To the Editor: Alveolar echinococcosis, which is caused by the fox tapeworm Echinococcus multilocularis, is an emerging disease in Europe that shows a high mortality rate (1). Humans can become infected after ingesting parasite eggs (e.g., through direct contact with dogs and red foxes [Vulpes vulpes] or with their contaminated feces). E. multilocularis tapeworm eggs are extremely resistant and can remain viable in the environment for years (2).
Numbers of red foxes have increased in many countries in Europe in recent decades, and the E. multilocularis tapeworm has also expanded its range. This tapeworm has recently been reported in 17 countries in Europe, including Lithuania, Latvia, and Estonia (1). Foxes and associated tapeworms are also increasingly found in urban areas, prompting considerable public health concern (1,3). Foxes began to colonize urban areas in Estonia in 2005, and they have since been reported in 33 of 47 towns nationwide (L. Plumer, et al. unpub. data). Because ≈30% of foxes are infected with the E. multilocularis tapeworm in natural habitats in Estonia (4), it is essential to monitor parasite spillover into urban areas, where it could become a serious public health risk. Consequently, there is an acute need for methods that can effectively detect the parasite and thereby help prevent human infection.
Although immunologic (2) and genetic methods (5–7) are available for identifying Echinococcus spp. parasites, a sensitive molecular diagnostic method that detects tapeworms and identifies their host species from degraded fecal samples would be useful. The purposes of this study were to develop a sensitive, noninvasive, genetic method to identify the host species by discriminating between feces of red foxes and dogs; detect E. multilocularis tapeworms in feces and distinguish them from the related parasite E. granulosus; and collect carnivore feces in an urban area in Estonia to identify this tapeworm.
Fecal samples suspected to be from red foxes were collected during January–March 2012 and January–March 2013 from streets and grassy areas of Tartu, Estonia. Tartu is a relatively small city (area 39 km2) with 98,000 human inhabitants. We surveyed 14 transects, each ≈4 km in length, that included all major districts in the city (Figure). Each transect was searched weekly during the study period (total ≈850 km surveyed).
Figure.
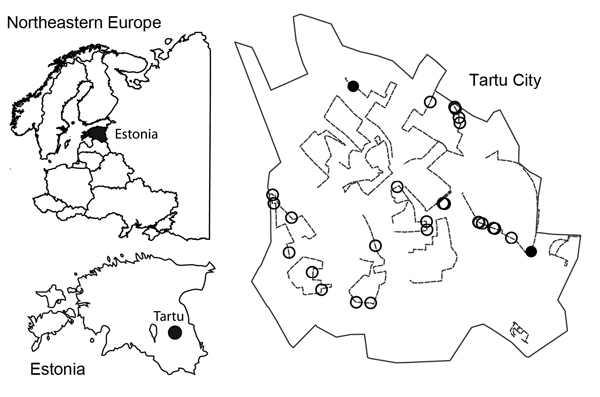
Location of Tartu in northeastern Europe, Estonia, and red fox feces sampling area in Tartu. The Tartu City boundary is indicated by a solid black line, survey transects are indicated by dashed lines, and fox fecal samples (n = 28) are indicated by circles. Filled circles (n = 2) indicate samples positive for Echinococcus multilocularis tapeworms.
A total of 137 fecal samples were collected and stored at −80°C for ≥1 week to avoid risk of infection from any Echinococcus spp. eggs present (2) because E. multilocularis (4) and E. granulosus (8,9) tapeworms have been found in Estonia. Samples of ≈250 mg were placed into 2-mL tubes, heated at 65°C for 15 min, and stored at −80°C. The heating and cooling procedure helps to break the parasite egg shells, enabling more efficient DNA extraction. DNA was extracted by using the QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.
Species-specific primers (Technical Appendix Table) were designed to amplify short sequences of mitochondrial DNA. On the basis of primer specificity and amplicon size, we determined host and parasite species (Technical Appendix Figure 1). DNA extraction and PCR were performed in a laboratory dedicated to environmental samples (for a complete description of methods, see Technical Appendix).
DNA was successfully extracted and amplified from 119 (86.9%) of 137 fecal samples. Of usable samples, 28 (23.5%) were from red foxes and 91 (76.5%) were from dogs. Two fox fecal samples (7.1%; 95% binomial CIs 0.9%–23.5%) were infected with E. multilocularis tapeworms; none of the dog samples were infected.
To verify parasite identification, we amplified DNA from the 2 E. multilocularis–positive samples with E. multilocularis–specific primers and sequenced the amplification products. To verify host species identification, we used primers that produced longer amplification products (327 and 197 bp) than the corresponding PCR primers and sequenced amplification products from 5 fox samples and 5 dog samples.
Sequencing procedures were performed according to the methods of Saarma et al. (10). Sequences from both E. multilocularis–positive samples showed 100% identity with an E. multilocularis tapeworm sequence (GenBank accession no. AB018440) (Technical Appendix Figure 2). All sequenced fox and dog samples also belonged to the corresponding species.
To estimate the sensitivity of this noninvasive genetic method, we determined the number of E. multilocularis eggs necessary to obtain a positive PCR result (Technical Appendix Figure 3). One egg was sufficient to give an E. multilocularis tapeworm–specific result.
In summary, we developed a noninvasive genetic method that identifies E. multilocularis tapeworms and their host species in carnivore fecal samples found in urban environments. Furthermore, these tapeworms can even be detected in fecal samples from red foxes when only 1 parasite egg is present. Thus, this method is highly sensitive and discriminatory and can be used with degraded fecal samples to monitor E. multilocularis tapeworms and their hosts.
Methods used to detect Echinococcus multilocularis tapeworms in red fox fecal samples in an urban area, Estonia.
Acknowledgments
This study was supported by grants from the Estonian Research Council (IUT-2032, ESF-8793, and ESF-8525), the European Union through the European Regional Development Fund (Centre of Excellence in Frontiers of Biodiversity Research), and the Estonian Doctoral School of Ecology and Environmental Sciences.
Footnotes
Suggested citation for this article: Laurimaa L, Davison J, Plumer L, Süld K, Oja R, Moks E, et al. Noninvasive detection of Echinococcus multilocularis tapeworm in urban area, Estonia [letter]. Emerg Infect Dis [Internet]. 2015 Jan [date cited]. http://dx.doi.org/10.3201/eid2101.140136
References
- 1.Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012;28:239–47. 10.1016/j.pt.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS, editors. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Geneva: World Health Organization and Paris: World Organisation for Animal Health; 2001. [cited 2014 Oct 23]. http://whqlibdoc.who.int/publications/2001/929044522X.pdf
- 3.Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. 10.1016/j.pt.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 4.Moks E, Saarma U, Valdmann H. Echinococcus multilocularis in Estonia. Emerg Infect Dis. 2005;11:1973–4. 10.3201/eid1112.050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–20. 10.1017/S0031182007002235 [DOI] [PubMed] [Google Scholar]
- 6.Boubaker G, Macchiaroli N, Prada L, Cucher MA, Rosenzvit MC, Ziadinov I, et al. A multiplex PCR for the simultaneous detection and genotyping of the Echinococcus granulosus complex. PLoS Negl Trop Dis. 2013;7:e2017. 10.1371/journal.pntd.0002017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinkel A, Kern S, Brinker A, Oehme R, Vaniscotte A, Giraudoux P, et al. A real-time multiplex-nested PCR system for coprological diagnosis of Echinococcus multilocularis and host species. Parasitol Res. 2011;109:493–8. 10.1007/s00436-011-2272-0 [DOI] [PubMed] [Google Scholar]
- 8.Moks E, Jõgisalu I, Saarma U, Talvik H, Järvis T, Valdmann H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J Wildl Dis. 2006;42:359–65. 10.7589/0090-3558-42.2.359 [DOI] [PubMed] [Google Scholar]
- 9.Moks E, Jõgisalu I, Valdmann H, Saarma U. First report of Echinococcus granulosus G8 in Eurasia and a reappraisal of the phylogenetic relationships of “genotypes” G5–G10. Parasitology. 2008;135:647–54. 10.1017/S0031182008004198 [DOI] [PubMed] [Google Scholar]
- 10.Saarma U, Jõgisalu I, Moks E, Varcasia A, Lavikainen A, Oksanen A, et al. A novel phylogeny for the genus Echinococcus, based on nuclear data, challenges relationships based on mitochondrial evidence. Parasitology. 2009;136:317–28 . 10.1017/S0031182008005453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods used to detect Echinococcus multilocularis tapeworms in red fox fecal samples in an urban area, Estonia.


