Abstract
Despite increasing reports that Blastocystis infection is associated with digestive symptoms, its pathogenicity remains controversial. We report appendicular peritonitis in a 9-year-old girl returning to France from Morocco. Only Blastocystis parasites were detected in stools, appendix, peritoneal liquid, and recto-uterine pouch. Simultaneous gastroenteritis in 26 members of the child’s family suggested an outbreak.
Keywords: Blastocystis, blastocystosis, parasite, gastrointestinal, appendicitis, peritonitis, Morocco, Casablanca, outbreak, subtypes
Blastocystis is a genus of anaerobic protozoan parasites that infect humans and a vast range of animal species. Prevalence in humans varies from 0.5%–24% in industrialized countries to 30%–76% in developing countries (1,2). Classic clinical features of infection include gastrointestinal symptoms such as nausea, anorexia, flatulence, and acute or chronic diarrhea. Fever is usually absent. An association with irritable bowel syndrome and extraintestinal manifestations such as urticaria has been suggested (2). Reports about invasive infection or disseminated diseases are rare (3). Here, we report the case of a pediatric patient infected with Blastocystis that was manifested by gastroenteritis associated with suppurative appendicitis and peritonitis.
The Study
In August 2013, a 9-year-old girl who was returning to France after a 1-month stay with her family in Casablanca, Morocco, was admitted to Lille University Hospital in Lille. Symptoms started in Casablanca 3 days before hospital admission and included fever, severe diarrhea (>10 liquid defecations/day), vomiting, and abdominal pain in the hypogastric area and in the right and left lower quadrants associated with bilateral dorsal pain, anorexia, and weakness.
Blood count showed 13,850/mm3 leukocytes (75.4% neutrophils, 15.9% lymphocytes, 8.5% monocytes). C-reactive protein level was increased at 266 mg/L (Low risk: <1.0mg/L; average risk: 1.0–3.0 mg/L; high risk >3.0 mg/L). Traveler’s gastroenteritis was diagnosed, and symptomatic treatment with acetaminophen, phloroglucinol glucoside, and acetorphan was prescribed. However, abdominal pain increased, and total food intolerance occurred in the following hours.
An abdominal ultrasound was performed, revealing appendicitis with suppuration in the recto-uterine pouch and a reflex ileus. Parasitologic examination of fecal matter revealed only abundant Blastocystis vacuolar forms, with >5 parasites per field. We further confirmed absence of Cryptosporidium spp. using glycerin assay and real-time PCR. Yeasts and a multimicrobial flora were present in the fecal material, but other infectious agents such as Salmonella spp., Shigella spp., Campylobacter spp., Yersinia enterocolitica, adenovirus, and rotavirus were not detected. Similarly, multimicrobial flora, but no pathogenic bacteria, were detected in the peritoneal liquid and recto-uterine pouch (Table). Histopathologic observation revealed acute suppurative appendicitis with ulcerations extending deep into the muscularis, which was covered with a suppurative and fibrinous exudate. We observed infiltration by numerous neutrophils, eosinophils, plasma cells, and lymphocytes through all layers and into the serous membrane (Figure, panel A).
Table. Microbiological examination of samples of feces, appendix, recto-uterine pouch, and peritoneal fluid from child who had peritonitis, Casablanca, Morocco*.
| Variable |
Date of sampling, 2013 |
||||||
| Aug 30 |
|
Sept 1 |
|
Nov 13 |
|||
| Procedure/result |
Feces |
Appendix |
Recto-uterine pouch |
Peritoneal fluid |
Feces |
||
| Microscopic examination |
Numerous Blastocystis vacuolar forms; no Cryptosporidium or other parasites |
|
Presence of rare Blastocystis† |
ND |
ND |
|
Absence of parasites |
| Real-time PCR | |||||||
| Blastocystis spp. | Positive | Positive | Positive | Positive | Negative | ||
|
Cryptosporidium spp. |
Negative |
|
Negative |
Negative |
Negative |
|
ND |
| Sequencing | |||||||
|
Blastocystis spp. genotype |
ST2, ST3 |
|
ST3 |
ST3 |
ST3 |
|
ND |
| Bacterial culture |
Negative for Salmonella, Shigella, Campylobacter, Yersinia enterocolitica |
|
ND |
Multimicrobial
flora |
Multimicrobial flora |
|
ND |
| Viral antigen detection |
Negative for aenovirus and rotavirus |
|
ND |
ND |
ND |
|
ND |
| *ND, not done; ST, subtype. †Figure, panels B,C. | |||||||
Figure.
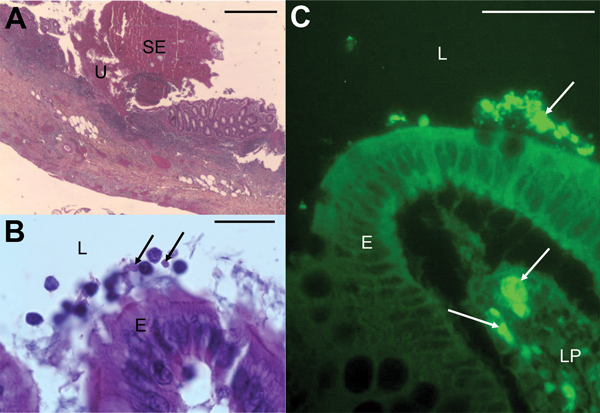
Micrographs showing histopathologic examination of appendix samples from a child who had peritonitis, Casablanca, Morocco, 2013. A) Ulceration (U) covered with suppurative and fibrinous exudates (SE) (hematoxylin-eosin stain). Scale bar indicates 200μm. B) Blastocystis parasites (arrows) in the lumen (L), and at the surface of the epithelium (E) (hematoxylin-eosin stain). Scale bar indicates 20μm. C) Blastocystis parasites (arrows) in the lumen, at the surface of the epithelium and in the lamina propria (LP) of the mucosa (immunofluorescence labeling with anti-Blastocystis ParaFlorB antibody). Scale bar indicates 50μm.
After hematoxylin-eosin staining and immunofluorescence labeling by using the anti-Blastocystis Paraflor B monoclonal antibody (Boulder Diagnostics, Marlborough, MA, USA), we detected parasitic forms in the lumen and in the lamina propria of the mucosa (Figure, panels B, C). We used real-time PCR for Blastocystis parasite detection as described (4), using DNA extracted from stools, appendix, peritoneal liquid, and the recto-uterine pouch, which all tested positive. We subsequently performed small subunit rDNA (SSU rDNA) amplification, then cloned the PCR product and sequenced 5 clones from all the DNA samples to subtype (ST) Blastocystis isolates and detect mixed infections (5). We identified ST3 in all the analyzed compartments. Mixed infection with ST2 and ST3 was detected only in the stools. The SSU rDNA gene sequences obtained in this study have been deposited in GenBank under accession nos. KJ605630–KJ605649.
The child completely recovered after an appendectomy, removal of a stercolith from the appendix lumen, and treatment with tinidazole, 20 mg/kg/d, and ceftriaxone, 50 mg/kg/d for 10 days, together with gentamicin, 5 mg/kg/d for 5 days. Although tinidazole is not the first line medication for treatment of Blastocystis infection, the child recovered completely and showed total clearance of parasites at day 73: using microscopy and real-time PCR on fecal samples, we found negative results for Blastocystis. Data obtained from the child’s mother revealed simultaneous cases of gastroenteritis in 26 family members: 13 adults, 34–98 years of age, and 13 children, 18 months–15 years of age, who lived in the same building at the residential “Mohammadi” area of Casablanca. Adults had mild or moderate diarrhea but symptoms were more severe in children, who all had abundant diarrhea, vomiting, and weight loss. Repatriation in France of an 18-month-old baby was considered, but his condition improved. None of the family members required hospitalization. Unfortunately, no explorations were performed, therfore the diagnosis could not be microbiologically documented.
Conclusions
Reports of Blastocystis infection associated with diarrhea and clinical symptoms in immunocompetent and immunocompromised patients have increased during the past 2 decades (2). Tissue invasion of Blastocystis parasites in the appendix (6) or in the colon mucosa (3), associated with acute or chronic inflammation, has been reported. However, controversy still exists over whether this parasite is commensal or pathogenic; this case further supports its invasive and inflammatory potential. Previous reports regarding the presence of Blastocystis parasites in 4 of 100 appendix specimens from patients with acute appendicitis (7), and of pseudoappendicular illness, which led to appendectomies in children with intestinal infection caused by this parasite (8), suggest that Blastocystis infection could be associated with appendicitis. Nevertheless, the actual role of Blastocystis in the pathogenesis of appendicitis remains inconclusive. In this report, the presence of a stercolith, which can be found in 50%–80% of appendicitis cases, suggested mechanical obstruction of the appendix’s lumen, which is the main etiology of appendicitis.
Here, we report dissemination of Blastocystis into the lumen, the mucosa, and the recto-uterine pouch exudate, associated with appendicular acute inflammation, and no other infectious agent was detected. These observations, together with the well-documented acute or chronic inflammation occurring in humans or animals with Blastocystis infections (3,9), likely support the contribution of this infection to the inflammatory process. Infection with ST3 further reinforced this hypothesis. Indeed, the presence of pathogenic strains among ST3 has been confirmed through experimental infections in rats (9). Additionally, a substantial inflammatory reaction and an increased propagation of human colorectal cancer cells exposed to Blastocystis ST3 antigens has been demonstrated in vitro (10). For humans, the pathogenicity of different STs is unclear and remains a debatable issue. ST1 isolates were found to be more prevalent among symptomatic patients in Lebanon (5), but ST3 was found to be the only ST that showed pathogenic potential in Malaysian patients when compared with ST1 and ST2 (11). ST3 was also found to be significantly associated with diarrhea in Libya (p = 0.008) (12). For this case, the fact that only ST3 was detected in all analyzed samples, whereas a mixed infection with ST2 and ST3 was found in the child’s stools, further supports the high invasive potential of ST3. ST3 is the most common ST in Europe, but, in African countries, its frequency varies from 17.8% in Libya (12) to 61.9% in Egypt (13). In Morocco, a 28.7% prevalence of blastocystosis has been reported, but data concerning the ST distribution of the parasite are not available (14). Furthermore, although Blastocystis infection could not be confirmed among the child’s relatives, the simultaneous occurrence of gastroenteritis cases in the same family and the absence of other infectious agents in the child’s stools suggest a potential outbreak of Blastocystis infection. Blastocystis parasites could have spread within the child’s family, as previously reported in Italy, where 2 adopted children originating from India and the Côte d’Ivoire transmitted Blastocystis parasites to their adoptive parents and grandmother (15). Possible acquisition of this parasite from a common source such as contaminated water could also explain family transmission in this report. Altogether, these data highlight 1) the need for both systematic parasitologic examinations of stools in patients with invasive infections who are traveling from countries with high Blastocystis prevalence and 2) the need for routine provision of imidazoles for empiric treatment of peritonitis.
Acknowledgments
We thank the members of the Biology and Diversity of Emerging Eukaryotic Pathogens team of the Pasteur Institute of Lille, especially Sadia Benamrouz and Karine Guyot, for their support.
This work was supported by grants from the Programme Orientations Stratégiques from the University of Lille, the Centre National de la Recherche Scientifique, and the Pasteur Institute of Lille. D.E.S. and M.O. were both supported by PhD fellowships from the Conseil National de la Recherche Scientifique and the Azm & Saade Association from Lebanon. A.C. was supported by a PhD fellowship from the Pasteur Institute of Lille and the University of Lille.
Biography
Dr Fréalle is a hospital practitioner and researcher in Lille University Hospital Center and Pasteur Institute of Lille. Her primary research interests are respiratory fungal and intestinal parasitic infections, with special emphasis on biodiversity and pathogenesis.
Footnotes
Suggested citation for this article: Fréalle E, El Safadi D, Cian A, Aubry E, Certad G, Osman M, et al. Acute Blastocystis-associated appendicular peritonitis in child, Casablanca, Morocco. Emerg Infect Dis. 2015 Jan [date cited]. http://dx.doi.org/10.3201/eid2101.140544
These authors contributed equally to this article.
References
- 1.Bart A, Wentink-Bonnema EMS, Gilis H, Verhaar N, Wassenaar CJA, van Vugt M, et al. Diagnosis and subtype analysis of Blastocystis sp. in 442 patients in a hospital setting in the Netherlands. BMC Infect Dis. 2013;13:389. 10.1186/1471-2334-13-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stensvold CR, Nielsen HV, Mølbak K, Smith HV. Pursuing the clinical significance of Blastocystis–diagnostic limitations. Trends Parasitol. 2009;25:23–9. 10.1016/j.pt.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 3.Janarthanan S, Khoury N, Antaki F. An unusual case of invasive Blastocystis hominis infection. Endoscopy. 2011;43(Suppl 2 UCTN):E185–6. [DOI] [PubMed]
- 4.Poirier P, Wawrzyniak I, Albert A, El Alaoui H, Delbac F, Livrelli V. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J Clin Microbiol. 2011;49:975–83. 10.1128/JCM.01392-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Safadi D, Meloni D, Poirier P, Osman M, Cian A, Gaayeb L, et al. Molecular epidemiology of Blastocystis in Lebanon and correlation between subtype 1 and gastrointestinal symptoms. Am J Trop Med Hyg. 2013;88:1203–6. 10.4269/ajtmh.12-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lintong PM, Sambuaga MK, Tambajong EH. Acute suppurative appendicitis with Blastocystis hominis. Asian Pac J Trop Dis 2012;S965–8.
- 7.Thanikachalam MP, Kasemsuk Y, Mak JW, Sharifah Emilia TS, Kandasamy P. A study of parasitic infections in the luminal contents and tissue sections of appendix specimens. Trop Biomed. 2008;25:166–72 . [PubMed] [Google Scholar]
- 8.Fleta Zaragozano J, Clavel Parrilla A, Castillo García FJ, Bueno Lozano M, Sarría Chueca A. Blastocystis hominis and abdominal pain in childhood. An Esp Pediatr. 1993;38:13–6. [PubMed]
- 9.Hussein EM, Hussein AM, Eida MM, Atwa MM. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res. 2008;102:853–60. 10.1007/s00436-007-0833-z [DOI] [PubMed] [Google Scholar]
- 10.Kumarasamy V, Kuppusamy UR, Samudi C, Kumar S. Blastocystis sp. subtype 3 triggers higher proliferation of human colorectal cancer cells, HCT116. Parasitol Res. 2013;112:3551–5. 10.1007/s00436-013-3538-5 [DOI] [PubMed] [Google Scholar]
- 11.Tan TC, Suresh KG, Smith HV. Phenotypic and genotypic characterisation of Blastocystis hominis isolates implicates subtype 3 as a subtype with pathogenic potential. Parasitol Res. 2008;104:85–93. 10.1007/s00436-008-1163-5 [DOI] [PubMed] [Google Scholar]
- 12.Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Al-Mekhlafi AM, Ahmed A, Surin J. Subtype distribution of Blastocystis isolates in Sebha, Libya. PLoS ONE. 2013;8:e84372 . 10.1371/journal.pone.0084372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souppart L, Moussa H, Cian A, Sanciu G, Poirier P, El Alaoui H, et al. Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol Res. 2010;106:505–11 . 10.1007/s00436-009-1693-5 [DOI] [PubMed] [Google Scholar]
- 14.El Guamri Y, Belghyti D, Barkia A, Tiabi M, Aujjar N, Achicha A, et al. Parasitic infection of the digestive tract in children in a regional hospital center in Gharb (Kenitra, Morroco): some epidemiological features. East Afr J Public Health. 2011;8:250–7 . [PubMed] [Google Scholar]
- 15.Guglielmetti P, Cellesi C, Figura N, Rossolini A. Family outbreak of Blastocystis hominis associated gastroenteritis. Lancet. 1989;334:1394 . 10.1016/S0140-6736(89)92000-X [DOI] [PubMed] [Google Scholar]


