Visual impairment is a key manifestation of multiple sclerosis, and correlates with reduced quality of life. Balcer et al. review current knowledge of vision and visual outcomes in multiple sclerosis, and provide recommendations for the management of visual impairment, the conduct of clinical trials, and topics for future research.
Keywords: multiple sclerosis, optic neuritis, vision, neuro-ophthalmology, clinical trials methodology
Abstract
Visual impairment is a key manifestation of multiple sclerosis. Acute optic neuritis is a common, often presenting manifestation, but visual deficits and structural loss of retinal axonal and neuronal integrity can occur even without a history of optic neuritis. Interest in vision in multiple sclerosis is growing, partially in response to the development of sensitive visual function tests, structural markers such as optical coherence tomography and magnetic resonance imaging, and quality of life measures that give clinical meaning to the structure-function correlations that are unique to the afferent visual pathway. Abnormal eye movements also are common in multiple sclerosis, but quantitative assessment methods that can be applied in practice and clinical trials are not readily available. We summarize here a comprehensive literature search and the discussion at a recent international meeting of investigators involved in the development and study of visual outcomes in multiple sclerosis, which had, as its overriding goals, to review the state of the field and identify areas for future research. We review data and principles to help us understand the importance of vision as a model for outcomes assessment in clinical practice and therapeutic trials in multiple sclerosis.
Introduction
Historically, multiple sclerosis clinical trials have lacked sensitive, vision-specific outcome measures. Low-contrast letter acuity (LCLA) has emerged as the leading candidate to measure visual impairment in multiple sclerosis. It correlates with vision-specific quality of life measures, providing information on clinical meaningfulness, and with the structural integrity of the retina measured by optical coherence tomography (OCT). Together, these factors have led to rapid accumulation of knowledge about visual impairment in optic neuritis and multiple sclerosis. The additional availability of MRI to provide further structural information and electrophysiological measures [visual-evoked potentials (VEPs) and electroretinography] make the afferent visual pathway a useful model system to elucidate inflammatory and neurodegenerative mechanisms in the CNS and to test novel agents for neuroprotection and repair in multiple sclerosis (Frohman et al., 2008a, b).
An international group of over 60 investigators in multiple sclerosis, neuro-ophthalmology, clinical trial design, and evaluation of clinical outcome measures from Europe, North America, Asia, and Australia met on 21–23 November 2013 in Dublin, Ireland (see Supplementary material for a list of attendees). This meeting was convened by the International Advisory Committee on Clinical Trials in Multiple Sclerosis and sponsored by the European Committee on Treatment and Research in Multiple Sclerosis (ECTRIMS) and the US National Multiple Sclerosis Society. Overriding goals were to review the state of the field of vision in multiple sclerosis and to identify areas for future research. The discussions focused on evaluating visual manifestations in multiple sclerosis and their impact on those with the disease, providing information to physicians for incorporating vision assessment into multiple sclerosis clinical practice, developing consensus on the design and administrative structure of multicentre multiple sclerosis clinical trials incorporating visual outcomes, and identifying priorities for vision research in multiple sclerosis.
This review is based on discussions at that meeting and a comprehensive search of the literature (PubMed search of English language publications, using search terms ‘vision’, ‘visual outcomes’, ‘specific visual measures’, and ‘multiple sclerosis’). We summarize the evolution of the role of vision assessment in multiple sclerosis and provide principles that help us understand the importance of vision as a model for outcomes assessment in the next generation of therapeutic trials.
Overview
Inflammatory, demyelinating, and neurodegenerative pathology in multiple sclerosis affects both afferent and efferent visual function. The incidence of optic neuritis in Europe and North America has been estimated at ∼5 cases per 100 000 person-years and may be increasing (Martinez-Lapiscina et al., 2014). About 20% of patients with multiple sclerosis present with optic neuritis (Costello, 2013). One study estimated that one-third have persistent visual symptoms (Jasse et al., 2013), but the proportion may be higher. Resulting impairment and disability lead to reductions in vision-related quality of life (Mowry et al., 2009; Garcia-Martin et al., 2013; Salter et al., 2013). The 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) and a 10-Item Neuro-Ophthalmic Supplement capture the most common symptoms, which include decreases in visual acuity and contrast sensitivity (Balcer and Frohman, 2010), defects in binocular vision, visual field abnormalities (Nakajima et al., 2010), reduced colour vision (Villoslada et al., 2012), blurred vision and diplopia.
Structural measures assessed by OCT, retinal nerve fibre layer (RNFL) thickness (Parisi et al., 1999; Trip et al., 2005; Fisher et al., 2006; Sepulcre et al., 2007; Petzold et al., 2010; Saidha et al., 2013), macular volume (Trip et al., 2005; Burkholder et al., 2009), and retinal ganglion cell/inner plexiform layer (GCL + IPL) thickness (Chen and Gordon, 2005; Graves and Balcer, 2010; Saidha et al., 2011; Sakai et al., 2011; Walter et al., 2012; Costello, 2013; Oberwahrenbrock et al., 2013) are affected in multiple sclerosis. Changes in optic nerve diffusion tensor imaging and other MRI measures are associated with multiple sclerosis-related visual dysfunction (Smith et al., 2011; Naismith et al., 2012). Visual manifestations in multiple sclerosis also may be captured by VEPs (Diem et al., 2003), electroretinography (Rodriguez-Mena et al., 2013), and electrophysiological recordings of eye movements (Tilikete et al., 2011).
Visual manifestations occur in the setting of acute optic neuritis, but may be present without a history of acute optic neuritis. As recovery following acute optic neuritis often is incomplete, with residual deficits in low-contrast vision, colour vision, vision-specific quality of life, and sometimes high-contrast visual acuity (HCVA) (Cole et al., 2000; Optic Neuritis Study Group, 2004, 2008b), more effective treatment of optic neuritis is itself an area of unmet need in multiple sclerosis therapeutics.
Afferent visual manifestations in optic neuritis and multiple sclerosis
Optic neuritis associated with multiple sclerosis
In adults, optic neuritis typically is unilateral, with visual loss evolving over several days, reaching a nadir within 2 weeks, and frequently associated with peri-orbital pain exacerbated by eye movements (Toosy et al., 2014). Reduction in HCVA ranges from minimal to severe, although complete loss (no light perception) is uncommon. In addition to decreased visual acuity resulting from central depression of the visual field, examination characteristically demonstrates a relative afferent pupillary defect in the affected eye or, in the case of bilateral optic neuritis, the more severely affected eye. Typically, colour vision and LCLA are more severely affected than is HCVA. In two-thirds of adult patients with optic neuritis, the optic disc appears normal on direct ophthalmoscopy during the acute phase; however, OCT reveals that many of these affected eyes have subclinical disc oedema (Kupersmith et al., 2012). When visible, optic disc swelling typically is mild, without evidence of haemorrhages or macular exudates. Such atypical findings indicate low risk for subsequent development of clinically definite multiple sclerosis, especially if the brain MRI is normal (Optic Neuritis Study Group, 2004, 2008a, b).
Improvement of vision after acute optic neuritis typically begins within 1 month following onset of visual symptoms. While patients with optic neuritis often are said to have ‘good recovery,’ with 95% of eyes achieving 20/40 or better HCVA, more sensitive measures indicate that visual recovery often is incomplete. Most patients have persistent deficits in vision-related quality of life 5–8 years later (Cole et al., 2000), likely related to the substantial thinning of RNFL and GCL + IPL detectable by OCT. The median loss of peripapillary RNFL is ∼20–40%, with most thinning occurring by 3 months and the full extent by 6 months (Costello et al., 2006, 2008; Henderson et al., 2010). The 10–15% of patients with severe persistent visual deficits tend to have more severe RNFL loss (Costello et al., 2012).
Although acute optic neuritis is often treated with a short course of high-dose intravenous methylprednisolone, which may speed visual recovery (Beck et al., 1992), there are no treatments that improve visual outcomes in general. Two small, uncontrolled studies of patients with optic neuritis and poor vision in spite of steroid therapy reported visual improvement in 70% of cases following a course of plasma exchange (Ruprecht et al., 2004; Roesner et al., 2012). Conflicting outcomes were reported in two trials investigating intravenous immunoglobulin for optic neuritis with poor visual recovery (Noseworthy et al., 2001; Tselis et al., 2008). This unmet need for treatment of optic neuritis itself, the wide range of available functional measures, and the structure-function correlations afforded by OCT make acute optic neuritis an attractive model system to test new therapies for neuroprotection and repair in multiple sclerosis.
Retinal findings and other afferent visual manifestations of multiple sclerosis
Some patients with previous optic neuritis or multiple sclerosis experience transient visual blurring associated with increase in body temperature with exercise, a hot bath, or fever—so-called Uhthoff phenomenon (Fraser et al., 2012). This symptom is caused by a temporary impairment of conduction by demyelinated axons in the afferent visual pathway. A similar phenomenon can affect other sensory and motor pathways.
Despite recovery of static measures of visual function, there may be impaired motion perception following optic neuritis—clinically known as the Pulfrich phenomenon. This symptom has been related to a sustained deficit in functional MRI during tasks that require motion perception (Raz et al., 2011). Development of a delayed latency VEP response in the clinically unaffected fellow eye following optic neuritis is associated with improvement in time-constrained binocular perception (Raz et al., 2013), suggesting an adaptive cortical response to improve synchronicity of input and, thereby, aid binocular vision. Binocular inhibition, the reduction in binocular vision compared to the better eye alone, has been observed in patients with multiple sclerosis and a history of acute unilateral optic neuritis (Pineles et al., 2011).
Abnormalities of the retinal layers other than the RNFL have been observed in post-mortem specimens from patients with multiple sclerosis (Green et al., 2010), where 79% of eyes exhibited ganglion cell loss and 40% showed amacrine and bipolar cell loss in the inner nuclear layer. These findings have been corroborated in vivo by OCT, demonstrating thinning of the GCL + IPL, and associated with reductions in visual function and vision-specific quality of life (Syc et al., 2012; Walter et al., 2012). These retinal findings demonstrated by OCT also correlate with more general clinical and imaging measures of multiple sclerosis disease activity and severity (Ratchford et al., 2013; Saidha et al., 2013).
About 5% of patients with early multiple sclerosis have evidence of microcystic macular oedema or thickening of the inner nuclear layer on OCT (Gelfand et al., 2012; Saidha et al., 2012). Microcystic macular oedema and inner nuclear layer thickening also occur in other inflammatory disorders associated with optic neuritis (Kaufhold et al., 2013) and do not appear merely to be due to vitreous traction (Brandt et al., 2014). In multiple sclerosis, the presence of microcystic macular oedema and inner nuclear layer thickening are associated with increased inflammatory disease activity, including gadolinium-enhancing lesions and new T2 lesions on brain MRI (Saidha et al., 2012). Because the retinal layers do not contain myelin, these observations suggest the inflammatory process in multiple sclerosis is not limited to myelinated CNS structures. However, the occurrence of microcystic macular oedema in non-inflammatory optic neuropathies (Burggraaff et al., 2014) indicates other mechanisms also may be involved.
Approximately 10% of patients with multiple sclerosis have predominantly macular thinning and relative preservation of other retinal layers on OCT (Saidha et al., 2011; Winges et al., 2013). Associated symptoms include photophobia, excessive glare, visual fading, and photopsias, which can occur with optic neuritis but are not typical. In one study, macular thinning was associated with a more rapidly disabling form of multiple sclerosis and hypothesized to reflect a primary neurodegenerative process (Saidha et al., 2011). These findings, however, were not reproduced in another series (Brandt et al., 2011).
Approximately 10% of patients with multiple sclerosis have retinal periphlebitis, the presence of which is associated with increased disease activity (Sepulcre et al., 2007; Ortiz-Perez et al., 2013). Multiple sclerosis is associated with uveitis or pars planitis in up to 15–20% of cases (Lightman et al., 1987; Biousse et al., 1999; Donaldson et al., 2007). These intraocular inflammatory conditions should be considered when chronic pain and photophobia are present. Non-inflammatory visual loss in multiple sclerosis may be a manifestation of comorbid ocular disease. In the NARCOMS registry, a large-scale questionnaire study of people in North America with multiple sclerosis, comorbid conditions, including refractive error, cataracts, strabismus, and glaucoma, were a common cause of visual dysfunction and reduced visual quality of life (Salter et al., 2013). The relative frequency of these comorbid conditions in patients with multiple sclerosis compared to the general population is uncertain.
Efferent visual manifestations of multiple sclerosis
Abnormalities of ocular motility are common in multiple sclerosis and can lead to transient or persistent impairment independent of or in addition to afferent visual pathway dysfunction. Efferent visual abnormalities are more common in progressive than relapsing multiple sclerosis and can be an indicator of posterior fossa lesions and worse neurologic prognosis. Visuomotor abnormalities most often are reported by patients as diplopia, oscillopsia, and blurred or ‘confused’ vision. Abnormal eye movements can be detected readily at the bedside but are not captured well by the standard multiple sclerosis disability rating scales such as the Expanded Disability Status Scale (EDSS); more detailed quantitative characterization requires sophisticated eye movement recording and analysis. Fatigue of adducting saccades in internuclear ophthalmoparesis in multiple sclerosis and improvement with dalfampridine have been demonstrated using such technology (Serra et al., 2014). Interestingly, abnormalities of saccades appeared to be associated with generalized fatigue in patients with multiple sclerosis (Finke et al., 2012).
The King-Devick Test, a brief rapid number-naming test new to the multiple sclerosis field, is a potential quantitative bedside performance measure of efferent visual dysfunction (Moster et al., 2014). This test takes <2 min to complete and is sensitive to dysfunction of saccadic and other eye movements; time scores are higher (worse) among patients with multiple sclerosis compared to disease-free controls. Further studies of this and other efferent visual function tests in multiple sclerosis are needed to bring assessment of this aspect of vision to the level of afferent system investigation.
Measurement of vision in multiple sclerosis trials
Ideally, visual measures used in multiple sclerosis clinical trials should be standardized, reliable, practical, tolerated by study participants, and applicable for both adult and paediatric populations. Aspects include visual function, vision-specific quality of life, structural markers, and electrophysiological tools.
Functional outcomes: high- and low-contrast visual acuity
The visual functional system component of the EDSS does not capture visual dysfunction optimally. This and other shortcomings of the EDSS were the main impetus for development of the Multiple Sclerosis Functional Composite (MSFC) as an alternative disability measure for multiple sclerosis clinical trials. However, the measures of vision available in the clinical trial data sets used to develop the MSFC were limited to non-standardized tests of HCVA (Rudick et al., 1996, 1997). In the evaluation of candidate MSFC visual components in those data sets, Snellen-formatted HCVA did not change over time or demonstrate concurrent changes with EDSS scores (Rudick et al., 1997). Therefore, the initial version of the MSFC did not include a vision test.
Measures of low-contrast (grey- rather than black-on-white) vision, tested by line gratings and letter charts in multiple sclerosis, and by Pelli-Robson charts in the North American Optic Neuritis Treatment Trial, were shown to be sensitive to visual impairment even among patients with Snellen acuities of 20/20 or better (Ashworth et al., 1989; Bodis-Wollner and Brannan, 1997; Mowry et al., 2009; Balcer and Frohman, 2010; Bock et al., 2012; Costello, 2013; Garcia-Martin et al., 2013; Jasse et al., 2013; Salter et al., 2013). In addition, measures of low-contrast vision predicted ‘real-world’ visual impairment of reading, facial recognition, and driving (Leat et al., 1999). Binocular LCLA testing with Sloan letter charts (Balcer et al., 2000) (Fig. 1) was incorporated as an exploratory outcome in several Phase 3 trials, including the AFFIRM trial of natalizumab versus placebo for relapsing-remitting multiple sclerosis (Table 1). Here, LCLA demonstrated changes over time and treatment effects manifested as reduced likelihood of sustained visual loss (Balcer et al., 2007) and greater likelihood of sustained visual improvement (Balcer et al., 2012) in the active treatment group. In contrast, HCVA did not detect sustained visual loss or improvement over time, or differences between treatment groups, similar to the analyses of the pooled data set used to develop the MSFC (Rudick et al., 1997). Based on these observations, LCLA shows promise as a vision-related outcome for multiple sclerosis clinical trials and as an additional component test for the MSFC.
Figure 1.
Low-contrast Sloan letter chart (Precision Vision). These charts have a standardized format based on Early Treatment Diabetic Retinopathy Study visual acuity charts, the standard used in ophthalmology clinical trials, and have several advantages over standard Snellen charts or near vision testing cards as traditionally used in multiple sclerosis trials: (i) letters (Sloan letters) are designed to be equally detectable for normal observers; (ii) each line has an equal number of letters (five per line); (iii) spacing between letters and lines is proportional to the letter size; (iv) change in visual acuity from one line to another occurs in equal logarithmic steps (change of three lines constitutes a doubling of the visual angle); and (v) visual acuity [for high-contrast (black letters on white) chart] may be specified by Snellen notation for descriptive purposes (i.e. 20/20), by the number of letters identified correctly. This figure shows the 25% contrast level for purposes of illustrating format; the actual contrast levels used in these trials, 2.5% and 1.25%, have substantially lighter grey letters. The charts measure 14 × 14 inches for easy use and portability in the multiple sclerosis clinical trial setting; charts may also be mounted on a retro-illuminated cabinet, thus eliminating the need for standardization of room lighting levels. Reprinted with permission (Balcer et al., 2007).
Table 1.
Visual outcomes reported from Phase 2 and 3 trials of approved agents for multiple sclerosis
| Agent | Trial | Visual outcome measures | Results | Reference |
|---|---|---|---|---|
| Alemtuzumab | CAMMS223: Phase 2 trial of alemtuzumab (two doses) or subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis, to assess relapse rate, 6 month confirmed EDSS disability progression and mean EDSS change | Visual contrast sensitivity measured using Pelli-Robson charts | Statistically significant difference favouring treatment with alemtuzumab in proportion of eyes with sustained improvement in contrast sensitivity at 3 and 6 months | Graves et al., 2013 |
| CARE-MS I and II: Phase 3 2-year studies of alemtuzumab or subcutaneous IB1A in relapsing-remitting multiple sclerosis patients, to assess relapse rate and time to 6-month confirmed accumulation of disability | Low contrast letter acuity measured using Sloan charts; visual acuity plus MSFC outcomes |
|
Balcer et al., 2013 | |
| 4-Aminopyridine | 10-week randomized placebo-controlled double blind cross-over trial of 4 aminopyridine in multiple sclerosis patients with optic neuropathy | VEPs, visual acuity, OCT measures of RNFL thickness | While treated, patients had:
|
Horton et al., 2013 |
| Fingolimod |
|
Visual acuity, central foveal thickness |
|
Cohen et al., 2010; Kappos et al., 2010 |
| Interferon beta-1b | BENEFIT: placebo controlled and open label follow-up trial in clinically isolated syndrome patients with two or more clinically silent brain MRI lesions, to assess time to clinically definite multiple sclerosis and to confirm EDSS progression | EDSS Visual Function System score | Small, insignificant change in Visual Functional System Score of the EDSS over 5 years | Kappos et al., 2009 |
| Natalizumab | AFFIRM: placebo controlled study in relapsing multiple sclerosis to assess relapse rate after 1 year and time to onset of sustained disability progression over 2 years measured by EDSS | Visual acuity measured with Sloan charts; 20% change in visual acuity |
|
Balcer et al., 2007 |
Technical factors that can affect LCLA assessment include optimal refraction of the study participant and luminance of the testing environment. Similarly, there are relative advantages and disadvantages of monocular versus binocular testing. Testing each eye individually would be expected to be more sensitive to monocular deficits and to relate to other monocular measures such as OCT but is more time-consuming. Conversely, binocular testing takes less time and is more analogous to visual function in the natural environment, but potentially could mask monocular deficits due to binocular summation. Interestingly, in some patients with a history of unilateral optic neuritis, binocular vision scores are worse than the better seeing eye, a phenomenon known as binocular inhibition. Testing both monocular and binocular vision is of value in this setting. A final consideration is the relative advantages of using 2.5% versus 1.25% contrast Sloan charts and potential ceiling and floor effects. The extent to which these factors need to be controlled depends on the study design and priority of visual assessment as an endpoint.
An additional issue is the magnitude of worsening of LCLA using Sloan charts that is appropriate as a clinical trial endpoint. Mowry and colleagues (2009) reported that worsening by two lines (10 letters) is associated with a clinically meaningful decrease in vision-related quality of life. Worsening by two lines (10 letters) confirmed at 3 months was used as the criterion for sustained worsening of LCLA in the AFFIRM clinical trial (Balcer et al., 2007). Ophthalmologic studies of HCVA support use of a one-line (five-letter) cut-off (Rosser et al., 2003; Beck et al., 2007). Examination of inter-rater and test-retest reliability of LCLA demonstrated that seven letters corresponds to two standard deviations of difference (Balcer et al., 2000). Change in LCLA by seven letters was used in a longitudinal study of vision in multiple sclerosis (Talman et al., 2010) and as the criterion for confirmed improvement in the AFFIRM clinical trial (Balcer et al., 2012). Further validation of the definition of clinically meaningful worsening and improvement in vision is needed and must be accompanied by discussion with regulatory agencies.
Vision-specific quality of life and patient-reported outcomes
Patient-reported outcomes related to visual function are important as, ultimately, the clinical relevance of measures of visual function, structure, and electrophysiology will be determined by how they relate to or predict measures of vision-specific quality of life. Scores for the NEI-VFQ-25, the most widely used and validated measure of vision-specific quality of life (Mangione et al., 2001), are reduced in patients with multiple sclerosis (Mowry et al., 2009). A 10-Item Neuro-Ophthalmic Supplement to the NEI-VFQ-25 was designed using multiple sclerosis cohorts to capture symptoms relevant to neurologic disease in a more sensitive manner (Ma et al., 2002; Raphael et al., 2006). The Impact of Visual Impairment Scale, a component of the Multiple Sclerosis Quality of Life Inventory (Fischer et al., 1999), also has shown association of reduced scores with worse performance on LCLA testing (Mowry et al., 2009). Collectively, data from these outcomes demonstrate that LCLA testing provides information on clinically relevant aspects of vision.
In considering use of patient-reported outcomes as therapeutic trial outcomes, attention must be paid to participant fatigue during the study visit, the currently limited availability of normative longitudinal data, and the differential sensitivity of the outcomes to treatment effects on decreased worsening versus augmented improvement. Vision-related patient-reported outcomes potentially are susceptible to context effects from co-existing damage from multiple sclerosis, cognitive impairment, comorbid medical conditions, depression, personality traits, dependence on vision, and the availability of social support (Submacular Surgery Trials Research Group, 2007; Wieder et al., 2013).
Structural assessment: optical coherence tomography
Confirmation that LCLA reflects visual pathway structure and disease burden was most firmly provided by the introduction of OCT to the multiple sclerosis field (Parisi et al., 1999). Studies of binocular LCLA had showed associations between worse scores and greater brain MRI lesion burden within the optic tracts, optic radiations, and occipital white matter (Wu et al., 2007). Through its ability to discern retinal anatomy at high resolution (Fig. 2), OCT showed in vivo that LCLA scores reflect the axonal and neuronal losses in the anterior visual pathways that characterize multiple sclerosis (Frohman et al., 2008b; Burkholder et al., 2009; Saidha et al., 2011, 2013).
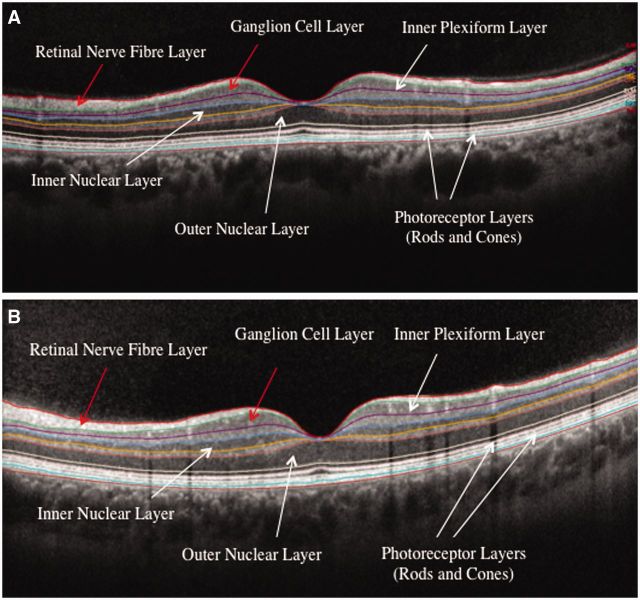
Figure 2.
Single frame of spectral-domain OCT images through the fovea and macular region of the left eye with retinal layers labelled. (A) A 41-year-old female with relapsing-remitting multiple sclerosis. (B) Research study volunteer with no history of ocular or neurological disease. Note visible relative thinning of the macular GCL in the patient with multiple sclerosis (total macular volume = 7.52 mm3) compared to the disease-free control (total macular volume = 8.67 mm3). Similarly, the peripapillary RNFL was thinner in the patient with multiple sclerosis (85 μm) compared to the disease-free control (98 μm). Images are courtesy of Rachel Nolan and Lisena Hasanaj, Neurology Vision Research Laboratory, New York University School of Medicine.
Trip and colleagues (2005) found a 33% reduction in RNFL thickness using time-domain (second generation technology) OCT in multiple sclerosis eyes with incomplete recovery from optic neuritis compared to eyes of matched controls. Costello and colleagues (2006) reported that up to 75% of patients with multiple sclerosis and acute optic neuritis develop 10–40 µm of RNFL loss within 3–6 months, a striking finding given that the RNFL is ∼110–120 µm thick by age 15 and most individuals without a history of glaucoma or macular degeneration lose only ∼0.27% per year in retinal thickness (∼10–20 µm over 60 years) (Kanamori et al., 2003; Harwerth et al., 2008). Costello et al. (2006) also provided compelling evidence for an injury threshold within the RNFL of ∼75 µm by time-domain OCT; thinning of the RNFL below this level was associated with impaired visual function measured by automated visual field testing. One of the most important findings from OCT in studies of patients with multiple sclerosis (both with and without a history of acute optic neuritis) is the correlation between RNFL thickness and visual function, both cross-sectionally (Costello et al., 2006; Fisher et al., 2006; Henderson et al., 2008; Zaveri et al., 2008) and longitudinally over time (Henderson et al., 2010; Talman et al., 2010). These findings suggest the possibility of screening potential neuroprotective or repair-promoting strategies in multiple sclerosis by their ability to prevent axonal loss measured by OCT RNFL thickness in acute optic neuritis. The trajectory and time course of RNFL axonal loss seen with OCT following an episode of acute optic neuritis is important for determining the ‘window of opportunity’ within which a neuroprotective or repair agent might be administered in a clinical trial setting.
Neuronal loss—observed directly (Cifelli et al., 2002) or inferred through detection of grey matter atrophy on MRI (Fisher et al., 2008; Fisniku et al., 2008)—is increasingly recognized as an important cause of worsening disability in multiple sclerosis. Spectral-domain OCT permits measurement of the GCL + IPL and other nucleus-containing retinal layers and has increased our understanding of disease mechanisms in multiple sclerosis (Ishikawa et al., 2005; Tan et al., 2008, 2009; Walter et al., 2012). These studies demonstrated that GCL + IPL thinning, suggesting ganglion cell loss, was significantly associated with reduced visual function and vision-specific quality of life. GCL + IPL thinning has been demonstrated 3 and 6 months following acute optic neuritis (Syc et al., 2012) by quantitative segmentation (Davies et al., 2011). Importantly, baseline GCL + IPL thickness did not demonstrate swelling as seen in the RNFL. GCL + IPL thickness correlates with cortical grey matter and caudate atrophy (Saidha et al., 2013). Thus, GCL + IPL thickness has rapidly emerged as a useful structural marker in multiple sclerosis, paralleling findings of MRI studies that associate grey matter disease (and by implication neuronal loss) with cognitive and neurologic disability.
Structural assessment: MRI
OCT and MRI provide complementary information about visual pathway integrity. MRI-detected anterior and posterior visual pathway lesion volumes correlate with binocular LCLA (Wu et al., 2007). MRI is able to assess brain structural integrity more globally. Five groups have shown RNFL thinning correlates with brain atrophy (Gordon-Lipkin et al., 2007; Sepulcre et al., 2007; Grazioli et al., 2008; Siger et al., 2008; Dorr et al., 2011).
MRI of the optic nerve has been a challenge, although a high signal lesion is visible in almost all cases of acute optic neuritis when using a fat-suppressed T2-weighted sequence that is focused on the optic nerves (Fig. 3). There is also evidence that diffusion tensor imaging may be valuable in quantifying tissue integrity of this structure. Optic nerves of eyes with remote optic neuritis history had abnormal diffusion tensor imaging, either increased radial diffusivity or decreased fractional anisotropy, which was associated with greater degrees of RNFL thinning by OCT and worse visual function (Naismith et al., 2010; Smith et al., 2011). In a recent study of acute optic neuritis, axial diffusivity measured by diffusion tensor imaging of the optic nerve correlated with 6-month outcomes of contrast sensitivity, HCVA, RNFL thickness by OCT, and VEP amplitude and latency (Naismith et al., 2012). Optic nerve diffusion tensor imaging may have the potential to enrich or stratify enrolment into optic neuritis clinical trials or guide allocation of new therapies for those patients who have the most dysfunctional axons and, therefore, might benefit most from treatment.
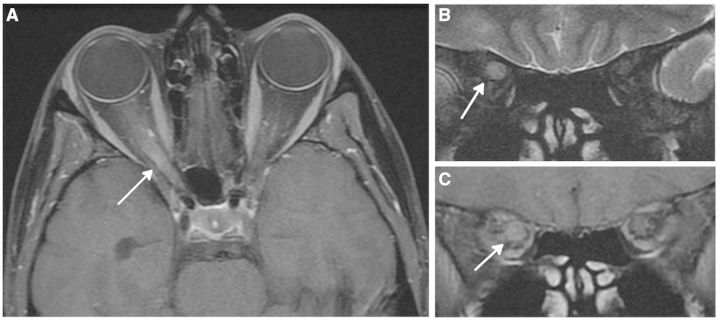
Figure 3.
Magnetic resonance optic nerve images acquired in a 30-year-old female with a 5-day history of acute right optic neuritis. (A) Axial post-contrast T1-weighted image shows swelling and enhancement of the intraorbital and intracanalicular parts of the right optic nerve. (B) Coronal T2-weighted image shows swollen hyperintense right optic nerve through posterior orbit. (C) Coronal post-contrast T1-weighted image shows gadolinium-enhancement of right optic nerve in posterior orbit. Images are courtesy of Dr Ahmed Toosy, UCL Institute of Neurology, London, UK.
Magnetization transfer imaging to measure the magnetization transfer ratio may correlate with myelin content in multiple sclerosis (Schmierer et al., 2004). A significant association between optic nerve magnetization transfer ratio and time-linked VEP latency following optic neuritis suggests a role for the former in detecting remyelination (Hickman et al., 2004). Further studies of diffusion tensor imaging and magnetization transfer imaging (Wang et al., 2012) will help refine MRI’s role and feasibility for use in multicentre optic neuritis and multiple sclerosis trials. Improvements continue to be made with regard to acquisition times and standardization across centres to make optic nerve diffusion tensor imaging and magnetization transfer imaging accessible for trials and clinical practice.
Evidence is conflicting regarding the prevalence of posterior visual pathway (i.e. optic radiation) axonal degeneration in the setting of anterior visual pathway demyelination in optic neuritis. Recent studies combining OCT and MRI provide evidence of trans-synaptic degeneration, both in the anterior and posterior visual pathways (Sriram et al., 2012; Gabilondo et al., 2014), which may appear months to years after optic neuritis. The link between functional recovery from optic neuritis and posterior visual pathway integrity and neuroplasticity also has been the topic of several functional MRI investigations (Werring et al., 2000; Toosy et al., 2005; Korsholm et al., 2007; Jenkins et al., 2010a, b; Raz et al., 2011, 2013; Costello, 2013). Some of these studies demonstrated that dynamic changes in functional connectivity are observed following acute optic neuritis, suggesting the potential for compensatory neuroplasticity in both lower and higher order visual areas of the brain.
Visual evoked potentials and electroretinography
Electrophysiological measures of visual pathway integrity have had increasing roles in the investigation of vision in optic neuritis and multiple sclerosis clinical trials. Demyelination results in both conduction delay and block. The former probably accounts for the characteristic finding in optic neuritis and multiple sclerosis of a main (P100) VEP wave form that is well-formed but of prolonged latency. Reduced VEP amplitude may reflect conduction block due to demyelination or damage to and/or loss of axons. A serial study of acute optic neuritis showed that early prolongation of VEP latency predicted subsequent retinal axonal loss measured by OCT (Henderson et al., 2011), suggesting that demyelinated axons are predisposed to degenerate in the setting of acute inflammation. However, one limitation of pattern VEP as an outcome measure for clinical trials is that the VEP may be undetectable early in the course of optic neuritis, so demonstrating changes in VEP latency from baseline may be challenging. Use of multifocal VEP, which captures a significantly larger area of the visual field than pattern VEP and can provide topographic assessment of amplitude and latency, may provide a useful adjunct or alternative in the setting of acute optic neuritis (Klistorner et al., 2008, 2009). Multifocal VEP may complement OCT measures in examining regional integrity of optic nerve axons and visual pathway structures.
The potential role for pattern electroretinography versus VEP latency in distinguishing macular disease from acute optic neuritis has been emphasized (Holder, 2004). The optic nerve head component of the multifocal electroretinography may provide an electrophysiological marker of axonal disruption in eyes of patients with multiple sclerosis (Schnurman et al., 2014). This novel approach examines the waveform of the multifocal electroretinogram signal as it travels from the unmyelinated retinal ganglion cell axon to the post-lamina cribosa myelinated segment of the optic nerve, and findings are highly correlated with both functional (LCLA) and structural (OCT-assessed RNFL thickness) measures of the visual pathway. Future studies are needed to investigate the ability of the optic nerve head component of the multifocal electroretinography to monitor visual function and predict outcome in optic neuritis and multiple sclerosis.
Fluid-based biomarkers and visual impairment in multiple sclerosis
Blood neurofilament heavy chain levels are elevated in patients with acute optic neuritis and other inflammatory optic neuropathies and correlate with visual outcome and treatment response (Petzold, 2005; Petzold and Plant, 2012). Neurofilament heavy and light chain levels and other validated biomarkers, such as anti-aquaporin 4 autoantibodies, need to be explored in longitudinal studies to determine their relation to structural measures of the visual pathways and prognostic value for neurodegeneration-related visual impairment in patients with optic neuritis and multiple sclerosis.
Optic neuritis and multiple sclerosis in children
Over the past decade, research in paediatric optic neuritis and multiple sclerosis has identified similarities and differences in the clinical manifestations and prognosis related to age. While the cardinal features of optic neuritis (blurred vision, pain with eye movements, dyschromatopsia, and visual field defects) are the same across the age spectrum, severe vision loss, bilateral involvement, and disc swelling are more common in paediatric optic neuritis (Waldman et al., 2011). In two paediatric cohorts, ∼70% of children had visual acuity of 20/200 or worse, and no light perception was relatively common (Wilejto et al., 2006; Bonhomme et al., 2009). However, visual recovery tends to be better than in adults. Younger children (<10 years of age) are more likely to have bilateral optic neuritis compared to adolescents, in whom unilateral optic neuritis is more common. The presence of unilateral or bilateral involvement does not predict the risk of multiple sclerosis in children, but multiple sclerosis risk does increase with age (Bonhomme et al., 2009; Waldman et al., 2011).
A few studies have assessed LCLA and OCT in paediatric demyelinating diseases (Yeh et al., 2009; Yilmaz et al., 2012; Waldman et al., 2014). Similar to adults, following optic neuritis in children, there is decreased LCLA and RNFL thinning compared to healthy control eyes. The data are conflicting on whether LCLA and RNFL thickness are decreased in the eyes of children with multiple sclerosis without a history of optic neuritis. Further studies are required, specifically, longitudinal studies of relation between visual function, OCT and MRI that account for increasing disease duration. Multiple sclerosis is rare in children, and thus, paediatric studies currently are limited by small sample sizes, emphasizing the importance of multi-centre collaborations.
Visual outcomes in optic neuritis and multiple sclerosis clinical trials
Secondary and exploratory outcomes in Phase 3 multiple sclerosis clinical trials and post-marketing studies
Several Phase 3 clinical trials and post-marketing studies in multiple sclerosis included visual outcomes as secondary or exploratory outcomes (Table 1). Many of these trials showed that measures such as LCLA and contrast sensitivity were able to detect treatment benefits with sensitivity similar to that of more traditional efficacy measures (clinical relapse rate and confirmed disability worsening). Addition of LCLA to the MSFC may increase sensitivity to changes in disability that are not detected by the original three-component MSFC (Balcer et al., 2003). Published studies to date have focused on clinically isolated syndromes or relapsing multiple sclerosis. More comprehensive studies of visual dysfunction as secondary outcomes in Phase 3 trials, particularly for progressive multiple sclerosis, are needed.
Visual outcomes in trials of acute optic neuritis
Early trials aimed at improving visual outcome following acute optic neuritis used HCVA as the primary outcome measure. The Optic Neuritis Treatment Trial (Beck et al., 1992; Beck and Gal, 2008) was important in establishing the role for other outcome measures. First, the Optic Neuritis Treatment Trial demonstrated that visual recovery following acute optic neuritis often is incomplete when assessed by measures of low-contrast vision, with Pelli-Robson contrast sensitivity demonstrating the greatest frequency of persistent abnormalities (Optic Neuritis Study Group, 2008b). Reduction in contrast sensitivity was associated with reduced vision-related quality of life (Cole et al., 2000) and provided the rationale for considering assessment of low-contrast vision as a sensitive visual outcome in multiple sclerosis clinical trials. Another key outcome measure in the Optic Neuritis Treatment Trial was automated (Humphrey) visual field testing (Keltner et al., 1994, 2010). This quantitative method remains an important aspect of visual assessment of optic neuritis and other neuro-ophthalmological disorders affecting the optic nerve.
More recently, visual outcomes have been used as the primary measure in Phase 2 clinical trials of putative neuroprotective or reparative agents designed to improve visual recovery following acute optic neuritis (Table 2). A variation on this design is to enrol patients with multiple sclerosis and a prior history of afferent visual system involvement to determine whether there is evidence of repair manifested as improvement in functional or structural measures. Two recent exploratory trials of autologous mesenchymal stem cell infusion used this approach (Connick et al., 2012; Cohen, 2013).
Table 2.
Acute optic neuritis trials recently completed or in progress
| Agent | Trial design | Visual outcome measures | Results | Reference |
|---|---|---|---|---|
| Simvastatin | Placebo-controlled trial to assess whether simvastatin 80/day for 6 months improves visual outcome at 6 months following an episode of acute optic neuritis (n = 64) | Contrast sensitivity (primary), visual acuity, colour vision, VEPs, visual analogue scale | In simvastatin-treated subjects: trend for improved contrast sensitivity (P = 0.06); improved VEP (latency shortened and amplitude increased) improved visual analogue scale (P = 0.04) | Tsakiri et al., 2012 |
| Erythropoietin | Placebo-controlled trial to assess whether erythropoietin 33 000 IU/day for 3 days prevents retinal axonal loss at 16 weeks following an episode of acute optic neuritis (n = 40) | OCT-measured change in RNFL thickness between baseline and 16 weeks (primary); visual acuity, visual fields, VEPs, optic nerve atrophy on MRI | In erythropoietin-treated subjects: smaller decrease in RNFL thickness and optic nerve diameter; shorter VEP latency at 16 weeks | Suhs et al., 2012 |
| Memantine | Placebo-controlled trial to assess whether memantine 5 mg/day for 1 week then 10 mg/day for 2 weeks prevents retinal axonal loss at 3 months following an episode of acute optic neuritis (n = 60) | OCT-measured RNFL thickness (primary); visual acuity; visual fields, contrast sensitivity; VEPs | In memantine-treated subjects: higher RNFL thickness (mean 91.3 versus 78.9 µm, P = 0.01); no difference in visual function measures | Esfahani et al., 2012 |
| Phenytoin | Placebo-controlled trial to assess whether phenytoin prevents retinal axonal loss following acute optic neuritis | OCT-measured RNFL thickness after 6 months (primary); visual acuity; low-contrast acuity; colour vision; VEPs; optic nerve area, lesion size, magnetization transfer ratio on MRI | Trial in progress | Neuroprotection with phenytoin in optic neuritis |
| Amiloride | Placebo-controlled trial to assess whether amiloride prevents retinal axonal loss at 6 months following an episode of acute optic neuritis | Scanning laser polarimetry-measured RNFL thickness after 6 months (primary); OCT-measured RNFL thickness; diffusion MRI of posterior visual pathways; MR spectroscopy of visual cortex; low-contrast acuity; visual acuity; colour vision; VEPs, QOL | Trial in progress | Amiloride clinical trial in optic neuritis (ACTION) |
| Anti-LINGO antibody | Placebo-controlled trial to assess whether anti-lingo antibody shortens visual evoked potential P100 latency at 24 weeks following an episode of acute optic neuritis | Whole field VEP latency after 6 months (primary); OCT-measured RNFL thickness; OCT-measured retinal ganglion cell/inner plexiform layer thickness; low-contrast acuity | Trial in progress | 215ON201 BIIB033 in acute optic neuritis (RENEW) |
| Erythropoietin | Placebo-controlled trial to assess whether erythropoietin improves visual function at 6 months after an episode of acute optic neuritis | Low-contrast letter acuity (co-primary); RNFL thickness (co-primary); macular volume; papillomacular bundle; contrast vision, visual field; VEPs, NEI-VFQ-25 | Trial in progress | Treatment of optic neuritis with erythropoietin |
| Adrenocorticotrophic hormone | Trial comparing the ability of adrenocorticotrophic hormone versus IV methylprednisolone to reduce RNFL loss at 6 months following an episode of acute optic neuritis | Mean RNFL thickness in adrenocorticotrophic-hormone-treated subjects after 6 months (primary); comparison of adrenocorticotrophic hormone and IV methylprednisolone for RNFL thickness at 1, 3 and 6 months; multifocal VEPs; pupillary diameter | Trial in progress | A Phase IV trial of neuroprotection with ACTH in acute optic neuritis |
NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire; QOL = quality of life.
Recommendations for assessment of vision in clinical trials and practice
Tools to measure visual function, vision-related quality of life, structure of the visual pathways, electrophysiology, and body fluid biomarkers in optic neuritis and multiple sclerosis are in various stages of development. Defining normative values for these tests in disease-free volunteers and patients with multiple sclerosis with and without a history of acute optic neuritis has been challenging (Petzold et al., 2010), but values for average LCLA and HCVA testing scores for monocular and binocular vision are now available for adult (Sakai et al., 2011) and paediatric (Waldman et al., 2014) multiple sclerosis.
Visual outcomes can be added to other clinical and imaging outcomes as secondary or exploratory outcomes in standard Phase 2 or 3 trial designs (Table 1). LCLA, either alone or as a component of the MSFC, could provide additional assessment of neurologic impairment. Similarly, OCT could be used to supplement MRI to provide an additional assessment of CNS tissue integrity, both loss and recovery.
Assessment of visual outcomes in acute optic neuritis shows promise as a model system for Phase 2 trials to screen putative neuroprotective or repair-promoting treatment strategies (Table 2). Acute optic neuritis lesions are representative of acute inflammatory demyelination elsewhere in the CNS and, thus, can serve as a more global model for neuroprotection and repair. The optic nerve is one of the few CNS locations where clinical function can be assessed in parallel with direct and non-invasive in vivo measures of structure and electrophysiology. Moreover, measures of visual function are more reliable, sensitive, and quantitative relative to functional measures of other anatomic sites in the CNS. While there is not yet consensus on the preferred primary outcome measure in optic neuritis trials, Phase 2 proof-of-concept trials will likely use a structural marker such as RNFL or GCL + IPL thickness, whereas Phase 3 trials will need to demonstrate clinically relevant benefit on vision as well as structural preservation.
It is imperative that trials focusing on acute optic neuritis have sufficiently stringent eligibility criteria to insure accurate diagnosis and to avoid enrolling participants with other forms of optic neuropathy or causes of visual loss. Similarly, it will be important to avoid participants with comorbidities that might affect clinician-assessed or patient-reported visual outcomes, or at least to collect sufficient information so that these factors can be accounted for in the statistical analyses. An appropriate primary endpoint of a Phase 2 trial in acute optic neuritis would be a between-group comparison of peripapillary RNFL thickness at 6 months (or as early as 4 months), when most of the acute thinning has occurred (Costello et al., 2006, 2008; Henderson et al., 2010). Henderson and colleagues (2010) estimated that, using time-domain OCT technology, a between-group difference of 40% reduction in the overall loss of RNFL thickness of the affected eye could be detected with 80% power with 90 eyes of participants with unilateral optic neuritis (Henderson et al., 2010). This sample size seems to be quite manageable for a multicentre clinical trial. Additional endpoints could include other OCT measures (GCL + IPL thickness and macular volume), LCLA, patient-reported outcomes, MRI measures (volume or length of optic nerve hyperintensity, optic nerve diffusion tensor imaging and magnetization transfer imaging, and possibly functional MRI), and electrophysiological measures (standard or multifocal VEP). It must be recognized that MRI studies of the optic nerve are limited technically by its small size and mobility in the orbit.
There are several caveats to this study design. As the window of therapeutic opportunity for neuronal recovery or protection is likely to be short, it is important to recruit potential study participants as soon as possible after the onset of acute optic neuritis. Body fluid biomarker levels at onset may provide diagnostic and prognostic information and help inform statistical analyses of outcome measures. Specimens should be collected and banked to permit future validation studies. For recruitment to be feasible, trial centres need to have an efficient referral process to identify potential participants and schedule evaluations expeditiously. This design also depends on having a therapeutic agent that can be initiated quickly and has a rapid onset of action. Optic nerve swelling and resultant increase in peripapillary RNFL thickness is often observed acutely in optic neuritis (Kupersmith et al., 2012) and precludes accurate measure of baseline RNFL thickness, which must be taken into consideration in the statistical analysis plan. One suggested approach is to use RNFL thickness in the clinically unaffected fellow eye as a baseline measure, which reduces sample size by around one-third (Henderson et al., 2010). However, this approach assumes the fellow eye is normal, which usually is the case in patients with acute optic neuritis as a clinically isolated syndrome but often is not the case in optic neuritis in the setting of multiple sclerosis (Fisher et al., 2006). Therefore, recent analyses to calculate potential sample sizes have used a variety of methods, including accounting for the fellow eye versus considering the affected eye only (Henderson et al., 2010). Analysing thinning of the GCL + IPL layer, which is not affected by this issue, is a potential alternative approach (Syc et al., 2012; Kupersmith, 2014). Finally, further studies are needed to assess how the neuroprotective or repair effects demonstrated in an acute lesion in optic neuritis (and relapsing multiple sclerosis) relate to the more gradual, presumably neurodegenerative processes that underlie progressive multiple sclerosis (Lublin et al., 2014).
Longitudinal studies of OCT underscore the value of a central OCT reading centre for clinical trials (Keltner et al., 2011). Analogous to a central MRI reading centre, which has become the standard approach in multiple sclerosis trials, a central OCT reading centre would be responsible for training sites in standardized image acquisition procedures, developing case report forms, and deploying computerized retinal segmentation software. Such multicentre studies will require transparent and validated quality control procedures (Schippling et al., 2014).
Clinical trials recently have been launched in paediatric multiple sclerosis. In comparison to adults, paediatric multiple sclerosis is rare, which creates many challenges for trial design. More importantly, investigators, pharmaceutical companies, and regulators must appreciate the differences between adult and paediatric multiple sclerosis when interpreting results. For example, the EDSS does not capture treatment effects in paediatric multiple sclerosis owing to the very low likelihood of accrual of physical disability within the first 10 years from disease onset in the paediatric population. The MSFC has not been validated in children. Specifically, there are no normative data for the component tests. With respect to vision, testing binocular acuity in paediatric trials may not detect subtle deficits or treatment effects due to the greater capacity for binocular summation in children compared to adults. Nevertheless, addition of LCLA and OCT assessments to paediatric trials would advance vision research in paediatric multiple sclerosis.
Future directions
The unique accessibility and structure-function correlations provided by the afferent visual system in multiple sclerosis, combined with additional understanding provided by electrophysiology, make vision a useful model system to test new multiple sclerosis therapies. Research over the past decade has expanded our understanding of vision in multiple sclerosis substantially; ongoing and future studies will take advantage of the growing and now well-organized network of investigators in this area. However, much work remains to be done in a number of areas, including practical aspects of implementing clinical outcome measures in multicentre studies, further validation of fluid-based biomarkers, development and application of new electrophysiological and imaging techniques, and assessing the inter-relationships among these measures both cross-sectionally and longitudinally. In particular, studies addressing how clinical measures of visual impairment correlate over time with or predict more general measures of neurologic disability are needed. It will be important to confirm the clinical meaningfulness of objective visual outcomes using patient-reported outcomes for them to be accepted by regulatory agencies for drug development and approval. In addition, development of normative values for the assessments will be essential for their ultimate application in clinical trials and clinical practice. Finally, although much is known about the neurophysiology of eye movements and the range of abnormalities in multiple sclerosis, substantial work is needed to develop practical methods to assess eye movements quantitatively in clinical trials.
The capacity for measures of visual function, quality of life, visual pathway structure, and electrophysiology to show not only deterioration but also improvement will be critical in the emerging era of agents that repair and protect the nervous system. For the moment, there will be continued reliance on structural outcomes of OCT and MRI to document benefit on reducing neuronal and axonal degeneration or improving tissue repair. Technological advances in both modalities should enable greater sensitivity and specificity in monitoring pathology in the anterior visual pathway and its modification by therapy. One such example is the development of improved techniques for quantitative diffusion tensor imaging of the optic nerve (Samson et al., 2013). Vision research in multiple sclerosis will continue to require and benefit from the collaborative approach that has contributed to its success over the past decade.
Acknowledgements
The International Conference on Vision and Vision-Related Outcomes in Multiple Sclerosis was organized under the auspices of the International Advisory Committee on Clinical Trials in Multiple Sclerosis. We are grateful for the active participation of the meeting attendees (Appendix), who were given the opportunity to review a draft of this manuscript and provide input.
Glossary
Abbreviations
- EDSS
Expanded Disability Status Scale
- GCL
ganglion cell layer
- HCVA
high contrast visual acuity
- IPL
inner plexiform layer
- LCLA
low-contrast letter acuity
- MSFC
Multiple Sclerosis Functional Composite
- OCT
optical coherence tomography
- RNFL
retinal nerve fibre layer
- VEP
visual-evoked potential
Funding
The conference and the activities of the Committee were funded by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the U.S. National Multiple Sclerosis Society.
Disclosures
Dr Balcer has received consulting and/or advisory board service fees from Biogen Idec, Genzyme, and Vaccinex.
Dr Cohen has received personal compensation for consulting from EMD Serono, Genentech, Genzyme, Innate Immunotherapeutics, Novartis, and Vaccinex. Dr Cohen received research support paid to his institution from Biogen Idec, Consortium of MS Centres, Department of Defense, Genzyme, National Institutes of Health, National MS Society, Novartis, Receptos, Synthon, Teva, and Vaccinex.
Dr Miller has received honoraria through payments to his employer, UCL Institute of Neurology, for advisory committee and/or consultancy advice in multiple sclerosis studies from Biogen Idec, GlaxoSmithKline, Novartis, Merck, Chugai, Mitsubishi Pharma Europe, and Bayer Schering Pharma. He also received compensation through payments to his employer for perform central MRI analysis of multiple sclerosis trials from Biogen Idec, GlaxoSmithKline, Merck, and Novartis. The Queen Square MS Centre at UCL Institute of Neurology is supported by the UK MS Society and UCL-UCLH Biomedical Research Centre.
Dr Reingold has received personal consulting fees and/or travel reimbursement from the National Multiple Sclerosis Society (NMSS), the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); Bayer HealthCare, Biogen Idec, Coronado Biosciences Inc., the Cleveland Clinic Foundation, Eli Lilly & Company, EMD Serono and Merck Serono, Genentech, F. Hoffmann-LaRoche, ISIS Pharmaceuticals Inc., Medimmune Inc., Novartis Pharmaceuticals Corporation, Observatoire Français de la Sclérosis en Plaques, Opexa Therapeutics, Sanofi-Aventis, SK Biopharmaceuticals, Synthon Pharmaceuticals Inc., TEVA Pharmaceutical Industries, and Fondation pour l'aide à la Recherche sur la Sclérosis en Plaques; and reports membership on the editorial board of the Multiple Sclerosis Journal over the past 3 years.
Supplementary material
Supplementary material is available at Brain online.
References
- Ashworth B, Aspinall PA, Mitchell JD. Visual function in multiple sclerosis. Doc Ophthalmol. 1989;73:209–24. doi: 10.1007/BF00155090. [DOI] [PubMed] [Google Scholar]
- Balcer LJ, Baier ML, Cohen JA, Kooijmans MF, Sandrock AW, Nano-Schiavi ML, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003;61:1367–73. doi: 10.1212/01.wnl.0000094315.19931.90. [DOI] [PubMed] [Google Scholar]
- Balcer LJ, Baier ML, Pelak VS, Fox RJ, Shuwairi S, Galetta SL, et al. New low-contrast vision charts: reliability and test characterisitics in patients with multiple sclerosis. Mult Scler. 2000;6:163–71. doi: 10.1177/135245850000600305. [DOI] [PubMed] [Google Scholar]
- Balcer LJ, Frohman EM. Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology. 2010;74(Suppl 3):S16–23. doi: 10.1212/WNL.0b013e3181dbb664. [DOI] [PubMed] [Google Scholar]
- Balcer LJ, Galetta SL, Calabresi PA, Confavreux C, Giovannoni G, Havrdova E, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68:1299–304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- Balcer LJ, Galetta SL, Polman CH, Eggenberger E, Calabresi PA, Zhang A, et al. Low-contrast acuity measures visual improvement in phase 3 trial of natalizumab in relapsing MS. J Neurol Sci. 2012:119–24. doi: 10.1016/j.jns.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Balcer LJ, Arnold DL, Cohen JA, Coles AJ, Confavreux C, Fox EJ, et al. Alemtuzumab improves visual outcomes in treatment-naive patients with relapsing-remitting multiple sclerosis (RRMS): analysis from the phase 3 CARE-MS I study. J Neurol Sci. 2013;333 (suppl 1):e375. [Google Scholar]
- Beck RW, Cleary PA, Anderson MM, Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–8. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- Beck RW, Gal RL. Treatment of acute optic neuritis. A summary of findings from the Optic Neuritis Treatment Trial. Arch Ophthalmol. 2008;126:994–5. doi: 10.1001/archopht.126.7.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Opththalmology. 2007:114. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Biousse V, Trichet C, Bloch-Michel E, Roullet E. Multiple sclerosis associated uveitis in two large clinic-based series. Neurology. 1999;52:179–81. doi: 10.1212/wnl.52.1.179. [DOI] [PubMed] [Google Scholar]
- Bock M, Brandt AU, Kuchenbecker J, Dorr J, Pfueller CF, Weinges-Evers N, et al. Impairment of contrast visual acuity as a functional correlate of retinal nerve fibre thinning and total macular colume reduction in multiple sclerosis. Br J Ophthalmol. 2012;96:62–7. doi: 10.1136/bjo.2010.193581. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Brannan JR. Hidden visual loss in optic neuropathy is revealed using Gabor patch contrast perimetry. Clin Neurosci. 1997;4:284–91. [PubMed] [Google Scholar]
- Bonhomme GR, Waldman AT, Balcer LJ, Daniels AB, Tennekoon GI, Forman S, et al. Pediatric optic neuritis. Brain abnormalities and risk of multiple sclerosis. Neurology. 2009;72:881–5. doi: 10.1212/01.wnl.0000344163.65326.48. [DOI] [PubMed] [Google Scholar]
- Brandt AU, Oberwahrenbrock T, Kadas EM, Lagreze WA, Paul F. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology. 2014;83:73–7. doi: 10.1212/WNL.0000000000000545. [DOI] [PubMed] [Google Scholar]
- Brandt AU, Oberwahrenbrock T, Ringelstein M, Young KL, Tiede M, Hartung HP, et al. Primary retinal pathology in multiple sclerosis as detected by optic coherence tomography (letter to the editor) Brain. 2011;134:1–3. doi: 10.1093/brain/awr095. [DOI] [PubMed] [Google Scholar]
- Burggraaff MC, Trieu J, de Vries-Knoppert WA, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci. 2014;55:952–61. doi: 10.1167/iovs.13-12912. [DOI] [PubMed] [Google Scholar]
- Burkholder BM, Osborne B, Loguidice MJ, Bisker ER, Frohman TC, Conger A, et al. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009;66:1366–72. doi: 10.1001/archneurol.2009.230. [DOI] [PubMed] [Google Scholar]
- Chen L, Gordon LK. Ocular manifestations of multiple sclerosis. Curr Opin Ophthalmol. 2005;16:315–20. doi: 10.1097/01.icu.0000179804.49842.e2. [DOI] [PubMed] [Google Scholar]
- Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. thalamic neurodegeneration in multiple sclerosis. Ann Neurol. 2002;52:650–3. doi: 10.1002/ana.10326. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci. 2013;333:43–9. doi: 10.1016/j.jns.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Beck RW, Moke PS, Gal RL, Long DT The Optic Neuritis Study Group. The National Eye Institute visual function questionnaire: experience of the ONTT. Invest Ophthalmol Vis Sci. 2000;41:1017–21. [PubMed] [Google Scholar]
- Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–6. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello F. The afferent visual pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol. 2013;2013:134858. doi: 10.1155/2013/134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- Costello F, Hodge W, Pan YI, Burton JM, Freedman MS, Stys PK, et al. Sex-specific differences in retinal nerve fiber layer thinning after acute optic neuritis. Neurology. 2012;79:1866–72. doi: 10.1212/WNL.0b013e318271f755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14:893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- Davies EC, Galetta KM, Sackel DJ, Talman LS, Frohman EM, Calabresi PA, et al. Retinal ganglion cell layer volumetric assessment by spectral-domain optical coherence tomography in multiple sclerosis: application of a high-precision manual estimation technique. J Neuroophthalmol. 2011;31:260–4. doi: 10.1097/WNO.0b013e318221b434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem R, Tschime A, Bahr M. Decreased amplitudes in multiple sclerosis patients with normal visual acuity: a VEP study. J Clin Neurosci. 2003;10:67–70. doi: 10.1016/s0967-5868(02)00172-8. [DOI] [PubMed] [Google Scholar]
- Donaldson MJ, Pulido JS, Herman DC, Diehl N, Hodge D. Pars planitis: a 20-year study of incidence, clinical features, and outcomes. Am J Ophthalmol. 2007;144:812–17. doi: 10.1016/j.ajo.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Dorr J, Wernecke KD, Bock M, Gaede G, Wuerfel J, Pfueller CF, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6:e18132. doi: 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani MR, Harandi ZA, Movasat M, Nikdel M, Adelpour M, Momeni A, et al. Memantine for axonal loss of optic neuritis. Graefes Arch Clin Exp Ophthalmol. 2012;250:863–9. doi: 10.1007/s00417-011-1894-3. [DOI] [PubMed] [Google Scholar]
- Finke C, Pech LM, Sommer C, Schlichting J, Stricker S, Endres M, et al. dynamics of saccade parameters in multiple sclerosis patients with fatigue. J Neurol. 2012;259:2656–63. doi: 10.1007/s00415-012-6565-8. [DOI] [PubMed] [Google Scholar]
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS) Mult Scler. 1999;5:251–9. doi: 10.1177/135245859900500410. [DOI] [PubMed] [Google Scholar]
- Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–65. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber thickness in multiple sclerosis. Opththalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–54. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- Fraser CL, Davagnanam I, Radon M, Plant GT. The time course and phenotype of Uthoff phenomenon following optic neuritis. Mult Scler J. 2012;18:1042–4. doi: 10.1177/1352458511431074. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Costello F, Stuve O, Calabresi PA, Miller DH, Hickman SJ, et al. Modeling axonal degeneration within the anterior visual system. Implications for demonstrating neuroprotection in multiple sclerosis. Arch Neurol. 2008a;65:26–35. doi: 10.1001/archneurol.2007.10. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter GR, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008b;4:664–75. doi: 10.1038/ncpneuro0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabilondo I, Martinez-Lapiscina EH, Martinez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75:98–107. doi: 10.1002/ana.24030. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Rodriguez-Mena D, Herrero R, Almarcegui C, Dolz I, Martin J, et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology. 2013;81:76–83. doi: 10.1212/WNL.0b013e318299ccd9. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in mjltiple sclerosis is associated with disease severity. Brain. 2012;135:1786–93. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Lipkin E, Chodklwski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–9. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- Graves J, Balcer LJ. Eye disorders in patients with multiple sclerosis: natural history and management. Clin Ophthalmol. 2010;4:1409–22. doi: 10.2147/OPTH.S6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J, Galetta SL, Palmer J, Margolin DH, Rizzo M, Bilbruck J, et al. Alemtuzumab improves contrast sensitivity in patients with relapsing-remitting multiple sclerosis. Mult Scler J. 2013;19:1302–9. doi: 10.1177/1352458513475722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazioli E, Zivadinov R, Weinstock-Guttman B, Lincoff N, Baier M, Wong JR, et al. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci. 2008;268:12–17. doi: 10.1016/j.jns.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49:4437–43. doi: 10.1167/iovs.08-1753. [DOI] [PubMed] [Google Scholar]
- Henderson APD, Altmann DR, Trip SA, Kallis C, Jones SJ, Schlottmann PG, et al. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain. 2010;133:2592–602. doi: 10.1093/brain/awq146. [DOI] [PubMed] [Google Scholar]
- Henderson APD, Altmann DR, Trip SA, Miszkiel KA, Schlottmann PG, Jones SJ, et al. Early factors associated with axonal loss after optic neuritis. Ann Neurol. 2011;70:955–63. doi: 10.1002/ana.22554. [DOI] [PubMed] [Google Scholar]
- Henderson APD, Trip SA, Schottman PG, Altmann DR, Garway-Heath DF, Plant GT, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277–87. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Toosy AT, Jones SJ, Altmann DR, Mizkiel KA, MacManus DG, et al. Serial magnetization transfer imaging in acute optic neuritis. Brain. 2004;127:692–700. doi: 10.1093/brain/awh076. [DOI] [PubMed] [Google Scholar]
- Holder GE. Electrophysiological assessment of optic nerve disease. Eye. 2004;18:1133–43. doi: 10.1038/sj.eye.6701573. [DOI] [PubMed] [Google Scholar]
- Horton L, Conger A, Conger D, Remington G, Frohman T, Frohman E, et al. Effect of 4-aminopyridine on vision in multiple sclerosis patients with optic neuropathy. Neurology. 2013;80:1862–6. doi: 10.1212/WNL.0b013e3182929fd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–17. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasse L, Vukusic S, Durand-Dubief F, Vartin C, Piras C, Bernard M, et al. Persistent visual impairment in multiple sclerosis: prevalence, mechanisms and resulting disability. Mult Scler J. 2013;19:1618–26. doi: 10.1177/1352458513479840. [DOI] [PubMed] [Google Scholar]
- Jenkins T, Ciccarelli O, Toosy A, Miszkiel K, Wheeler-Kingshott C, Altmann D, et al. Dissecting structure-function interactions in acute optic neuritis to investigate neuroplasticity. Hum Brain Mapp. 2010a;31:276–86. doi: 10.1002/hbm.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TM, Toosy AT, Ciccarelli O, Miszkiel KA, Wheeler-Kingshott CA, Henderson AP, et al. Neuroplasticity predicts outcome of optic neuritis independent of tissue damage. Ann Neurol. 2010b;67:99–113. doi: 10.1002/ana.21823. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Escano MF, Eno A, Nakamura M, Maeda H, Seya R, et al. Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica. 2003;217:273–8. doi: 10.1159/000070634. [DOI] [PubMed] [Google Scholar]
- Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Long-term effect of early treatment with interferon-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8:987–97. doi: 10.1016/S1474-4422(09)70237-6. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue E-W, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Kaufhold F, Zimmerman H, Schneider E, Ruprecht K, Paul F, Oberwahrenbrock T, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic edema independently of multiple sclerosis. PLoS One. 2013;8:e71145. doi: 10.1371/journal.pone.0071145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JL, Cello KE, Balcer LJ, Calabresi PA, Markowitz CE, Werner JS. Stratus OCT quality control in two multi-centre multiple sclerosis clinical trials. Neuro-ophthalmology. 2011;35:57–64. doi: 10.3109/01658107.2011.557760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JL, Johnson CA, Cello KE, Dontchev M, Gal RL, Beck RW, et al. Visual field profile of optic neuritis. A final follow-up report from the Optic Neuritis Treatment Trial from baseline through 15 years. Arch Ophthalmol. 2010;128:330–7. doi: 10.1001/archophthalmol.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JL, Johnson CA, Spurr JO, Beck RW for the Optic Neuritis Study Group. Visual field profile of optic neuritis. One-year follow-up in the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1994;112:946–53. doi: 10.1001/archopht.1994.01090190094027. [DOI] [PubMed] [Google Scholar]
- Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, et al. Multifocal VEP and OCT in optic neuritis: a topographical study of the structure-function relationship. Doc Ophthalmol. 2009;118:129–37. doi: 10.1007/s10633-008-9147-4. [DOI] [PubMed] [Google Scholar]
- Klistorner A, Fraser C, Garrick R, Graham S, Arvind H. Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol. 2008;116:19–27. doi: 10.1007/s10633-007-9072-y. [DOI] [PubMed] [Google Scholar]
- Korsholm K, Madsen KH, Frederiksen JL, Skimminge A, Lund TE. Recovery from optic neuritis: an ROI-based analysis of LGN and visual cortical areas. Brain. 2007;130:1244–53. doi: 10.1093/brain/awm045. [DOI] [PubMed] [Google Scholar]
- Kupersmith MJ, Wang J-K, Garvin M, Kardon R. Retinal ganglion cell layer thinning and vision outcome in optic neuritis over six months. Mult Scler J. 2014;20:(S1)358–9. doi: 10.1177/1352458515598020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersmith MJ, Kardon R, Durbin MK, Shulman J. Scanning laser polarimetry reveals status of RNFL integrity in eyes with optic nerve head swelling by OCT. Invest Ophthalmol Vis Sci. 2012;53:1962–70. doi: 10.1167/iovs.11-9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leat SJ, Legge GE, Bullimore MA. What is low vision? A re-evaluation of definitions. Optom Vis Sci. 1999;76:198–211. doi: 10.1097/00006324-199904000-00023. [DOI] [PubMed] [Google Scholar]
- Lightman S, McDonald WI, Bird AC, Francis DA, Hoskins A, Batchelor JR, et al. Retinal venous sheathing in optic neuritis. Its significance for the pathogenesis of multiple sclerosis. Brain. 1987;110:405–14. doi: 10.1093/brain/110.2.405. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–86. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SL, Shea JA, Galetta SL, Jacobs DA, Markowitz CE, Maguire MG, et al. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. 2002;133:686–92. doi: 10.1016/s0002-9394(02)01337-5. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- Martinez-Lapiscina EH, Fraga-Pumar E, Pastor X, Gomez M, Conesa A, Lozano-Rubi R, et al. Is the incidence of optic neuritis rising? Evidence from an epidemiological study in Barcelona (Spain), 2008-2012. J Neurol. 2014;261:759–67. doi: 10.1007/s00415-014-7266-2. [DOI] [PubMed] [Google Scholar]
- Moster S, Wilson JA, Galetta SL, Balcer LJ. The King-Devick (K-D) test of rapid eye movements: a bedside correlate of disability and quality of life in MS. J Neurol Sci. 2014;343:105–9. doi: 10.1016/j.jns.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Mowry EM, Loguidice MJ, Daniels AB, Jacobs DA, Markowitz CE, Galetta SL, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high conrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80:767–72. doi: 10.1136/jnnp.2008.165449. [DOI] [PubMed] [Google Scholar]
- Naismith RT, Xu J, Tutlam NT, Lancia S, Trinkaus K, Song S-K, et al. Diffusion tensor imaging in acute optic neuropathies. Arch Neurol. 2012;69:65–71. doi: 10.1001/archneurol.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Xu J, Tutlam NT, Trinkaus K, Cross AH, Song SK. Radial diffusivity in remote optic neuritis discriminates visual outcomes. Neurology. 2010;74:1702–10. doi: 10.1212/WNL.0b013e3181e0434d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Hosokawa T, Sugino M, Kimura F, Sugasawa J, Hanafusa T, et al. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol. 2010;10:45. doi: 10.1186/1471-2377-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, O'Brien PC, Petterson TM, Weis J, Stevens L, Peterson WK, et al. A randomized trial of intravenous immunoglobulin in inflammatory demyelinating optic neuritis. Neurology. 2001;56:1514–22. doi: 10.1212/wnl.56.11.1514. [DOI] [PubMed] [Google Scholar]
- Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler J. 2013;19:1887–95. doi: 10.1177/1352458513489757. [DOI] [PubMed] [Google Scholar]
- Optic Neuritis Study Group. Visual function more than 10 years after optic neuritis: experieince of the optic neuritis treatmetn trial. Am J Ophthalmol. 2004;137:77–83. doi: 10.1016/s0002-9394(03)00862-6. [DOI] [PubMed] [Google Scholar]
- Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008a;65:727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the Optic Neuritis Treatment Trial. Opththalmology. 2008b;115:1079–82. doi: 10.1016/j.ophtha.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Ortiz-Perez S, Martinez-Lapiscina E, Gabilondo I, Fraga-Pumar E, Martinez-Heras E, Saiz A, et al. Retinal periphlebitis is associated with multiple sclerosis severity. Neurology. 2013;81:877–81. doi: 10.1212/WNL.0b013e3182a3525e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7. [PubMed] [Google Scholar]
- Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–98. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–32. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- Petzold A, Plant GT. The diagnostic and prognostic value of neurofilament heavy chain levels in immune-mediated optic neuropathies. Mult Scler Int. 2012;2012:217802. doi: 10.1155/2012/217802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Birch EE, Talman LS, Sackel DJ, Frohman EM, Calabresi PA, et al. One eye or two: a comparison of binocular and monocular low-contrast acuity testing in multiple sclerosis. Am J Ophthalmol. 2011;152:133–40. doi: 10.1016/j.ajo.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, et al. Validation and test characterisitics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142:1026–35. doi: 10.1016/j.ajo.2006.06.060. [DOI] [PubMed] [Google Scholar]
- Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80:47–54. doi: 10.1212/WNL.0b013e31827b1a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Chokron S, Ben-Hur T, Levin N. Temporal reorganization to overcome monocular demyelination. Neurology. 2013;81:702–9. doi: 10.1212/WNL.0b013e3182a1aa3e. [DOI] [PubMed] [Google Scholar]
- Raz N, Dotan S, Benoliel T, Chokron S, Ben-Hur T, Levin N. Sustained motion perception deficint following optic neuritis. Behavioral and cortical evidence. Neurology. 2011;76:2103–111. doi: 10.1212/WNL.0b013e31821f4602. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mena D, Almarcegui C, Dolz I, Herrero R, Bambo MP, Fernandez J, et al. Electrophysiologic evaluation of the visual pathway in patients with multiple sclerosis. J Clin Neurophysiol. 2013;30:376–81. doi: 10.1097/WNP.0b013e31829d75f7. [DOI] [PubMed] [Google Scholar]
- Roesner S, Appel R, Gbadamosi J, Martin R, Heesen C. Treatment of steroid-unresponsive optic neuritis with plasma exchange. Acta Neurol Scand. 2012;126:103–8. doi: 10.1111/j.1600-0404.2011.01612.x. [DOI] [PubMed] [Google Scholar]
- Rosser AE, Cousens SN, Murdoch IE, Fitzke FW, Laidlaw DAH. How sensitive to clinical change are ETDRS logMAR visual acuity measurements. Invest Ophthalmol Vis Sci. 2003;44:3278–81. doi: 10.1167/iovs.02-1100. [DOI] [PubMed] [Google Scholar]
- Rudick R, Antel J, Confavreux C, Cutter G, Ellison G, Fischer J, et al. Clinical outcomes assessment in multiple sclerosis. Ann Neurol. 1996;40:469–79. doi: 10.1002/ana.410400321. [DOI] [PubMed] [Google Scholar]
- Rudick R, Antel J, Confavreux C, Cutter G, Ellison G, Fischer J, et al. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol. 1997;42:379–82. doi: 10.1002/ana.410420318. [DOI] [PubMed] [Google Scholar]
- Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R. Plasma exchange for severe optic neuritis. Treatment of 10 patients. Neurology. 2004;63:1081–3. doi: 10.1212/01.wnl.0000138437.99046.6b. [DOI] [PubMed] [Google Scholar]
- Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–72. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70:34–43. doi: 10.1001/jamaneurol.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Syc SB, Ibrahim MA, Eckstein C, WARNER CV, Farrell SK, et al. Primary retinal pathology in multple sclerosis as detected by optical coherence tomography. Brain. 2011;134:518–33. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- Sakai RE, Feller DJ, Galetta KM, Galetta SL, Balcer LJ. Vision in multiple sclerosis: the story, structure-function correlations, and models for neuroprotection. J Neuroophthalmol. 2011;31:362–73. doi: 10.1097/WNO.0b013e318238937f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter AR, Tyry T, Vollmer T, Cutter GR, Marrie RA. “Seeing” in NARCOMS: a look at vision-related quality of life in the NARCOMS registry. Mult Scler J. 2013;9:953–60. doi: 10.1177/1352458512469694. [DOI] [PubMed] [Google Scholar]
- Samson RS, Kolappan M, Thomas DL, Symms MR, Connick P, Miller DH, et al. Development of a high-resolution fat and CSF-suppressed optic nerve DTI protocol at 3T: application in multiple sclerosis. Funct Neurol. 2013;28:93–100. doi: 10.11138/FNeur/2013.28.2.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippling S, Balk L, Costello F, Albrecht P, Balcer L, Calabresi P, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler 2014 Jun 16. doi: 10.1177/1352458514538110. pii: 1352458514538110. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–15. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- Schnurman ZS, Frohman TC, Beh SC, Conger D, Conger A, Saidha S, et al. Retinal architecture and mfERG. Optic nerve head component response characteristics in MS. Neurology. 2014;82:1888–96. doi: 10.1212/WNL.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layanna A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–94. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- Serra A, Skelly MM, Jacobs JB, Walker MF, Cohen JA. Improvement of internuclear ophthalmoparesis in multiple sclerosis with dalfampridine. Neurology. 2014;83:192–4. doi: 10.1212/WNL.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siger M, Dziegielewski K, Jasek L, Bieniek M, Nicpan A, Nawrocki JW, et al. Optical coherence tomography in multiple sclerosis. The thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol. 2008;255:1555–60. doi: 10.1007/s00415-008-0985-5. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams ZR, Ratchford JN, Newsome SD, Farrell SK, Farrell JAD, et al. Diffusion tensor imaging of the optic nerve in multiple sclerosis: association with retinal damage and visual disability. AJNR. 2011;32:1662–8. doi: 10.3174/ajnr.A2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram P, Graham SI, Wang C, Yiannikas C, Garrick R, Klistorner A. Transsynaptic retinal degeneration in optic neuropathies: optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012;53:1271–75. doi: 10.1167/iovs.11-8732. [DOI] [PubMed] [Google Scholar]
- Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST Report Number 19. Ophthalmic Epidemiol. 2007;14:205–15. doi: 10.1080/09286580701502970. [DOI] [PubMed] [Google Scholar]
- Suhs K-W, Hein K, Sattler MB, Gorlitz A, Ciupka C, Scholz K, et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72:199–210. doi: 10.1002/ana.23573. [DOI] [PubMed] [Google Scholar]
- Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–33. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman LS, Bisker ER, Sackel DJ, Long DA, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–60. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–14. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan O, Li G, Lu AT, Varma R, Huang D Advanced Imaging for Glaucoma Study Group. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008;115:949–56. doi: 10.1016/j.ophtha.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilikete C, Jasse L, Vukusic S, Durand-Dubief F, Vardanian C, Pelisson D, et al. Persistent ocular motor manifestations and related visual consequences in multiple sclerosis. Ann N Y Acad Sci. 2011;1233:327–34. doi: 10.1111/j.1749-6632.2011.06116.x. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Hickman SJ, Miszkiel KA, Jones SJ, Plant GT, Altmann DR, et al. Adaptive cortical plasticity in higher visual areas after acute optic neuritis. Ann Neurol. 2005;57:622–33. doi: 10.1002/ana.20448. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13:83–99. doi: 10.1016/S1474-4422(13)70259-X. [DOI] [PubMed] [Google Scholar]
- Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–91. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- Tsakiri A, Kallenbach K, Fuglo D, Wanscher B, Larsson H, Frederiksen J. Simvastatin improves final visual outcome in acute optic neuritis: a randomized study. Mult Scler J. 2012;18:72–81. doi: 10.1177/1352458511415452. [DOI] [PubMed] [Google Scholar]
- Tselis A, Perumal J, Caon C, Hreha S, Ching W, Din M, et al. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur J Neurol. 2008;15:1163–7. doi: 10.1111/j.1468-1331.2008.02258.x. [DOI] [PubMed] [Google Scholar]
- Villoslada P, Cuneo A, Gelfand J, Hauser SL, Green A. Color vision is strongly associated with retinal thinning in multiple sclerosis. Mult Scler J. 2012;18:991–9. doi: 10.1177/1352458511431972. [DOI] [PubMed] [Google Scholar]
- Waldman AT, Hiremath G, Avery RA, Conger A, Pineles SL, Loguidice MJ, et al. Monocular and binocular low-contrast visual acuity and optical coherence tomography in pediatric multiple sclerosis. Mult Scler Rel Dis. 2014;3:326–34. doi: 10.1016/j.msard.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman AT, Stull LB, Galetta SL, Balcer LJ, Liu GT. Pediatric optic neuritis and risk of multiple sclerosis: meta-analysis of observational studies. J AAPOS. 2011;15:441–6. doi: 10.1016/j.jaapos.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–7. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, van der Walt A, Paine M, Klistorner A, Butzkueven H, Egan GF, et al. Optic nerv e magnetisation transfer ratio after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2012;7:e52291. doi: 10.1371/journal.pone.0052291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring DJ, Bullmore ET, Toosy AT, Miller DH, Barker GJ, MacManus DG, et al. Recovery from optic neuritis is associated in the distribution of cerebral response to visual stimulation: a functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2000;68:441–9. doi: 10.1136/jnnp.68.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieder L, Gade G, Pech LM, Zimmerman H, Wernecke KD, Dorr JM, et al. Low contrast visual acuity testing is associated with cognitive performance in multiple sclerosis: a cross-sectional pilot study. BMC Neurol. 2013;13:167. doi: 10.1186/1471-2377-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilejto M, Shroff M, Buncic J K, JR, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67:258–62. doi: 10.1212/01.wnl.0000224757.69746.fb. [DOI] [PubMed] [Google Scholar]
- Winges KM, Werner JS, Harvey DJ, Cello KE, Durbin MK, Balcer LJ, et al. Baseline retinal nerve fiber layer thickness and macular volume quantified by OCT in the North American phase 3 fingolimod trial for relapsing-remitting multiple sclerosis. J Neuroophthalmol. 2013;33:322–9. doi: 10.1097/WNO.0b013e31829c51f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Schwartz ED, Lei T, Souza A, Mishra S, Jacobs DA, et al. Relation of vision to global and regional brain MRI in multiple sclerosis. Neurology. 2007;69:2128–35. doi: 10.1212/01.wnl.0000278387.15090.5a. [DOI] [PubMed] [Google Scholar]
- Yeh EA, Weinstock-Guttman B, Lincoff N, Reynolds J, Weinstock A, Madurai N, et al. Retinal nerve fiber thickness in inflammatory demyelinating diseases of childhood onset. Mult Scler. 2009;15:802–10. doi: 10.1177/1352458509104586. [DOI] [PubMed] [Google Scholar]
- Yilmaz U, Gucuyener K, Erin DM, Yazar Z, Gurkas E, Serdaroglu A, et al. Reduced retinal nerve fiber layer thickness and macular volume in pediatric multiple sclerosis. J Child Neurol. 2012;27:1517–23. doi: 10.1177/0883073812447683. [DOI] [PubMed] [Google Scholar]
- Zaveri MS, Conger A, Salter A, Frohman TC, Galetta SL, Markowitz CE, et al. Retinal imaging by laser polarimetry and optic coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch Neurol. 2008;65:924–8. doi: 10.1001/archneur.65.7.924. [DOI] [PubMed] [Google Scholar]