Unique virulence factors of certain strains facilitate central nervous system invasion, regardless of infection route.
Keywords: neurologic, melioidosis, Burkholderia pseudomallei, route of infection, virulence, neurotropism, bacteria, central nervous system, bioterrorism, melioidosis
Abstract
The death rate for neurologic melioidosis is high. Whether certain Burkholderia pseudomallei strains are more likely than other strains to cause central nervous system infection and whether route of infection influences the neurotropic threat remain unclear. Therefore, we compared the virulence and dissemination of Australian clinical isolates collected during October 1989–October 2012 from patients with neurologic and nonneurologic melioidosis after intranasal and subcutaneous infection of mice in an experimental model. We did not observe neurotropism as a unique characteristic of isolates from patients with neurologic melioidosis. Rather, a distinct subset of B. pseudomallei strains appear to have heightened pathogenic potential for rapid dissemination to multiple tissues, including the central nervous system, irrespective of the infection route. This finding has valuable public health ramifications for initiating appropriate and timely therapy after exposure to systemically invasive B. pseudomallei strains. Increasing understanding of B. pseudomallei pathology and its influencing factors will further reduce illness and death from this disease.
Melioidosis is caused by the gram-negative bacterium Burkholderia pseudomallei. It incorporates a wide spectrum of clinical disease that ranges from severe, rapidly fatal, invasive disease to asymptomatic latent infection; thus, diagnosis is immensely challenging (1). No vaccine against melioidosis is currently available. The ability of B. pseudomallei to cause severe, rapidly fatal, invasive infections and to persist in the environment for extended periods, plus its intrinsic resistance to many antibacterial drugs, make B. pseudomallei a desirable candidate for use as a bioterrorism agent (1). Furthermore, B. pseudomallei can invade host cells, including macrophages, neutrophils, and other cells of the immune system, and persist within them (2,3). Without appropriate drug therapy, the death rate for melioidosis can exceed 90% (4,5).
Neurologic abnormalities occur in 3%–5% of melioidosis cases, and more than one quarter of those are fatal (5–8). Many similarities have been described regarding the clinical features of neurologic melioidosis in naturally infected animals and humans and in animal models infected with B. pseudomallei (6,9–15). Cranial nerve palsies and unilateral limb weakness are frequently described in patients with neurologic melioidosis (6,7,9,13,14). Flaccid paraparesis, commonly documented in animals with B. pseudomallei infection, also has been reported in humans (6,10,15). Often, microscopic and macroscopic abscesses are evident, and a predilection of B. pseudomallei for the brainstem and spinal cord has been suggested (9,12–14).
In contrast to patients with other forms of melioidosis, those with melioidosis with central nervous system (CNS) involvement are less likely to have predisposing risk factors, such as diabetes and chronic lung disease (6,8,9). Relatively little is known about the potential for different B. pseudomallei strains to cause severe disease, including whether particular strains are more likely to cause neurologic sequelae or whether CNS involvement is a consequence of the mode of delivery of B. pseudomallei. Neurologic melioidosis can result from direct invasion or through hematogenous spread (3,6,11,16,17). Initial suggestions that neurologic melioidosis might result from damage from immune or toxin-mediated mechanisms (6,12) has been supplanted by the recognition that direct invasion of brain and spinal cord by bacteria is evident on histologic examination of samples from case-patients who died (16). Furthermore, direct invasion of the brain by B. pseudomallei was recently demonstrated in an experimental model of melioidosis meningitis after delivery of intracellular bacteria by CNS-infiltrating CD11b+ immune cells (3).
Given the high rate of death from neurologic melioidosis, interest is increasing in improving understanding of its pathogenesis, particularly the potential for different B. pseudomallei isolates to cause neurologic melioidosis and the influence of the route of transmission on entry into and dissemination within the CNS. Therefore, using a well-characterized animal model of melioidosis (18) and clinical isolates of B. pseudomallei collected in the Northern Territory, Australia, during October 1989–October 2012 (7), we sought to determine whether strains isolated from patients with neurologic melioidosis (neurologic isolates) showed higher virulence levels and neurotropism than isolates from patients with nonneurologic melioidosis (nonneurologic isolates) after respiratory and percutaneous exposure.
Methods
B. pseudomallei Isolates
Eleven B. pseudomallei clinical isolates were analyzed. Six of these isolates were from patients with moderate to severe neurologic melioidosis, and 5 were from patients with nonneurologic melioidosis, both disseminated and localized cases (Table 1).
Table 1. Clinical features of Burkholderia pseudomallei strains isolated from patients with neurologic and nonneurologic melioidosis and their virulence in C57BL/6 and BALB/c mice, Northern Territory, Australia, October 1989–October 2012*.
| MSHR ID no. | Age, y/sex | Clinical feature | Risk factor | ID50 in mice (CFU)† |
||||
|---|---|---|---|---|---|---|---|---|
| Intranasal |
Subcutaneous |
|||||||
| C57BL/6 | BALB/c | C57BL/6 | BALB/c | |||||
| Neurologic | ||||||||
| 668‡ | 53/M | Severe neurologic signs | None | 4.1 × 104 | 2.9 × 102 | 7.1 × 103 | <10 | |
| 305§ | 64/M | Severe neurologic signs | Alcohol use | 2.6 × 102 | 2.6 × 102 | 3.7 × 104 | <10 | |
| 62‡ | 24/M | Severe neurologic signs | None | 2.2 × 102 | 6.3 × 101 | 2.4 × 102 | <10 | |
| 435‡ | 37/M | Severe neurologic signs | Kava | 5.0 × 102 | 1.3 × 102 | <10 | <10 | |
| 1153§ | 60/M | Severe neurologic signs | Diabetes mellitus | 1.7 | <10 | <10 | <10 | |
| 3709‡ |
14/M |
Moderate neurologic signs |
None |
4.4 × 104 |
1.8 × 104 |
|
2.2 × 102 |
1.3 × 102 |
| Nonneurologic | ||||||||
| 1655‡ | 61/F | Chronic pulmonary | Bronchiectasis | >108 | >108 | >2 × 108 | >2 × 108 | |
| 465§ | 67/M | Septicemia | Diabetes mellitus, chronic obstructive pulmonary disease | 1.3 × 105 | 1.1 × 103 | 8.3 × 105 | <10 | |
| 2138‡ | 49/F | Septicemia | Diabetes mellitus | 3.6 × 103 | <10 | 4.2 × 103 | 1.2 × 101 | |
| 346‡ | 49/M | Chronic pulmonary | Alcohol use | 8.5 × 104 | 6.0 × 104 | 6.0 × 105 | 7.0 × 105 | |
| 543‡ | 22/F | Skin ulcer | None | 2.9 × 102 | 8.5 × 101 | 1.2 × 101 | <10 | |
*ID50, 50% infectious dose; MSHR ID, neurologic isolate identification. †ID50 determined after intranasal and subcutaneous infection of C57BL/6 and BALB/c mice ‡Nonfatal. §Fatal.
Animals
The C57BL/6–BALB/c mouse model is a well-characterized model of differential susceptibility to B. pseudomallei (18). We included C57BL/6 and BALB/c mice to enable comparison of disease progression within immunocompetent and immune-impaired hosts, respectively. We purchased 8- to 12-week-old BALB/c and C57BL/6 mice from the Small Animal Breeding Facility, James Cook University (Townsville, QLD, Australia). Experiments were conducted in accordance with National Health and Medical Research Council guidelines and were approved by the institutional ethics committee (A1500).
Preparation and Delivery of B. pseudomallei Isolates
B. pseudomallei isolates were cultured in tryptic soy broth at 37°C to logarithmic phase. After washing in phosphate-buffered saline (PBS), pH 7.2, bacteria were suspended to 108 CFU/mL. Serial 10-fold dilutions were prepared in sterile PBS to obtain required infectious doses. Doses were confirmed retrospectively by plating serial dilutions onto Ashdown agar (AA). To mimic natural routes of infection, intranasal or subcutaneous routes were used for inoculation with B. pseudomallei by previously described methods (18).
Determination of 50% Infectious Dose
Groups of 5 mice were inoculated intranasally and subcutaneously at 10-fold increasing doses of B. pseudomallei, ranging from 100 CFU to 107 CFU (18). Survival was monitored for 21 days, and moribund mice were euthanized. At necropsy, organs were observed for presence of visible abscesses. Spleens were homogenized and plated on AA to confirm the presence of B. pseudomallei in mice that died from their infection within the experiment period. Mice that survived to 21 days after infection were euthanized and underwent necropsy to determine whether abscesses were visible in spleen and liver. For mice infected subcutaneously, the subcutaneous adipose tissue at the site of infection was also assessed. Tissue homogenates were cultured on AA to confirm the presence or absence of persistent B. pseudomallei infection. We determined the 50% infectious dose (ID50) from the total number of mice that either died of their infection or had evidence of persistent infection 21 days postinfection (dpi) using a modified version of the Reed and Meunch method (19). ID50 for neurologic and nonneurologic isolates are expressed as mean log10 CFU ± the standard error of the mean (SEM). Virulence, as defined by the ID50 of B. pseudomallei isolates derived from patients with neurologic and nonneurologic melioidosis, were compared in BALB/c and C57BL/6 mice after both intranasal and subcutaneous infection.
Determination of Bacterial Load
At specified time points, 5 mice were euthanized by cardiac puncture, and blood was collected into sterile tubes containing lithium heparin. Bacterial load in blood was determined by plating serial dilutions of whole blood in PBS onto AA and counting colonies after 24–48 h incubation at 37°C. Immediately after collection of blood, the liver, spleen, lung, lymph nodes (cervical and inguinal), brain, and nasal-associated lymphoid tissue were aseptically excised. Tissue bacterial load was determined by homogenizing tissue in 1 mL of PBS and plating serial dilutions onto AA for colony counts. The detection limit of bacteria in tissues was 2 CFU. Data were expressed as the mean log10 CFU ± SEM.
Statistical Analysis
For statistical analysis, we used Graphpad Prism version 6 (http://www.graphpad.com). Kaplan–Meier survival curves were used to compare susceptibility to infection with B. pseudomallei isolates after infection by different routes. ID50 for neurologic and nonneurologic B. pseudomallei isolates were compared by using Student t test. Bacterial loads in organs after B. pseudomallei infection by different routes were tested for significance using 1-way analysis of variance based on normally distributed sets of data. Comparisons were considered to be significant at p<0.05.
Results
Although neurologic isolates tended to be more virulent (lower ID50) than nonneurologic isolates after intranasal infection, this finding did not reach statistical significance (Table 1). Consistent with previous evidence for differential susceptibility, C57BL/6 mice demonstrated greater resistance than BALB/c mice to B. pseudomallei infection, as indicated by their 10-fold higher ID50 (18).
Signs of CNS involvement (i.e., head tilt and/or circling, difficulty walking, limb paresis) developed after intranasal infection of BALB/c and C57BL/6 mice, typically 8–12 dpi. This feature was not unique to neurologic isolates; head tilt and limb paralysis also were observed in mice infected with non-neurologic isolates. The development of neurologic signs corresponded with bacterial loads in brain, reaching >103 CFU. However, the data suggest that development of neurologic signs did not depend on the initial infectious dose because serial increases in the inoculating dose failed to cause a stepwise increase in number of C57BL/6 mice with neurologic signs (Table 2). Similar trends were observed after intranasal infection of BALB/c mice (data not shown).
Table 2. Development of signs of neurologic involvement* in C57BL/6 mice after intranasal infection with Burkholderia pseudomallei strains isolated from patients with neurologic and non-neurologic melioidosis, Northern Territory, Australia, October 1989–October 2012.
| MSHR ID no.† | Inoculating dose, CFU | No. mice with neurologic signs/total mice infected |
|---|---|---|
|
Neurologic
|
|
|
|
668
|
2.9 × 103 |
1/5 |
|
|
2.9 × 104 |
0/5 |
|
|
2.9 × 105 |
2/5 |
|
|
2.9 × 106 |
2/5 |
|
305
|
2.6 × 103 |
0/5 |
|
|
2.6 × 104 |
1/5 |
|
|
2.6 × 105 |
3/5 |
|
|
2.6 × 106 |
0/5 |
|
62
|
2.2 × 103 |
1/5 |
|
|
2.2 × 104 |
1/5 |
|
|
2.2 × 105 |
2/5 |
|
|
2.2 × 106 |
1/5 |
|
435
|
3.0 × 103 |
0/5 |
|
|
3.0 × 104 |
3/5 |
|
|
3.0 × 105 |
2/5 |
|
|
3.0 × 106 |
2/5 |
|
1153
|
5.3 × 101 |
1/5 |
|
|
5.3 × 102 |
3/5 |
|
|
5.3 × 103 |
0/5 |
|
|
5.3 × 104 |
1/5 |
|
3709
|
2.2 × 103 |
0/5 |
|
|
2.2 × 104 |
0/5 |
|
|
2.2 × 105 |
0/5 |
|
|
2.2 × 106 |
0/5 |
|
Non-neurologic
|
|
|
|
1655
|
1.1 × 104 |
0/5 |
|
|
1.1 × 105 |
0/5 |
|
|
1.1 × 106 |
0/5 |
|
|
1.1 × 107 |
0/5 |
|
465
|
6.6 × 103 |
0/5 |
|
|
6.6 × 104 |
0/5 |
|
|
6.6 × 105 |
2/6 |
|
|
6.6 × 106 |
1/6 |
|
2138
|
2.4 × 102 |
0/5 |
|
|
2.4 × 103 |
0/5 |
|
|
2.4 × 104 |
1/5 |
|
|
2.4 × 105 |
2/4 |
|
346
|
4.2 × 103 |
0/5 |
|
|
4.2 × 104 |
0/5 |
|
|
4.2 × 105 |
2/5 |
|
|
4.2 × 106 |
0/5 |
|
543
|
9.4 × 102 |
1/5 |
|
|
9.4 × 103 |
1/5 |
|
|
9.4 × 104 |
3/5 |
| 9.4 × 105 | 2/5 |
*Head tilt, difficulty walking, limb paresis. †MSHR ID, neurologic isolate identification.
After subcutaneous infection with B. pseudomallei isolates, ID50 ranged from <10 CFU to >2 × 108 CFU in BALB/c and C57BL/6 mice. Consistent with intranasal infection, BALB/c mice were more susceptible than C57BL/6 mice to subcutaneous infection with B. pseudomallei, as indicated by their 10–100-fold lower ID50. Similar to findings after intranasal infection, neurologic isolates tended to be more virulent (lower ID50) than nonneurologic isolates after subcutaneous infection, although this finding did not reach statistical significance.
Mean ID50 was comparable for C57BL/6 mice after intranasal (3.2 × 104 CFU) or subcutaneous (9.3 × 104 CFU) infection with neurologic isolates. Similarly, mean ID50 for BALB/c mice did not differ between intranasal (7.6 × 102 CFU) and subcutaneous (8.1 × 102 CFU) infection. Neurologic isolates appeared to be more infectious for BALB/c mice when delivered subcutaneously rather than intranasally (p = 0.04). However, we did not observe this phenomenon for C57BL/6 mice.
We conducted a second series of studies to compare early bacterial load kinetics within tissues after intranasal and subcutaneous infection with B. pseudomallei. The purpose of these studies was to determine whether infection of the brain occurred more rapidly after intranasal than subcutaneous infection with isolates from neurologic melioidosis. The neurologic isolates, MSHR435 and MSHR1153, were associated with severe neurologic melioidosis whereby the CNS was suspected to be the primary site of infection and therefore represent isolates with high potential for direct CNS invasion after intranasal exposure in an animal model. BALB/c mice were inoculated with equivalent doses of B. pseudomallei intranasally or subcutaneously. Survival was monitored for 21 days. In addition, bacterial loads were determined in nasal-associated lymphoid tissue, draining lymph nodes (cervical or inguinal), blood, lung, brain, spleen, liver, and subcutaneous adipose tissue at the site of infection at 1, 3, and 7 dpi. BALB/c mice tended to be more susceptible to MSHR435 when infected subcutaneously; the rate of death reached 100% within 18 days, although this finding was not statistically significant (p = 0.29) (Figure 1, panel A). However, the death rate for infection of BALB/c with MSHR1153 was significantly higher after subcutaneous inoculation; hind leg paresis developed within the second week after infection that necessitated euthanasia of 6 of the 10 mice (p = 0.03, Figure 1, panel B). In contrast to subcutaneous infection, greater variability in disease progression was associated with intranasal inoculation of mice with B. pseudomallei, ranging from rapid systemic dissemination in some mice to low-level persistence in the respiratory tract with potential for clearance within a week after exposure. Infection was established in 100% of mice when MSRH435 or MSHR1153 was delivered subcutaneously. Consequently, the differences in overall death rates for mice after intranasal and subcutaneous infection reflect the variability in dissemination of B. pseudomallei after respiratory exposure.
Figure 1.
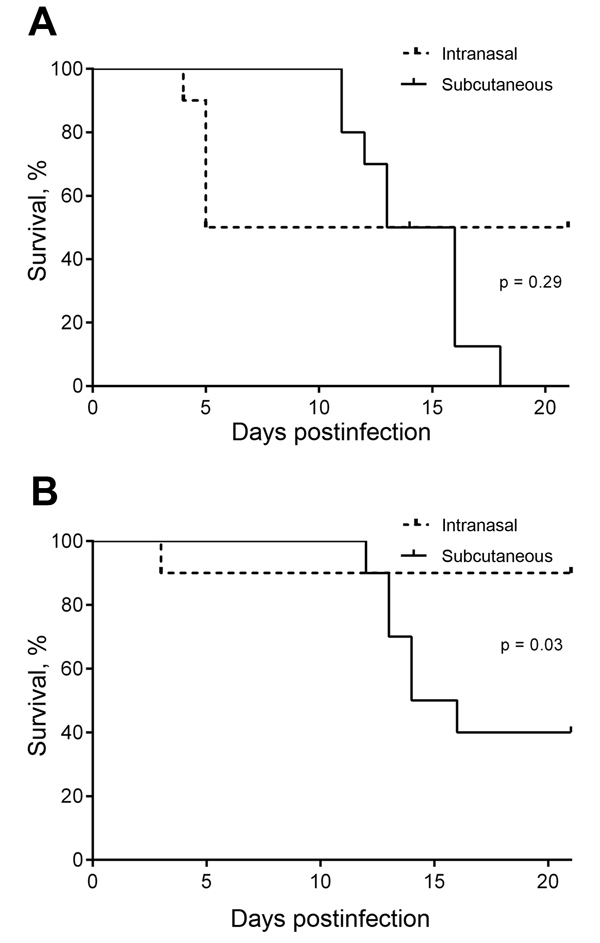
Comparison of survival after intranasal and subcutaneous infection of BALB/c mice with equivalent doses of the neurologic Burkholderia pseudomallei isolates MSHR435 (5 × 102 CFU) (A) and MSHR1153 (4.5 × 102 CFU) (B), Northern Territory, Australia, October 1989–October 2012. This inoculation dose was >50× the 50% infectious dose for MSHR435 and MSHR1153, delivered by intranasal or subcutaneous inoculation. Data are expressed as percentage survival; 10 mice were monitored within each group for 21 days postinfection.
Regardless of the route of infection, dissemination occurred rapidly; bacteria were detected not only at sites of infection but also in draining lymph nodes and spleen by 1 dpi (Figure 2, panels A and B). Bacterial loads continued to increase significantly from 3 dpi (Figure 2, panels C, D) through 7 dpi (Figure 2, panels E, F), with comparable levels in spleen, liver, and lung after intranasal or subcutaneous infection for MSHR435 and MSHR1153. Bacterial loads in the brain of mice infected with MSHR435 and MSHR1153 were low or undetectable within the first week after infection, consistent with the tendency for neurologic signs to develop after 8 dpi. The levels of bacteria recovered from brain after intranasal or subcutaneous infection with MSHR435 or MSHR1153 did not differ between 3 dpi and 7 dpi.
Figure 2.
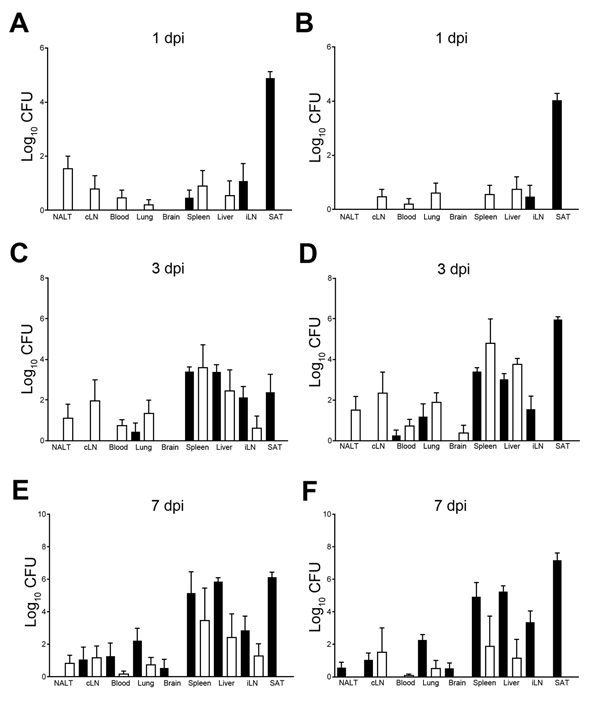
Comparison of Burkholderia pseudomallei loads in organs of BALB/c mice at days 1 (A, B), 3 (C, D) and 7 (E, F) after intranasal (white bars) and subcutaneous (black bars) infection with the neurologic isolates MSHR435 (5 × 102 CFU; panels A, C, E) and MSHR1153 (4.5 × 102 CFU; panels B, D, F) , Northern Territory, Australia, October 1989–October 2012. Bacterial loads were assessed in NALT, cLN, iLN, blood, lung, brain, spleen, liver, and SAT at the indicated dpi. Five mice were assessed at each time point. Data are expressed as mean l;og10 CFU per tissue ± SEM. cLN, cervical lymph nodes; dpi, days postinfection; iLN, inguinal lymph nodes; NALT, nasal-associated lymphoid tissue; SAT, subcutaneous adipose tissue. Error bars indicate standard error of the mean.
Discussion
Neurologic melioidosis is a serious, potentially fatal form of B. pseudomallei infection (5–7,16). The increased incidence of neurologic melioidosis in persons without recognized risk factors emphasizes the potential public health threat from this form of the disease. The contribution of bacteria- and host-specific factors in the pathogenesis of neurologic melioidosis is poorly understood, as are the potential roles of different modes of infection, such as percutaneous versus respiratory inoculation. Our study evaluated whether B. pseudomallei strains isolated from patients with neurologic melioidosis showed higher virulence in animal models of melioidosis than did strains isolated from patients with nonneurologic melioidosis. We found a trend for higher virulence for neurologic isolates than for nonneurologic isolates in an animal model, regardless of route of infection. However, neurotropism was not a unique characteristic of isolates from patients with neurologic melioidosis.
Consistent with the spectrum of clinical presentations of melioidosis, dissemination of B. pseudomallei in mice varied substantially. Neurologic signs developed in BALB/c and C57BL/6 mice 8–12 dpi. CNS involvement was not unique to neurologic isolates; we also observed head tilt and limb paralysis in mice infected with nonneurologic isolates. Although neurologic involvement was more commonly associated with intranasal inoculation, signs of CNS infection also occurred after subcutaneous infection. In most instances, subcutaneous infection resulted in localized abscesses in joints of hind limbs and vertebral column, causing hind leg paresis. However, neurologic signs occasionally developed in the absence of lesions at the subcutaneous injection site. When we compared intranasal and subcutaneous infection, we found a similar pattern of dissemination of neurologic isolates, which suggests that no predilection exists for neurologic isolates to invade by the respiratory route.
Our study provides evidence that B. pseudomallei isolates from patients with neurologic melioidosis do not demonstrate selective neurotropism in an experimental model. Rather, a subset of B. pseudomallei isolates appear to have unique virulence factors that facilitate rapid dissemination to multiple tissues, including the CNS, after both intranasal and subcutaneous exposure. We propose that this group of isolates is associated with severe disease progression and increased rates of death. This finding has valuable public health ramifications for initiating appropriate and timely therapy after exposure to systemically invasive B. pseudomallei strains. Studies focused on identifying virulence factors of B. pseudomallei that influence systemic spread, together with an improved understanding of the host–pathogen interactions that influence the progression to different forms of melioidosis, will be instrumental in identifying and evaluating future vaccine candidates and novel therapeutics for this potentially life-threatening disease.
Acknowledgments
We thank Christopher Davis and Ifor Beacham for their helpful discussions and contribution to the work described in this article.
This research was financially supported in part by the Commonwealth of Australia through the National Security Science and Technology Centre within the Defence Science and Technology Organisation and the US Department of Homeland Security. This support does not represent an endorsement of the contents or conclusions of the research. The authors have no financial interests in the results of this study.
Biography
Dr Morris is a postdoctoral researcher in the Australian Institute of Tropical Health and Medicine. Her research interests include the immunopathogenesis of B. pseudomallei infection.
Footnotes
Suggested citation for this article: Morris J, Fane A, Rush C, Govan B, Mayo M, Currie et al. Neurotropic threat characterization of Burkholderia pseudomallei strains. Emerg Infect Dis [Internet]. 2015 Jan [date cited]. http://dx.doi.org/10.3201/eid2101.131570
References
- 1.Ketheesan N, editor. Melioidosis: a century of observation and research. Amsterdam: Elsevier; 2012. [Google Scholar]
- 2.Stevens MP, Galyov EE. Exploitation of host cells by Burkholderia pseudomallei. Int J Med Microbiol. 2004;293:549–55 . 10.1078/1438-4221-00292 [DOI] [PubMed] [Google Scholar]
- 3.Liu PJ, Chen YS, Lin HH, Ni WF, Hsieh TH, Chen HT, et al. Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harbouring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis. 2013;7:e2363. 10.1371/journal.pntd.0002363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng AC, Hanna JN, Norton R, Hills SL, Davis J, Krause VL, et al. Melioidosis in northern Australia, 2001–2. Commun Dis Intell. 2003;27:272–7. [PubMed] [Google Scholar]
- 5.Malczewski AB, Oman KM, Norton RE, Ketheesan N. Clinical presentation of melioidosis in Queensland, Australia. Trans R Soc Trop Med Hyg. 2005;99:856–60. 10.1016/j.trstmh.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 6.Currie BJ, Fisher DA, Howard DM, Burrow JNC. Neurological melioidosis. Acta Trop. 2000;74:145–51. 10.1016/S0001-706X(99)00064-9 [DOI] [PubMed] [Google Scholar]
- 7.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. 10.1371/journal.pntd.0000900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmond KM, Bauert P, Currie BJ. Paediatric melioidosis in the Northern Territory of Australia: an expanding clinical spectrum. J Paediatr Child Health. 2001;37:337–41. 10.1046/j.1440-1754.2001.00660.x [DOI] [PubMed] [Google Scholar]
- 9.Chadwick DR, Ang B, Sitoh YY, Lee CC. Cerebral melioidosis in Singapore: a review of five cases. Trans R Soc Trop Med Hyg. 2002;96:72–6. 10.1016/S0035-9203(02)90248-8 [DOI] [PubMed] [Google Scholar]
- 10.Laws L, Hall WTK. Melioidosis in animals in north Queensland. I. Incidence and pathology, with special reference to central nervous system lesions. Qld J Agric Sci. 1963;20:499–513. [Google Scholar]
- 11.Omar AR. Pathology of melioidosis in pigs, goats and a horse. J Comp Pathol. 1963;73:359–72 . 10.1016/S0368-1742(63)80038-7 [DOI] [PubMed] [Google Scholar]
- 12.Woods ML, Currie BJ, Howard DM, Tierney A, Watson A, Anstey NM, et al. Neurological melioidosis: seven cases from the Northern Territory of Australia. Clin Infect Dis. 1992;15:163–9. 10.1093/clinids/15.1.163 [DOI] [PubMed] [Google Scholar]
- 13.Bartley PP, Pender MP, Woods ML, Walker D, Douglas JA, Allworth AM, et al. Spinal cord disease due to melioidosis. Trans R Soc Trop Med Hyg. 1999;93:175–6 . 10.1016/S0035-9203(99)90299-7 [DOI] [PubMed] [Google Scholar]
- 14.Howe PW, Holland HM, Burrow JCN, Currie BJ. Neurological melioidosis (Burkholderia pseudomallei) mimicking Guillain-Barré syndrome. Anaesth Intensive Care. 1997;25:166–7 . [DOI] [PubMed] [Google Scholar]
- 15.Haran MJ, Jenney AW, Keenan RJ, Flavell HD, Anstey NM, Currie BJ. Paraplegia secondary to Burkholderia pseudomallei myelitis: a case report. Arch Phys Med Rehabil. 2001;82:1630–2. 10.1053/apmr.2001.25074 [DOI] [PubMed] [Google Scholar]
- 16.Koszyca B, Currie BJ, Blumbergs PC. The neuropathology of melioidosis: two cases and a review of the literature. Clin Neuropathol. 2004;23:195–203 . [PubMed] [Google Scholar]
- 17.Owen SJ, Batzloff M, Chehrehasa F, Meedeniya A, Casart Y, Logue C-A, et al. Nasal-associated lymphoid tissue and olfactory epithelium as portals of entry for Burkholderia pseudomallei in murine melioidosis. J Infect Dis. 2009;199:1761–70. 10.1086/599210 [DOI] [PubMed] [Google Scholar]
- 18.Barnes JL, Ketheesan N. Melioidosis: routes of infection. Emerg Infect Dis. 2005;11:638–9. 10.3201/eid1104.041051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed LJ, Meunch HA. Simple method of estimating fifty per-cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]


