Neurosurgery. 1994;35:52–6. doi: 10.1227/00006123-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Hirni DI, Kivisaari SL, Monsch AU, Taylor KI. Distinct neuroanatomical bases of episodic and semantic memory performance in Alzheimer's disease. Neuropsychologia. 2013;51:930–7. doi: 10.1016/j.neuropsychologia.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Jambaque I, Dellatolas G, Fohlen M, Bulteau C, Watier L, Dorfmuller G, et al. Memory functions following surgery for temporal lobe epilepsy in children. Neuropsychologia. 2007;45:2850–62. doi: 10.1016/j.neuropsychologia.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organisation in medial temporal lobe epilepsy. Brain. 2003;126:2043–51. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Kuehn SM, Keene DL, Richards PMP, Ventureyra ECG. Are there changes in intelligence and memory functioning following surgery for the treatment of refractory epilepsy in childhood? Child Nerv Syst. 2002;18:306–10. doi: 10.1007/s00381-002-0599-7. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Ehsan S, Baker GA, Rogers TT. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain. 2012;135:242–58. doi: 10.1093/brain/awr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex. 2009;19:832–8. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, Baldeweg T. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17:1861–7. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Smith ML. Memory in children with temporal or extra-temporal excisions. Neuropsychologia. 2003;41:995–1007. doi: 10.1016/s0028-3932(02)00318-4. [DOI] [PubMed] [Google Scholar]
- Meekes J, Braams O, Braun KP, Jennekens-Schinkel A, van Nieuwenhuizen O. Verbal memory after epilepsy surgery in childhood. Epilepsy Res. 2013;107:146–55. doi: 10.1016/j.eplepsyres.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Phil Trans R Soc. 1998;352:1461–7. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T, et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95–100. doi: 10.1016/j.yebeh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Oitment C, Vriezen E, Smith ML. Everyday memory in children after resective epilepsy surgery. Epilepsy Behav. 2013;28:141–6. doi: 10.1016/j.yebeh.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Ozkara C, Uzan M, Benbir G, Yeni N, Oz B, Hanoglu L, et al. Surgical outcome of patients with mesial temporal lobe epilepsy related to hippocampal sclerosis. Epilepsia. 2008;49:696–9. doi: 10.1111/j.1528-1167.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- Pahs G, Rankin P, Cross JH, Croft L, Northam GB, Liegeois F, et al. Asymmetry of planum temporale constrains interhemispheric language plasticity in children with focal epilepsy. Brain. 2013;136:3163–75. doi: 10.1093/brain/awt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Rev Neurosci. 2007;8:976–88. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Rantanen K, Eriksson K, Nieminen P. Cognitive impairment in preschool children with epilepsy. Epilepsia. 2011;52:1499–505. doi: 10.1111/j.1528-1167.2011.03092.x. [DOI] [PubMed] [Google Scholar]
- Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology. 2003;60:951–9. doi: 10.1212/01.wnl.0000048203.23766.a1. [DOI] [PubMed] [Google Scholar]
- Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132:570–82. doi: 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- Sherman EM, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52:857–69. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76:1330–7. doi: 10.1212/WNL.0b013e31821527f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Lah S. One declarative memory system or two? The relationship between episodic and semantic memory in children with temporal lobe epilepsy. Neuropsychology. 2011;25:634–44. doi: 10.1037/a0023770. [DOI] [PubMed] [Google Scholar]
- Smith ML, Olds J, Snyder T, Elliott I, Lach L, Whiting S. A follow-up study of cognitive function in young adults who had resective epilepsy surgery in childhood. Epilepsy Behav. 2014;32:79–83. doi: 10.1016/j.yebeh.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stroup E, Langfitt J, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy (ATL) Neurology. 2003;60:1266–73. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- Szabo CA, Wyllie E, Stanford LD, Geckler C, Kotagal P, Comair YG, et al. Neuropsychological effect of temporal lobe resection in preadolescent children with epilepsy. Epilepsia. 1998;39:814–9. doi: 10.1111/j.1528-1157.1998.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Talley JL. Children’s auditory verbal learning test-2. Professional manual. Odessa, FL: Psychological Assessment Resources Inc.; 1993. [Google Scholar]
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–98. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsych. 1999;14:167–77. [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–5. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Tulving E, Markowitsch HJ. Epidosic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vaz SA. Nonverbal memory functioning following right anterior temporal lobectomy: a meta-analytic review. Seizure. 2004;13:446–52. doi: 10.1016/j.seizure.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Viggedal G, Kristjansdottir R, Olsson I, Rydenhag B, Uvebrant P. Cognitive development from two to ten years after pediatric epilepsy surgery. Epilepsy Behav. 2012;25:2–8. doi: 10.1016/j.yebeh.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Stone CP. Wechsler memory scale. San Antonio, TX: Psychological Corporation; 1945. [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Meth. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Williams J, Griebel ML, Sharp GB, Boop FA. Cognition and behavior after temporal lobectomy in pediatric patients with intractable epilepsy. Pediatr Neurol. 1998;19:189–94. doi: 10.1016/s0887-8994(98)00053-8. [DOI] [PubMed] [Google Scholar]