Abstract
The feasibility of de novo everolimus without calcineurin inhibitor (CNI) therapy following liver transplantation was assessed in a multicenter, prospective, open-label trial. Liver transplant patients were randomized at 4 weeks to start everolimus and discontinue CNI, or continue their current CNI-based regimen. The primary endpoint was adjusted estimated GFR (eGFR; Cockcroft-Gault) at month 11 postrandomization. A 24-month extension phase followed 81/114 (71.1%) of eligible patients to month 35 postrandomization. The adjusted mean eGFR benefit from randomization to month 35 was 10.1 mL/min (95% confidence interval [CI] −1.3, 21.5 mL/min, p = 0.082) in favor of CNI-free versus CNI using Cockcroft-Gault, 9.4 mL/min/1.73 m2 (95% CI −0.4, 18.9, p = 0.053) with Modification of Diet in Renal Disease (four-variable) and 9.5 mL/min/1.73 m2 (95% CI −1.1, 17.9, p = 0.028) using Nankivell. The difference in favor of the CNI-free regimen increased gradually over time due to a small progressive decline in eGFR in the CNI cohort despite a reduction in CNI exposure. Biopsy-proven acute rejection, graft loss and death were similar between groups. Adverse events led to study drug discontinuation in five CNI-free patients and five CNI patients (12.2% vs. 12.5%, p = 1.000) during the extension phase. Everolimus-based CNI-free immunosuppression is feasible following liver transplantation and patients benefit from sustained preservation of renal function versus patients on CNI for at least 3 years.
The beneficial effect on renal function achieved by early CNI withdrawal and treatment with everolimus after liver transplantation is still evident after three years.
Keywords: Calcineurin inhibitor, everolimus, liver transplantation, long-term, withdrawal
Introduction
Development of chronic renal failure is a well-documented complication following liver transplantation 1. Despite awareness of the issue, recent analyses have continued to show a significant deterioration of renal function in liver transplant recipients 2–4, with up to 18% of patients ultimately progressing to end-stage renal disease 2,5. The etiology of declining renal function after transplantation is complex, but nephrotoxicity associated with long-term calcineurin inhibitor (CNI) therapy is one important modifiable risk factor 6. This has prompted investigation into a potential role for mammalian target of rapamycin (mTOR) inhibitors in minimizing CNI exposure without compromising efficacy.
To date, few studies have evaluated the effect of everolimus on renal function after liver transplantation 7–10. Two randomized trials have investigated late conversion from CNI therapy to everolimus 7,8, at a mean of more than 3 years posttransplant, in populations with a mean estimated GFR (eGFR) of 50 7 and 65 mL/min 8 when the switch took place. Consistent with findings in kidney transplantation 11,12, neither study showed a renal benefit after such a late switch to mTOR inhibitor–based immunosuppression. In contrast, a trial of 78 liver transplant patients randomized to conversion from CNI to everolimus by day 30 demonstrated a significant improvement in renal function versus CNI-treated controls, with no increase in rejection 9. In the randomized H2304 trial, CNI-free everolimus therapy by month 4 was also associated with excellent renal function, but the treatment group was discontinued prematurely due to a higher rate of acute rejection versus everolimus with reduced-exposure tacrolimus or standard tacrolimus therapy 10. In the large PROTECT (Preservation of Renal functiOn in liver Transplant rEcipients with Certican Therapy) trial, conversion from CNI to everolimus took place over an 8-week period starting on day 30, with complete CNI withdrawal by month 4 13. At 12 months posttransplant, there was a clinically relevant increase in eGFR in favor of everolimus.
Data on outcomes following conversion of liver transplant recipients to an mTOR inhibitor beyond 1 year posttransplant remain limited 8. The extended effect of conversion is of particular interest in view of evidence that eGFR during the first year is predictive of subsequent renal function 4,14,15. Moreover, based on early trial results, concerns were expressed about the safety of everolimus therapy in liver transplant recipients, and long-term safety data are required 16. Here, 3-year data from the PROTECT study population were examined with the aim of determining the long-term feasibility of CNI-free everolimus-based immunosuppression.
Methods
Study design and conduct
PROTECT was a multicenter, prospective, open-label, parallel-group trial in which de novo liver transplant patients were randomized at 4 weeks posttransplant in a 1:1 ratio to start everolimus and discontinue CNI therapy, or to continue their current CNI-based regimen (NCT NCT00378014). Starting in August 2006, patients were recruited at 16 transplant centers in Germany, Austria, Switzerland and the Netherlands.
Following the core 12-month study, all 10 centers in Germany were invited to take part in a follow-up extension study, two of which declined to do so. Participation of the remaining six centers outside Germany was not possible due to logistic reasons. The final extension study visit took place in January 2012.
Inclusion and exclusion criteria
The core study population comprised adult (18–70 years) de novo recipients of a liver transplant from a deceased or living donor. Exclusion criteria included multi-organ transplantation, previous transplantation, severe systemic infection and preexisting renal dysfunction with eGFR expected to be <50 mL/min (Cockcroft-Gault formula 17) by the time of randomization. At week 4, randomization took place if the following criteria were met: (i) no rejection for at least the preceding 2 weeks; (ii) platelet count >50 000/mm3, white blood cell count >2500/mm3 and hemoglobin level >8 g/dL; and (iii) eGFR >50 mL/min.
All patients who completed the 12-month core study at German centers and were still receiving the immunosuppression regimen to which they were randomized were asked to enter the follow-up extension study.
Immunosuppression
As described previously 13, during the core study all patients received basiliximab induction. CNI therapy (tacrolimus or cyclosporine) with or without corticosteroids was administered according to local practice. After randomization, the treatment group started everolimus to achieve a target trough level of 5–12 ng/mL. Thereafter, CNI dose was lowered by 70% and then withdrawn entirely 8 weeks later if the patient had remained rejection-free for at least the preceding 4 weeks. If CNI discontinuation could not be achieved by month 4 posttransplant, study treatment was withdrawn. In the control arm, patients continued to receive their CNI-based regimen. Corticosteroid therapy was permitted in both treatment arms. Accordingly, at the start of the extension phase, patients were receiving either everolimus plus steroids or tacrolimus/cyclosporine plus steroids (controls). Use of mycophenolic acid was not part of either treatment regimen but was not specifically excluded by protocol.
Evaluation
During the extension phase, patients were to continue their randomized immunosuppression regimen as per the core study protocol.
Statistical analysis
Efficacy and safety analyses were based on all patients entering the extension phase.
The primary endpoint (superior renal function in the CNI-free group [eGFR, Cockcroft-Gault 17]) was assessed by analysis of covariance (ANCOVA) with treatment and center as factors and eGFR at randomization as covariate (two-sided α = 0.05). The primary analyses of renal function during the core study were based on the randomized period (months 1–12 posttransplant) by use of last observation carried forward analyses. An adjusted mean (least squares) was presented for eGFR at month 11. As a preplanned sensitivity analysis, the primary comparison was also undertaken using the four-variable Modification of Diet in Renal Disease (MDRD4) formula 18, with an additional post hoc comparison based on the Nankivell formula 19. Analysis of the primary variable was repeated for data obtained at month 35 postrandomization in the extension study. The sample size estimation for the core study assumed a mean difference of at least 8 mL/min for the primary endpoint between treatment groups with a standard deviation of 20 mL/min, and resulted in 100 patients per arm with a statistical power of 80% and a two-sided significance level of 5%. Based on this calculation, the extension phase was underpowered for the primary endpoint.
All other analyses were exploratory. Observed values for eGFR were compared between groups using a t-test.
All analyses were performed using the software package SAS® (Version 9.2; SAS Institute, Cary, NC).
Results
Patient population
The core trial was completed by 177 of the 203 randomized patients (87.2%); 114 (56.2%) were still receiving their randomized study medication at the final visit at month 12 posttransplant (Figure 1). Of these 114 patients, 81 entered the extension study (71.1%; 41 CNI-free, 40 CNI) of whom 33 patients (80.45%) in the CNI-free cohort and 27 (67.5%) in the CNI arm completed the extension study visit at month 35 after randomization. The demographics and baseline characteristics of the extension study population are shown in Table 1. Patients randomized to the CNI-free arm who took part in the extension study had experienced significantly more frequent biopsy-proven acute rejection (BPAR) during the core study versus CNI-treated patients (19.5% [8/41] vs. 2.5% [1/40], p = 0.029).
Figure 1.
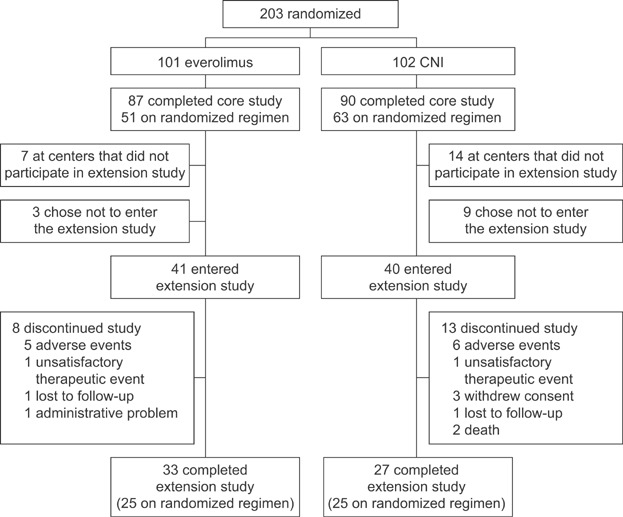
Patient disposition.
Demographic and baseline characteristics of the extension study population
| CNI-free (n = 41) | CNI (n = 40) | |
|---|---|---|
| Recipient age (years), mean (SD) | 53.0 (10.3) | 52.0 (10.9) |
| Male gender, n (%) | 24 (58.5) | 25 (62.5) |
| Body mass index (kg/m2), mean (SD) | 26.0 (4.4) | 26.6 (3.9) |
| Primary reason for transplantation (n, %) | ||
| Alcoholic cirrhosis | 14 (34.1) | 13 (32.5) |
| Hepatitis B | 3 (7.3) | 3 (7.5) |
| Hepatitis C | 1 (2.4) | 5 (12.5) |
| Sclerosing cholangitis | 2 (4.9) | 2 (5.0) |
| Cryptogenic cirrhosis | 4 (9.8) | 2 (5.0) |
| Primary biliary cirrhosis | 0 (0.0) | 2 (5.0) |
| Other | 17 (41.5) | 13 (32.5) |
| MELD score (points), mean (SD) | 16.6 (9.3) | 14.0 (6.5) |
| Time from liver transplant to randomization (days), mean (SD) | 46.7 (11.6) | 42.1 (11.5) |
| Type of transplant, n (%) | ||
| Complete liver | 36 (87.8) | 36 (90.0) |
| Split liver | 5 (12.2) | 4 (10.0) |
| Donor type, n (%) | ||
| Deceased heart beating | 39 (95.1) | 38 (95.0) |
| Living related | 2 (4.9) | 1 (2.5) |
| Living unrelated | 0 (0.0) | 1 (2.5) |
| Donor hepatitis C positive, n (%) | 0 (0.0) | 4 (10.0) |
| BPAR during core study, n (%) | 8 (19.5) | 1 (2.5) |
*p = 0.029.
BPAR, biopsy-proven acute rejection; CNI, calcineurin inhibitor; MELD, Model for End-Stage Liver Disease; SD, standard deviation.
Immunosuppression
During the extension study, the mean achieved everolimus trough concentration remained stable, in the range 8.3–8.9 ng/mL. In the CNI group, 28 patients were receiving tacrolimus and 12 patients were receiving cyclosporine at the start of the extension study. Mean (SD) trough concentrations of tacrolimus and cyclosporine decreased from 8.4 (2.6) and 154 (32) ng/mL, respectively, at month 11 postrandomization to 6.7 (1.5) and 110 (22) ng/mL at month 35. Between months 11 and 35, oral corticosteroids were administered to 23/41 (56.1%) of CNI-free patients and 15/40 (37.5%) of CNI-treated patients. During the extension study, one patient in the CNI-free group and four patients in the CNI group started mycophenolate mofetil therapy.
Renal function
Unadjusted mean (SD) eGFR (Cockcroft-Gault) at the start of the extension study was 88.2 (29.0) mL/min versus 81.4 (22.1) mL/min in the CNI-free and CNI cohorts (p = 0.240). This was similar to month 12 values in the total population of patients who completed the core phase (87.9 [32.0] mL/min vs. 84.1 [34.9] mL/min). During the extension study, unadjusted eGFR was numerically or significantly higher in the CNI-free cohort than in the CNI group at all time points using the Cockcroft-Gault, Nankivell or MDRD4 formulae (Figure 2). Despite a reduction in CNI exposure from months 11 to 35, there was a small but progressive decline in eGFR over time in the CNI group compared to stable values in the CNI-free group (Figure 2). At month 35 postrandomization, mean eGFR was 10.5 mL/min higher with the CNI-free regimen versus CNI using the Cockcroft-Gault formula (p = 0.096), 10.5 mL/min/1.73 m2 using the Nankivell formula (p = 0.015) and 9.6 mL/min/1.73 m2 using the MDRD4 formula (p = 0.059).
Figure 2.
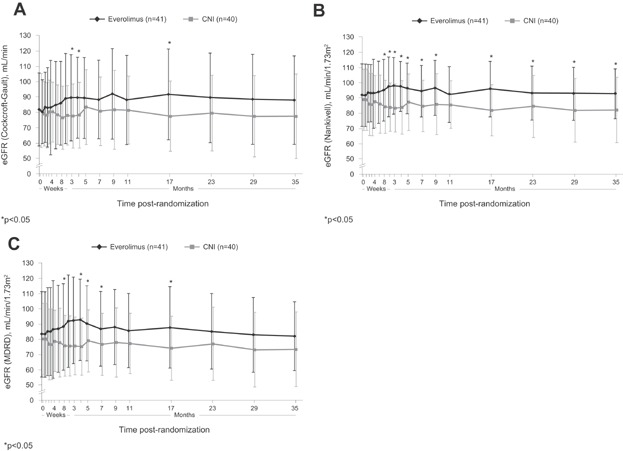
eGFR from the time of randomization to month 35 after randomization according to (A) Cockcroft-Gault, (B) Nankivell and (C) MDRD4 formulae in patients randomized to everolimus or CNI therapy. Values are shown as mean (SD). CNI, calcineurin inhibitor; eGFR, estimated GFR; MDRD4, Modification of Diet in Renal Disease (four-variable).
The difference in adjusted eGFR values obtained from the ANCOVA model (with treatment and center as factors and eGFR at randomization as covariate) at month 35 postrandomization was 10.1 mL/min in favor of the CNI-free regimen with the Cockcroft-Gault formula (95% confidence interval [CI] −1.3, 21.5 mL/min, p = 0.082) and 9.4 mL/min/1.73 m2 with the MDRD4 formula (95% CI −0.4, 18.9, p = 0.053). The difference was significant when using the Nankivell formula (difference 9.5 [95% CI 1.1, 17.9] mL/min/1.73 m2, p = 0.028; Table 2). The mean difference in adjusted change from randomization between the CNI-free and CNI groups increased during the extension phase, from 6.2 mL/min at month 11 to 10.1 mL/min at month 35 by the Cockcroft-Gault formula (Figure 3A). Similar increases in the between-group difference were also seen over time using the Nankivell formula (Figure 3B) and MDRD4 formula (Figure 3C).
Renal endpoints in the extension study population
| CNI-free (n = 41) | CNI (n = 40) | Difference [95% CI] | p-Value | |
|---|---|---|---|---|
| Randomization | ||||
| eGFR, Cockcroft-Gault (mL/min) | 81.8 (23.6) | 81.2 (24.1) | – | 0.909 |
| eGFR, Nankivell (mL/min/1.73 m2) | 92.0 (20.5) | 89.2 (20.1) | – | 0.535 |
| eGFR, MDRD4 (mL/min/1.73 m2) | 79.0 (23.5) | 77.8 (24.4) | – | 0.814 |
| Month 11 postrandomization | ||||
| eGFR, Cockcroft-Gault (mL/min) | ||||
| Unadjusted | 88.2 (29.0) | 81.4 (22.1) | – | 0.240 |
| Adjusted | 88.2 [79.4; 97.0] | 82.0 [72.3; 91.8] | 6.2 [−4.2; 16.6] | 0.236 |
| Adjusted change from randomization | 6.8 [−2.1; 15.6] | 0.5 [−9.2; 10.3] | – | – |
| eGFR, Nankivell (mL/min/1.73 m2) | ||||
| Unadjusted | 92.5 (18.3) | 85.9 (15.5) | – | 0.084 |
| Adjusted | 92.6 [86.4; 98.8] | 87.8 [81.0; 94.6] | 4.8 [−2.4; 12.1] | 0.189 |
| Adjusted change from randomization | 2.0 [−4.2; 8.2] | −2.8 [−9.6; 4.0] | – | – |
| eGFR, MDRD4 (mL/min/1.73 m2) | ||||
| Unadjusted | 78.8 (23.9) | 72.0 (16.7) | – | 0.143 |
| Adjusted | 78.7 [71.5; 85.9] | 72.7 [64.7; 80.6] | 6.0 [−2.5; 14.6] | 0.164 |
| Adjusted change from randomization | 0.3 [−6.9; 7.5] | −5.7 [−13.7; 2.2] | – | – |
| Month 23 postrandomization | ||||
| eGFR, Cockcroft-Gault (mL/min) | ||||
| Unadjusted | 89.4 (29.2) | 79.5 (24.7) | – | 0.104 |
| Adjusted | 88.1 [78.7; 97.4] | 77.5 [67.2; 87.9] | 10.5 [−0.5; 21.5] | 0.061 |
| Adjusted change from randomization | 6.6 [−2.8; 15.9] | −3.9 [−14.3; 6.4] | – | – |
| eGFR, Nankivell (mL/min/1.73 m2) | ||||
| Unadjusted | 93.5 (17.7) | 84.7 (20.3) | – | 0.039 |
| Adjusted | 92.6 [86.4; 98.8] | 84.0 [76.3; 91.8] | 8.6 [0.2; 16.9] | 0.044 |
| Adjusted change from randomization | 2.0 [−5.1; 9.0] | −6.6 [−14.3; 1.2] | – | – |
| eGFR, MDRD4 (mL/min/1.73 m2) | ||||
| Unadjusted | 78.7 (24.7) | 70.5 (20.9) | – | 0.110 |
| Adjusted | 77.5 [69.4; 85.7] | 68.6 [59.7; 77.7] | 8.9 [−0.8; 18.5] | 0.071 |
| Adjusted change from randomization | −0.9 [−9.0; 7.3] | −9.8 [−18.7; −0.8] | – | – |
| Month 35 postrandomization | ||||
| eGFR, Cockcroft-Gault (mL/min) | ||||
| Unadjusted | 88.0 (28.7) | 77.5 (27.4) | – | 0.096 |
| Adjusted | 88.2 [78.6; 97.9] | 78.1 [67.4; 88.8] | 10.1 [−1.3, 21.5] | 0.082 |
| Adjusted change from randomization | 6.8 [−2.9; 16.4] | −3.4 [−14.1; 7.3] | – | – |
| eGFR, Nankivell (mL/min/1.73 m2) | ||||
| Unadjusted | 92.9 (16.3) | 82.4 (21.5) | – | 0.015 |
| Adjusted | 93.6 [86.5; 100.8] | 84.1 [76.3; 91.9] | 9.5 [1.1, 17.9] | 0.028 |
| Adjusted change from randomization | 3.0 [−4.1; 10.2] | −6.5 [−14.3; 1.4] | – | – |
| eGFR, MDRD4 (mL/min/1.73 m2) | ||||
| Unadjusted | 77.5 (23.4) | 67.9 (21.8) | – | 0.059 |
| Adjusted | 78.2 [70.1; 86.2] | 68.8 [60.0; 77.7] | 9.4 [−0.4, 18.9] | 0.053 |
| Adjusted change from randomization | −0.2 [−8.3; 7.8] | −9.6 [−18.4; −0.7] | – | – |
Significant p-values are shown in bold.
Table 1t-Test (between-group comparison).
Table 2ANCOVA.
Unadjusted values are shown as mean (SD). Adjusted data are obtained from ANCOVA analysis, with treatment and center as factors and eGFR at randomization as covariate, and are presented as least square mean values [95% CI].
ANCOVA, analysis of covariance; CI, confidence interval; CNI, calcineurin inhibitor; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease (four-variable).
Figure 3.
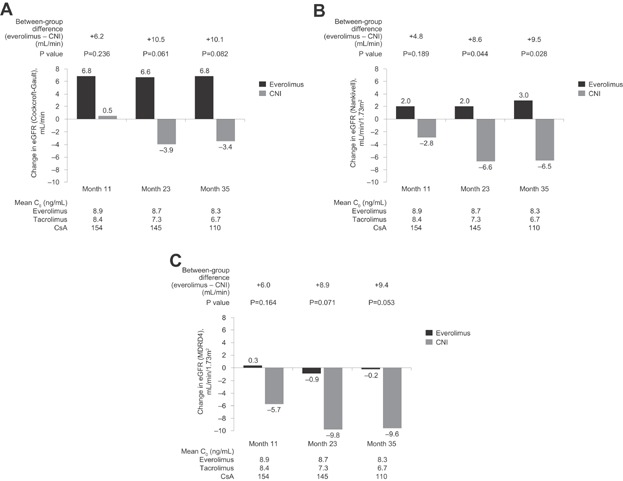
Mean change in adjusted estimated GFR (eGFR) from baseline to months 11, 23 and 35 postrandomization according to (A) Cockcroft-Gault, (B) Nankivell and (C) MDRD4 formulae in patients randomized to everolimus or CNI therapy. The between-group differences in the adjusted change from baseline at each time point are indicated above the bars. p-Values refer to the comparison between groups. Values below the graphs show mean trough (C0) concentrations of everolimus in the everolimus group and tacrolimus or cyclosporine (CsA) in the CNI cohort at each time point. CNI, calcineurin inhibitor; MDRD4, Modification of Diet in Renal Disease (four-variable).
Efficacy
During the extension study two patients in the CNI-free group experienced one episode of BPAR (Table 3), both of which were graded mild and resolved after steroid treatment. The most recent measurement of everolimus C0 level prior to BPAR was 8.4 ng/mL in both patients. There were no cases of BPAR in the CNI group. Two patients in the CNI group died (one from septic shock, one from peritoneal carcinosis and stomach cancer), and none in the CNI-free group. A combined endpoint of BPAR, graft loss, death or follow-up occurred in two patients in each group during the extension phase.
Efficacy endpoints (ITT population)
| Randomization to month 11 (ITT population) | Randomization to month 11 (extension study population) | Months 11–35 (extension study population) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CNI-free (n = 96) | CNI (n = 98) | p-Value | CNI-free (n = 41) | CNI (n = 40) | p-Value | CNI-free (n = 41) | CNI (n = 40) | p-Value | |
| BPAR, n (%) | 17 (17.7) | 15 (15.3) | 0.702 | 8 (19.5) | 1 (2.5) | 0.029 | 2 (4.9) | 0 (0.0) | 0.494 |
| Treated BPAR, n (%) | 13 (13.5) | 10 (10.2) | 0.512 | 7 (17.1) | 0 (0.0) | 0.012 | 2 (4.9) | 0 (0.0) | 0.494 |
| Graft loss, n (%) | 2 (2.1) | 2 (2.0) | 1.000 | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 0 (0.0) | – |
| Death, n (%) | 4 (4.2) | 4 (4.1) | 1.000 | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 2 (5.0) | 0.241 |
| Treatment failure (BPAR, graft loss, death or loss to follow-up) | 20 (20.8) | 20 (20.4) | 1.000 | 8 (19.5) | 1 (2.5) | 0.029 | 2 (4.9) | 2 (5.0) | 1.000 |
Table 1Fisher’s exact test.
BPAR, biopsy-proven acute rejection; CNI, calcineurin inhibitor; ITT, intent-to-treat.
Safety
During the extension phase, the most frequent adverse events in the CNI-free group were diarrhea, peripheral edema, incisional hernia and back pain, with peripheral edema (22.0% vs. 5.0%, p = 0.048) and back pain (22.0% vs. 2.5%, p = 0.014) occurring significantly more frequently in the CNI-free group versus the CNI group (Table 4). Three cases of proteinuria were observed in CNI-free patients during months 11–35, all of which were graded mild by the investigator and no action was taken. During the 35 months after randomization, proteinuria occurred in seven patients (17.1%) but only led to a change of immunosuppressive treatment in one case, where the investigator withdrew everolimus. In two of the seven cases, proteinuria resolved spontaneously. There were no cases of hepatic artery thrombosis in either group. Neoplasms (benign or malignant) were reported in 17.1% and 19.8% of patients in the CNI-free and CNI groups, respectively, during the extension phase (p = 0.587) with no apparent differences in the type of neoplasms. Serious adverse events were reported in 23 CNI-free patients (56.1%) and 23 CNI patients (57.5%) (p = 1.00), the most frequent of which was incisional hernia (four CNI-free, five CNI; p = 0.74).
Adverse events occurring in ≥20% of patients in either treatment group in the extension study population from randomization to month 11 postrandomization and from months 11 to 35, n (%)
| Randomization to month 11 (extension study population) | Months 11–35 (extension study population) | |||||
|---|---|---|---|---|---|---|
| CNI-free (n = 41) | CNI (n = 40) | p-Value | CNI-free (n = 41) | CNI (n = 40) | p-Value | |
| Blood and lymphatic system disorders | ||||||
| Anemia | 9 (22.0) | 5 (12.5) | 0.379 | 2 (4.9) | 2 (5.0) | 1.000 |
| Gastrointestinal disorders | ||||||
| Diarrhea | 12 (29.3) | 7 (17.5) | 0.295 | 10 (24.4) | 4 (10.0) | 0.140 |
| Nausea | 3 (7.3) | 9 (22.5) | 0.067 | 0 | 0 | – |
| General disorders and administration site conditions | ||||||
| Peripheral edema | 11 (26.8) | 5 (12.5) | 0.162 | 9 (22.0) | 2 (5.0) | 0.048 |
| Infections | ||||||
| Nasopharyngitis | 9 (22.0) | 9 (22.5) | 1.000 | 6 (14.6) | 7 (17.5) | 0.770 |
| Injury, poisoning and procedural complications | ||||||
| Incisional hernia | 10 (24.4) | 7 (17.5) | 0.587 | 10 (24.4) | 6 (15.0) | 0.404 |
| Investigations | ||||||
| Increased hepatic enzyme | 10 (24.4) | 4 (10.0) | 0.140 | 1 (2.4) | 2 (5.0) | 0.616 |
| Metabolism and nutrition disorders | ||||||
| Hypercholesterolemia | 13 (31.7) | 3 (7.5) | 0.011 | 4 (9.8) | 1 (2.5) | 0.359 |
| Musculoskeletal and connective tissue disorders | ||||||
| Arthralgia | 5 (12.2) | 8 (20.0) | 0.379 | 2 (4.9) | 2 (5.0) | 1.000 |
| Back pain | 6 (14.6) | 10 (25.0) | 0.276 | 9 (22.0) | 1 (2.5) | 0.014 |
| Skin and subcutaneous tissue disorders | ||||||
| Pruritus | 9 (22.0) | 6 (15.0) | 0.569 | 5 (12.2) | 4 (10.0) | 1.000 |
| Vascular disorders | ||||||
| Hypertension | 9 (22.0) | 6 (15.0) | 0.569 | 5 (12.2) | 5 (12.5) | 1.000 |
Table 1Fishers’ exact test (between-group comparison).
CNI, calcineurin inhibitor.
At the end of the extension phase, hematological parameters were similar between treatment groups (Table 5). Levels of liver enzymes (ASAT, ALAT and gamma-glutamyl transferase) did not differ significantly between the two treatment arms (Table 5). Total cholesterol was significantly higher with CNI-free versus CNI (mean 5.6 [1.2] mmol/L vs. 4.8 [1.0] mmol/L; p = 0.006).
Hematology and laboratory parameters at month 35 postrandomization (extension study population)
| CNI-free (n = 41) | CNI (n = 40) | p-Value | |
|---|---|---|---|
| Hematology, mean (SD) | |||
| White blood cell count, 109/L | 6.3 (2.3) | 6.5 (2.3) | 0.826 |
| Platelets, 109/L | 201 (86) | 186 (65) | 0.349 |
| Neutrophils, % | 61 (12) | 65 (10) | 0.189 |
| Hemoglobin, g/dL | 13.2 (1.6) | 14.0 (1.6) | 0.061 |
| Liver function | |||
| Aspartate transaminase (ASAT), U/L | |||
| Mean (SD) | 32 (13) | 44 (37) | 0.090 |
| Median (range) | 31 (14–71) | 27 (11–129) | – |
| Alanine transaminase (ALAT), U/L | |||
| Mean (SD) | 32 (9) | 46 (43) | 0.061 |
| Median (range) | 33 (17–49) | 27 (11–195) | – |
| Gamma-glutamyl transferase | |||
| Mean (SD) | 50 (71) | 89 (143) | 0.168 |
| Median (range) | 28 (0.4–367) | 47 (7–717) | – |
| Total bilirubin, µmol/L | |||
| Mean (SD) | 7.7 (4.4) | 15.8 (19.4) | 0.023 |
| Median (range) | 6.8 (3.0–23.9) | 10.3 (5.1–109.4) | – |
| Metabolic, mean (SD) | |||
| Total cholesterol, mmol/L | 5.6 (1.2) | 4.8 (1.0) | 0.006 |
| LDL-cholesterol, mmol/L | 3.2 (0.9) | 2.9 (0.8) | 0.293 |
| HDL-cholesterol, mmol/L | 1.5 (0.5) | 1.3 (0.5) | 0.446 |
| Triglycerides, mmol/L | 2.4 (1.7) | 1.7 (1.1) | 0.076 |
Values are shown as mean (SD).
Table 1t-Test (between-group comparison).
CNI, calcineurin inhibitor; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
During the extension study, adverse events led to discontinuation of study drug in five CNI-free patients and five CNI patients (12.2% vs. 12.5%, p = 1.000).
Discussion
Three-year follow-up data from the randomized PROTECT trial show that renal function is preserved long term in de novo liver transplant patients who remain on everolimus after early conversion from CNI therapy, without loss of efficacy. After 3 years, eGFR was approximately 10 mL/min higher in CNI-free patients than in patients receiving a CNI. Such a benefit is considered clinically meaningful, and highly encouraging in view of evidence that early deterioration in renal function predicts progression to advanced chronic kidney disease or renal failure after liver transplantation 15,20.
The rate of renal deterioration in CNI-treated liver transplant patients is greatest at 1 month posttransplant 21. Consistent with this, the CNI group in the PROTECT study showed an early decline in eGFR shortly after randomization 13. In contrast, the CNI-free group showed an increase in eGFR during the period of CNI tapering and withdrawal. Subsequently, mean eGFR values in the two groups gradually diverged as the CNI cohort experienced a progressive decline in renal function, even though CNI levels were tapered over time, while renal function in the CNI-free group remained stable (Figure 2). The difference in unadjusted and adjusted eGFR between the treatment groups did not achieve statistical significance by 3 years posttransplant in the smaller extension population using the prespecified Cockcroft-Gault formula but attained significance in favor of the CNI-free regimen in a post hoc analysis based on the Nankivell formula. These results suggest that preemptive early conversion of liver transplant patients from CNI therapy to everolimus may help to avoid loss of renal function, and may be a more effective strategy than selective use of mTOR inhibition only after pronounced renal deterioration. It is important to note, however, that recruitment to the PROTECT study started before organ allocation was based on Model for End-Stage Liver Disease (MELD) scoring, and as a result the renal function of the population at time of transplant was higher than might be observed under the current allocation process.
In the recent H2304 trial, de novo liver transplant patients who were randomized to everolimus with tacrolimus elimination and who continued on this regimen showed strikingly good renal function at 2 years posttransplant 10. However, this treatment group was terminated prematurely due to a significantly higher rate of acute rejection versus either CNI continuation or everolimus with reduced tacrolimus 10. In the current study, BPAR during the first year posttransplant was comparable in the CNI-free and CNI groups. It is noteworthy that compared to the CNI-free group, significantly fewer patients in the CNI group who experienced BPAR during the core study entered the extension phase (CNI-free 8/41, CNI 1/40; p = 0.029). Thus, as has been shown in kidney transplantation 22, early mild BPAR after liver transplantation does not predict poor long-term outcomes, and continuing an everolimus-based CNI-free regimen after an early rejection episode is feasible without increasing the risk of subsequent rejection.
The discrepancy in early efficacy in the CNI-free group in the current study and the H2304 trials is unlikely to have been due to substantial differences in the characteristics of the patient populations, which exhibited no marked disparity in terms of rejection risk. In the H2304 study, patients did not receive induction with an IL-2 receptor antibody, in contrast to the current trial, a factor that may have contributed. One further possibility is that the protocol for conversion to an mTOR inhibitor may be important in maintaining immunosuppressive potency. Although conversion to everolimus started at 1 month posttransplant in both PROTECT and H2304 trials, tacrolimus withdrawal was more abrupt in H2304 (over a maximum of 4 weeks compared with 8 weeks in PROTECT) and the upper threshold for the everolimus exposure range was 10 ng/mL compared with 12 ng/mL in the current study. Rejection episodes in the H2304 study were clustered after tacrolimus withdrawal 10, indicating that an extended CNI tapering period and adequate mTOR inhibitor exposure are important.
The safety profile of everolimus was acceptable. Peripheral edema was more frequent in the CNI-free arm, a recognized side effect of mTOR inhibitors that is dose dependent 23. In our population, there was no apparent association between peripheral edema and proteinuria. Incisional hernias were numerically more frequent in the CNI-free group during the study. Last, no patient had anemia at month 35 and the hematological profile was similar between treatment groups.
The incidence of proteinuria in the CNI-free group after randomization (17.1%) was similar to that reported following switch of maintenance liver transplant patients to everolimus 24. Regrettably, urinary protein values were available in only a small proportion of patients in the extension population (e.g. nine patients at month 35 postrandomization) so a more detailed analysis was not feasible.
These results should be interpreted in the context of the extension study design. Notably, only patients who remained on their randomized medication at the end of the core study were included. During the core study, 49.5% of CNI-free patients and 38.2% of CNI patients discontinued study medication 13, most frequently due to adverse events (27.2% and 15.7% 13), so the extension study population represented only a selected cohort of patients. This is likely to have contributed to the relatively low rate of discontinuations due to adverse events during the extension study (∼12%). Furthermore, the fact that six of the initial 16 centers were excluded for logistical reasons meant that the extension phase was underpowered to detect any difference in renal function: The sample size calculation for the core study estimated a need for 100 patients in each treatment arm to detect a difference in eGFR (Cockcroft-Gault) of 8 mL/min 13. It should also be borne in mind that the core study randomized only patients without severe renal dysfunction, and without acute rejection during the 2 weeks prior to randomization. Therefore, the results cannot be extrapolated to all liver transplant recipients.
This study represents the longest evaluation to date of liver transplant recipients after early conversion to CNI-free everolimus-based immunosuppression. Its findings suggest that in liver transplant patients with compensated baseline renal function, gradual conversion from CNI to everolimus between months 1 and 4 posttransplant is a promising therapeutic approach that preserves renal function with manageable side effects and no loss of efficacy to 3 years posttransplant.
Acknowledgments
The study was funded by Novartis Pharma GmbH. Support for manuscript preparation was provided by a freelance medical writer (Caroline Dunstall) with funding from Novartis Pharma GmbH.
Glossary
- ANCOVA
analysis of covariance
- BPAR
biopsy-proven acute rejection
- CI
confidence interval
- CNI
calcineurin inhibitor
- eGFR
estimated GFR
- ITT
intent-to-treat
- LOCF
last observation carried forward
- MDRD4
Modification of Diet in Renal Disease (four-variable)
- MELD
Model for End-Stage Liver Disease
- mTOR
mammalian target of rapamycin
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. M. Sterneck has received travel grants from Novartis Pharma GmbH, Astellas Pharma GmbH and Biotest AG and has been a member of an advisory board for Merck KGaA. G.M. Kaiser has received research funding and/or travel grants from Novartis, Astellas, Roche and Pfizer. N. Heyne has received research funding and travel grants from Novartis Pharma GmbH. N. Richter has no conflicts of interest to declare. F. Rauchfuss has received travel grants from Novartis, Astellas, Wyeth, Biotest and Roche. A. Pascher has received speaker’s fees, research funding and travel grants from Astellas, Novartis, Roche, Genzyme and Sanofi-Aventis. P. Schemmer has received speaker’s fees, research funding and travel grants from Novartis, Astellas and Sanofi-Aventis. L. Fischer has received speaker’s fees, research funding and travel grants as member of advisory boards from Novartis Pharma GmbH, and has received speaker’s fees and travel grants from Astellas Pharma GmbH. C.G. Klein has received research funding and/or travel grants from Astellas, Biotest, Novartis, Pfizer and Roche. S. Nadalin is an advisory board member and has received travel grants from Novartis Pharma GmbH. F. Lehner has no conflicts of interest to declare. U. Settmacher has received travel grants from Novartis, Astellas, Wyeth and Roche. P. Neuhaus has received research funding from Astellas, Novartis and Roche. D. Gotthardt has received speaker’s fees, unrestricted research grants and travel grants from Novartis. M. Loss has received consulting fees and lecture fees and travel/accommodation/meeting expenses from Novartis, Astellas, Roche, Genzyme, Covidien, Ethicon, Merck Serono and Biotest. S. Ladenburger, E.M. Paulus and M. Mertens are employees of Novartis. H.J. Schlitt has received research support and honoraria as a speaker and/or as advisory board member from Novartis, Roche, Genzyme and BMS.
References
- 1.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Lamattina JC, Mezrich JD, Fernandez LA, et al. Native kidney function following liver transplantation using calcineurin inhibitors: Single-center analysis with 20 years of follow-up. Clin Transplant. 2013;27:193–202. doi: 10.1111/ctr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JP, Heo NJ, Joo KW, et al. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant. 2010;25:2772–2785. doi: 10.1093/ndt/gfq093. [DOI] [PubMed] [Google Scholar]
- 4.Herlenius G, Fistouris J, Olausson M, Felldin M, Bäckman L, Friman S. Early renal function post-liver transplantation is predictive of progressive chronic kidney disease. Scand J Gastroenterol. 2008;43:344–349. doi: 10.1080/00365520701679264. [DOI] [PubMed] [Google Scholar]
- 5.Garces G, Contreras G, Carvalho D, et al. Chronic kidney disease after orthotopic liver transplantation in recipients receiving tacrolimus. Clin Nephrol. 2011;75:150–157. doi: 10.5414/cnp75150. [DOI] [PubMed] [Google Scholar]
- 6.Fabrizi F, Dixit V, Martin P, Messa P. Chronic kidney disease after liver transplantation: Recent evidence. Int J Artif Organs. 2010;33:803–811. [PubMed] [Google Scholar]
- 7.De Simone P, Metselaar HJ, Fischer L, et al. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: A prospective, randomized, multicenter trial. Liver Transpl. 2009;15:1262–1269. doi: 10.1002/lt.21827. [DOI] [PubMed] [Google Scholar]
- 8.Abdelmalek MF, Humar A, Stickel F, et al. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: A randomized trial. Am J Transplant. 2012;12:694–705. doi: 10.1111/j.1600-6143.2011.03919.x. [DOI] [PubMed] [Google Scholar]
- 9.Masetti M, Montalti R, Rompianesi G, et al. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252–2262. doi: 10.1111/j.1600-6143.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 10.De Simone P, Nevens F, De Carlis L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schena FP, Pascoe MD, Alberu J, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-Month efficacy and safety results from the CONVERT trial. Transplantation. 2009;87:233–242. doi: 10.1097/TP.0b013e3181927a41. [DOI] [PubMed] [Google Scholar]
- 12.Holdaas H, Rostaing L, Serón D, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: A randomized, multicenter, 24-month study. Transplantation. 2011;92:410–418. doi: 10.1097/TP.0b013e318224c12d. [DOI] [PubMed] [Google Scholar]
- 13.Fischer L, Klempnauer J, Beckebaum S, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation—PROTECT. Am J Transplant. 2012;12:1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 14.Burra P, Senzolo M, Masier A, et al. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350–356. doi: 10.1016/j.dld.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Cantarovich M, Tchervenkov J, Paraskevas S, et al. Early changes in kidney function predict long-term chronic kidney disease and mortality in patients after liver transplantation. Transplantation. 2011;92:1358–1363. doi: 10.1097/TP.0b013e3182384aff. [DOI] [PubMed] [Google Scholar]
- 16.Everson GT. Everolimus and mTOR inhibitors in liver transplantation: Opening the “box. Liver Transpl. 2006;12:1571–1573. doi: 10.1002/lt.20845. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Nankivell BJ, Gruenewald SM, Allen R, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995;59:1683–1689. doi: 10.1097/00007890-199506270-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez EQ, Melton LB, Chinnakotla S, et al. Predicting renal failure after liver transplantation from measure glomerular filtration rate: Review of up to 15 years of follow-up. Transplantation. 2010;89:232–235. doi: 10.1097/TP.0b013e3181c42ff9. [DOI] [PubMed] [Google Scholar]
- 21.Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9:741–747. doi: 10.1053/jlts.2003.50113. [DOI] [PubMed] [Google Scholar]
- 22.Nashan B. Is acute rejection the key predictor for long-term outcomes after renal transplantation when comparing calcineurin inhibitors. Transplant Rev. 2009;23:47–52. doi: 10.1016/j.trre.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Shihab FS, Cibrik D, Chan L, et al. Association of clinical events with everolimus exposure in kidney transplant patients receiving reduced cyclosporine. Clin Transplant. 2013;27:217–226. doi: 10.1111/ctr.12045. [DOI] [PubMed] [Google Scholar]
- 24.Vallin M, Guillaud O, Morard I, et al. Tolerability of everolimus-based immunosuppression in maintenance liver transplant recipients. Clin Transplant. 2011;25:660–669. doi: 10.1111/j.1399-0012.2010.01370.x. [DOI] [PubMed] [Google Scholar]


