Abstract
Background
This study evaluated the benefit of Bifidobacterium bifidum OLB6378 (B. bifidum) in very low-birthweight (VLBW) infants (birthweight <1500 g) for the acceleration of enteral feeding.
Methods
A cluster-randomized, double-blind, placebo-controlled trial was conducted in 19 hospitals, divided into two groups: the B group (n = 10 hospitals; B. bifidum given to infants within 48 h of birth) and the P group (n = 9 hospitals; infants received a placebo). The primary outcome was establishment of enteral feeding after birth, defined as the postnatal day at which enteral feeding exceeded 100 mL/(kg/day). Secondary outcomes were defined as incidence of morbidity and somatic growth before discharge.
Results
Overall, 283 VLBW infants were enrolled in the study: B group, n = 153; and P group, n = 130. Enteral feeding was established within 21 days after birth in 233 infants, of whom 119 received B. bifidum and 114 received placebo until their bodyweight reached 2000 g. Enteral feeding was established significantly earlier in the B group, at 11.0 ± 3.6 days versus 12.1 ± 3.8days in P group (P < 0.05). Infant growth during the stay in the neonatal intensive care unit was not different between groups, but the incidence of late-onset sepsis among all enrolled infants was significantly lower in the B group (3.9%, 6/153) than in the P group (10.0%, 13/130; P < 0.05). No differences were observed in the incidence of other adverse outcomes including mortality.
Conclusions
B. bifidum in VLBW infants accelerated the establishment of enteral feeding after birth without increasing the incidence of adverse effects.
Keywords: establishment of enteral feeding, necrotizing enterocolitis, neonate, probiotics, sepsis
Establishment of enteral feeding after birth is often delayed in preterm infants because of their immature intestinal function. These infants also have difficulty maintaining normal gut flora, which further prevents functional maturation of the intestine.1 A relatively high incidence of cesarean delivery and prophylactic use of antibiotics after birth among preterm infants inhibits the activity of beneficial bacteria such as Lactobacilli and Bifidobacteria.2 Bacterial translocation from the gastrointestinal tract is an important pathway in the initiation of late-onset sepsis and necrotizing enterocolitis (NEC) in very low-birthweight (VLBW) infants.3 The emerging intestinal microbiota, nascent intestinal epithelia, naive immunity, and suboptimal nutrition play a role in facilitating this bacterial translocation.
Probiotics have been shown, in many clinical trials, to promote establishment of normal gut flora and prevent diseases in preterm infants,4 but few studies have examined the effect of probiotics on other benefits, such as support of enteral feeding and growth and prevention of sepsis. Here, we carried out a small-scale pilot study to evaluate the efficacy and safety of Bifidobacterium bifidum OLB6378 (B. bifidum) for VLBW infants prior to initiating a larger multicenter clinical trial, and found that the daily bodyweight gain was significantly higher in infants who received B. bifidum supplementation within 48 h of birth.5 We subsequently conducted the present study on a larger group of preterm infants to determine the effect of B. bifidum within 48 h after birth on the establishment of enteral feeding in VLBW infants.
Methods
Design
This study was conducted as a cluster-randomized multicenter, double-blind, placebo-controlled trial. The institutional review board of Tokyo Women's Medical University approved the study protocol, under clinical trial registration number UMIN000002543.
Patients
Nineteen neonatal intensive care units (NICU) in Japan, none of which had previously used probiotics, participated in the trial. In order to avoid cross contamination and infant-to-infant dissemination of B. bifidum within the same NICU, the 19 NICUs were divided into two groups. All participating hospitals were stratified according to patient volume in order to balance the number of patients between the groups. Pair-matched randomization of clusters was done using computer-generated random numbers, designated B and P. In the 10 B group NICUs, infants received B. bifidum, while infants in the nine P group NICUs received a placebo.
The study group consisted of VLBW infants (birthweight <1500 g) who were born or transferred within 24 h to the participating hospitals during the period from January 2010 to March 2011. Exclusion criteria were lack of parental consent or presence of major congenital malformation, systemic infection, or failure to give B. bifidum or placebo within 48 h due to a clinical condition that precluded oral feeding. Data from all infants who received at least one dose of B. bifidum or placebo were analyzed. Any infant who died or was transferred before the establishment of enteral feeding was designated as a failure of enteral feeding.
All clinical variables were registered through the website at each participating hospital into the neonatal research network database.6 No additional parameters specific to this study were added to the database.
Interventions
Probiotic supplement, containing approximately 2.5 × 109 viable cells of B. bifidum/500 mg, was supplied as a freeze-dried powder in dextrin (Meiji, Tokyo, Japan), as previously reported.5 The probiotic was approximately divided in half, and each portion was suspended in 0.5 mL warm water, breast milk, or infant formula. B. bifidum was then given to infants in B group through an enteral nutrition catheter within 48 h after birth. After the first dose, B. bifidum was given to infants twice a day until the bodyweight reached 2000 g. Placebo consisting of 500 mg dextrin was supplied, prepared, given to infants of P group in the same manner as described for the probiotic. Treatment and nutrition were otherwise given according to conventional practice in each NICU.
Outcome measures
The primary outcome was establishment of enteral feeding, defined as the postnatal day at which the amount of enteral feeding exceeded 100 mL/(kg/day). Using the neonatal research network database value of 14 ± 4 postnatal days for mean age at establishment of enteral feeding in VLBW infants in 2003,6 we considered inability to reach an enteral feeding volume of 100 mL/(kg/day) within 21 days (mean + 2SD) as failure to achieve enteral feeding. Once enteral feeding was established, VLBW infants received either B. bifidum or placebo until the bodyweight reached 2000 g.
Secondary outcomes specifically evaluated in this study were length of hospital stay, bodyweight at discharge, bodyweight gain per day (g/day), head circumference at discharge (cm), and increase in head circumference/hospital days (cm).
Morbidity as recorded in the database was also analyzed as a secondary outcome in all enrolled infants as previously defined in the neonatal research network data base.6 Especially, NEC was diagnosed in cases of stage ≥I disease according to the Bell classification. Late-onset sepsis was defined as sepsis occurring ≥1 week after birth, proven on positive blood culture.
Sample size calculation
The intracluster correlation coefficient was calculated using the mean age and standard deviation at which enteral feeding was established, expressed as the day feeding volume reached 100 mL/(kg/day) and was set at 0.02.6 We set a sample size of 19 hospitals in each of the two study arms before starting the trial, in order to achieve a statistical power of 80% (two sided = 0.05) to detect a decrease of 2 days to reach full feeding in the proportion of infants receiving B. bifidum during the stay in NICU. The resulting sample size was calculated as 77 infants for each arm.
Statistical analysis
Student's t-test was used for all normally distributed data with equal variance, and results are expressed as mean ± SD. For data not conforming to normal distribution or equal variance, medians were compared using the Mann–Whitney test. The day at which enteral feeding was established in both groups was compared using the Kaplan–Meier method. Frequency data for mortality and morbidity were analyzed on chi-squared test. All statistical analysis was carried out using StatView (version 5.0; SAS Institute, Cary, NC, USA). P was adjusted using a design effect determined by average cluster size. Statistical significance was set at the 0.05 level.
Results
Background characteristics
Overall, 585 VLBW infants were admitted during the study period, of whom 298 were assigned to B group and 287 to P group. Of these, 145 and 157 infants were excluded from the B and P groups, respectively, due to lack of parental consent, congenital anomalies, systemic infection or failure to give B. bifidum or placebo within 48 h after birth for clinical reasons. Of the infants not enrolled in the study, almost 80% were not enrolled due to lack of parental consent within 48 h after birth. Finally, 283 VLBW infants were enrolled in the study: 153 in B group and 130 in P group. Among the enrolled infants, 19 in B group and 11 in P group died during their stay in NICU or were transferred to another hospital before establishment of enteral feeding, and were designated as failure to establish enteral feeding. Of the remaining infants, 233 (119 in B group and 114 in P group) began enteral feeding within 21 days after birth and received B. bifidum or placebo until their bodyweight reached 2000 g. These results are illustrated as a flow chart in Figure 1.
Fig 1.
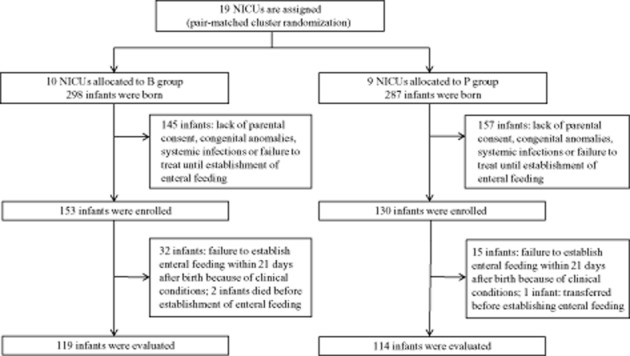
Study flowchart. B, Bifidobacterium bifidum; NICU, neonatal intensive care unit; P, placebo.
Table 1 summarizes the background characteristics and outcomes of the enrolled infants. Because this study randomized the groups at the hospital level rather than the infant level, the power to detect a difference between groups was affected by the average number of infants in the cluster, known as the design effect. Thus, a design effect of 1.21 in this study was calculated according to an average sample size of 11.5 infants per hospital as described in the previous section. Further calculation of P was done by dividing both the number of participants and the number of events by this design effect.
Table 1.
Subject characteristics and outcomes
| Enrolled infants | |||
|---|---|---|---|
| B group (n = 153) Mean ± SD or n (%) | Placebo group (n = 130) Mean ± SD or n (%) | P | |
| Gestational age (weeks) | 28.6 ± 2.9 | 28.5 ± 3.3 | NS† |
| Birthweight (g) | 1016 ± 289 | 998 ± 281 | NS† |
| Multiple-birth | 29 (19.0) | 27 (20.8) | NS‡ |
| Antenatal steroid | 101 (66.0) | 66 (50.8) | <0.05‡ |
| Cesarean section | 91 (59.5) | 103 (79.2) | <0.05‡ |
| Male | 87 (56.9) | 71 (54.6) | NS‡ |
| Apgar score at 1 min, median (25th–75th%) | 6 (4–8) | 5 (3–7) | NS§ |
| Apgar score at 5 min, median (25th–75th%) | 8 (7–9) | 7 (6–9) | NS§ |
| Outborn | 2 (1.3) | 5 (3.8) | NS‡ |
| Respiratory distress syndrome | 101 (66.0) | 82 (63.1) | NS‡ |
| Chronic lung disease at 28 days | 79 (51.6) | 61 (46.9) | NS‡ |
| Chronic lung disease at 36 weeks | 53 (34.6) | 30 (23.1) | NS‡ |
| Symptomatic PDA treated with indomethacin | 73 (47.7) | 56 (43.1) | NS‡ |
| PDA ligation | 8 (5.2) | 8 (6.2) | NS‡ |
| Intraventricular hemorrhage | 20 (13.1) | 24 (18.5) | NS‡ |
| Intraventricular hemorrhage grade III and IV | 6 (3.9) | 7 (5.4) | NS‡ |
| Periventricular leukomalacia | 7 (4.6) | 5 (3.8) | NS‡ |
| Sepsis | 13 (8.5) | 17 (13.1) | NS‡ |
| Sepsis ≥1 week after birth | 6 (3.9) | 10 (10.0) | <0.05‡ |
| Necrotizing enterocolitis | 0 (0.0) | 0 (0.0) | NS‡ |
| ROP treatment | 20 (15.4) | 25 (19.2) | NS‡ |
| Failure to establish enteral feeding within 21 days after birth | 32 (20.9) | 16 (12.3) | NS‡ |
| Dead at discharge | 2 (1.3) | 0 (0.0) | NS‡ |
Student's t-test
χ2-test; §Mann–Whitney test. B, Bifidobacterium bifidum; PDA, patent ductus arteriosus; ROP, retinopathy of prematurity.
No significant difference in gestational age or birthweight was detected between groups, but there was a significant difference between the groups in the rates of antenatal steroid and cesarean section.
Primary outcome
Table 2 summarizes clinical outcome in both study groups. The postnatal day at which the amount of enteral feeding exceeded 100 mL/(kg/day) was significantly earlier in B group infants compared with P group infants (11.0 ± 3.6 days vs 12.1 ± 3.8 days; P < 0.05). No other significant differences in outcome were observed.
Table 2.
Outcome of establishment of enteral feeding before 21 days after birth
| Infants evaluated | |||
|---|---|---|---|
| B group (n = 119) Mean ± SD or median (IQR) | Placebo group (n = 114) Mean ± SD or median (IQR) | P | |
| Postnatal day at which enteral feeding exceeded 100 mL/kg/day | 11.0 ± 3.6 | 12.1 ± 3.8 | <0.05† |
| Length of hospital days | 92.3 ± 44.5 | 92.9 ± 40.2 | NS‡ |
| Bodyweight at the time of discharge (g) | 2831.8 ± 581.0 | 2876.8 ± 499.2 | NS‡ |
| Bodyweight gain/hospital days (g/day) | 20.1 ± 3.7 | 20.8 ± 4.0 | NS‡ |
| Head circumference at the time of discharge (cm) | 34.5 (33.8–35.5) | 34.8 (33.7–36.0) | NS§ |
| Increased head circumference/hospital days (cm) | 0.10 (0.09–0.11) | 0.10 (0.09–0.12) | NS§ |
Student's t-test and Kaplan–Meier method
Student's t-test
Mann–Whitney test. B, Bifidobacterium bifidum.
Figure 2 plots days at which infants in each group reached enteral feeding at a volume of 100 mL/(kg/day). The difference between the groups was significant (P < 0.05), most evident at approximately 10 days after birth.
Fig 2.
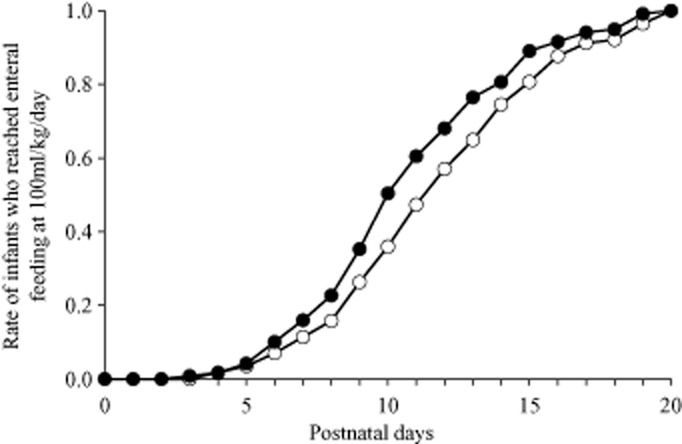
Day at which infants reached enteral feeding at 100 mL/kg/day. (∼), Bifidobacterium bifidum group (n = 119); (○) placebo group (n = 114).
Secondary outcomes
No significant differences were observed between groups in terms of length of hospital stay, bodyweight at discharge, bodyweight gain per day, head circumference at discharge, or head circumference increase.
No difference was observed in the mortality and morbidities among the infants enrolled, except the incidence of late-onset sepsis (Table 1). The incidence of sepsis ≥1 week after birth was significantly lower in B group (6/153, 3.9%) than in P group (13/130, 10.0%; P < 0.05).
Subgroup analysis
The frequency of use of parenteral hyperalimentation was greater in P group (88.6%) than in B group (77.3%; P < 0.05). Because this difference might influence outcome, the VLBW infants who received parenteral hyperalimentation were re-evaluated. The postnatal day at the establishment of enteral feeding was significantly earlier in B group infants after re-evaluation (B group, 11.0 ± 3.4 days; P group, 12.4 ± 3.8 days; P < 0.05).
Discussion
This study clearly shows the benefit of early use of B. bifidum on enteral feeding among VLBW infants. Early introduction of preterm infants to enteral feeding results in earlier achievement of full feeding without apparent increase in risk of morbidity including NEC.7 Probiotics have been tested in clinical trials as a way to improve feeding tolerance in preterm infants.8–12 Some studies report that probiotics have beneficial effects in preterm infants, such as acceleration of enteral feeding and growth improvement.8–10 This trial was performed as a multicenter clinical trial, following a previous single-center study that showed enhancement of enteral feeding and growth in VLBW infants treated with B. bifidum.5 The pilot study showed that probiotics given within 48 h after birth can very effectively colonize the immature bowel without increasing morbidity,5 and supported the benefit of the early use of B. bifidum in enteral feeding. The pilot study was also performed in one hospital only, with all infants treated in a similar manner according to hospital practice. In contrast, the present study was performed in 19 hospitals, and treatment and nutrition protocols conformed to the methods of each NICU. This multicenter study was also performed as a cluster-randomized trial, taking into account how growth of the infant seemed to be affected by the nutrition method. Given that enrolled infants were followed up, a long-term growth benefit might be able to be observed.
Probiotics protect the immature intestine from NEC, an effect associated with reduction of inflammatory reaction in the ileum, regulation of the main components of the mucus layer, and improvement of intestinal integrity.13 The ability of probiotics to downregulate apoptosis in the rat NEC model and in intestinal epithelial cell line-6 cells appear to be a cyclo-oxygenase-2-mediated phenomenon.14 Probiotics have also been shown to maintain integrity of the mucosal barrier, reducing its permeability, and to strengthen intestinal cell tight junctions.15,16 B. bifidum may also influence the mucosal barrier, and support early enteral feeding, but this is not well studied in Japan due to the low incidence of NEC.17 Because we encountered no cases of NEC, the preventive effect of probiotics on this disease was not evaluated in the present study.
There was a significant difference in the frequency of use of parenteral hyperalimentation in this study. Infants who receive hyperalimentation may be more likely to experience delayed enteral feeding, and might be more susceptible to any intestinal effects of B. bifidum. For this reason, we re-evaluated the VLBW infants who received parenteral hyperalimentation separately.
Bifidobacterium bifidum resulted in a significant reduction in late-onset sepsis, possibly related to its influence on the mucosal barrier. More importantly, an in vitro study showing upregulation of IgA and pIgR expression suggests that the probiotic may increase intestinal soluble IgA (sIgA).18,19 sIgA was detectable in the early postnatal period in the saliva of preterm infants.20,21 In addition, use of probiotics for preterm infants has been shown to upregulate transforming growth factor-β, which in turn upregulates mucosal IgA expression.22 Dietary supplementation of preterm infants with probiotics starting early after birth has been shown to produce an increase in fecal IgA.23,24 The increase in sIgA by B. bifidum may explain the observed decrease in late-onset sepsis. Reduction in bacterial translocation is an alternative mechanism by which probiotics help to maintain the integrity of the mucosal barrier.
The comparatively high number of B group infants failing to establish enteral feeding is a potential limitation of this study. Because we observed no adverse effect related directly to the use of B. bifidum or placebo, clinical conditions appear to be the only factors correlated with failed enteral feeding. In addition, mortality did not differ between the study groups, and was similar to what was previously reported.17 These findings suggest that B. bifidum may be used safely for VLBW infants.
Difference in antenatal steroid use was another concern of this study, because this might favor reduced morbidity in the B group. Given that this was a multicenter clustered clinical trial, adjustment of antenatal steroid usage equally between the groups was difficult. Nevertheless, the incidence of morbidity was similar in B and P group infants, suggesting that any such effect was not clinically significant.
Conclusion
Use of B. bifidum in VLBW infants accelerated the establishment of enteral feeding without increasing morbidity. A significant decrease in late-onset sepsis was observed among all enrolled infants.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Bifidobacterium bifidum OLB6378 was generously provided by Meiji Dairies Corporation, Odawara, Japan.
Appendix I
List of Probiotics Study Group in Japan
Takeo Kasai, Iwate Medical University; Hirokazu Arai, Japanese Red Cross Akita Hospital; Maki Sato, Fukushima Medical University; Niro Ujiie, Tsutomu Kawahara, Tsutomu Isii, Fukushima National Hospital; Junichi Arai, Ibaraki Children's Hospital; Atsushi Baba, Shinshi University; Kinuko Kojima, Niigata Prefectural Central Hospital; Junya Onozuka, Nagaoka Red Cross Hospital; Hiroaki Imamura, Takaoka Hospital; Yoshinori Kono, Gifu Prefecture General Medical Hospital; Takahiro Sugiura, Shizuoka Saiseikai General Hospital; Sumio Fukuda, Nagoya City West Medical Center; Takahide Yanagi, Sayuri Nakahara, Shiga University of Medical Science; Shinichiro Tanaka, Hitoshi Awakuni, Uji-Tokusyukai Medical Center; Fumihide Kato, Shimane Prefectural Central Hospital; Shinichi Watabe, Akihito Takahashi, Kurashiki Central Hospital; Moriharu Sugimoto, Tsuyama Cyuo Hospital; Yutaka Kawamoto, Kawasaki Medical School; Mikihiro Aoki, National Hospital Organization Nagasaki Medical Center.
References
- 1.Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum. Dev. 2012;88:S41–49. doi: 10.1016/j.earlhumdev.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Westerbeek EA, van den Berg A, Lafeber HN, et al. The intestinal bacterial colonisation in preterm infants: A review of the literature. Clin. Nutr. 2006;25:361–368. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Sherman MP. New concepts of microbial translocation in the neonatal intestine: Mechanisms and prevention. Clin. Perinatol. 2010;37:565–579. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfaleh K, Anabrees J, Bassler D, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2011;(3):CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki C, Totsu S, Uchiyama A, et al. Effect of Bifidobacterium administration on very-low-birthweight infants. Pediatr. Int. 2012;54:651–656. doi: 10.1111/j.1442-200X.2012.03649.x. [DOI] [PubMed] [Google Scholar]
- 6.Kusuda S, Fujimura M, Sakuma I, et al. Morbidity and mortality of infants with very low birth weight in Japan: Center variation. Pediatrics. 2006;118:e1130–1138. doi: 10.1542/peds.2005-2724. [DOI] [PubMed] [Google Scholar]
- 7.Leaf A, Dorling J, Kempley S, et al. Early or delayed enteral feeding for preterm growth-restricted infants: A randomized trial. Pediatrics. 2012;129:e1260–1268. doi: 10.1542/peds.2011-2379. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hosni M, Duenas M, Hawk M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 2012;32:253–259. doi: 10.1038/jp.2011.51. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima H, Sumida Y, Tanaka R, et al. Early administration of Bifidobacterium breve to preterm infants: Randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 1997;76:F101–107. doi: 10.1136/fn.76.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Cho SJ, Park EA. Effects of probiotics on enteric flora and feeding tolerance in preterm infants. Neonatology. 2007;91:174–179. doi: 10.1159/000097449. [DOI] [PubMed] [Google Scholar]
- 11.Chou IC, Kuo HT, Chang JS, et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J. Pediatr. 2010;156:393–396. doi: 10.1016/j.jpeds.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 12.Underwood MA, Salzman NH, Bennett SH, et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: Impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Pediatr. Gastroenterol. Nutr. 2009;48:216–225. doi: 10.1097/MPG.0b013e31818de195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khailova L, Dvorak K, Arganbright KM, et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G940–949. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khailova L, Mount Patrick SK, Arganbright KM, et al. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1118–1127. doi: 10.1152/ajpgi.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 16.Stratiki Z, Costalos C, Sevastiadou S, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007;83:575–579. doi: 10.1016/j.earlhumdev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Kusuda S, Fujimura M, Uchiyama A, et al. Trends in morbidity and mortality among very low birth weight infants from 2003 to 2008 in Japan. Pediatr. Res. 2012;72:531–538. doi: 10.1038/pr.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura Y, Terahara M, Yajima M, et al. Effect of Bifidobacterium bifidum OLB6377 and Bifidobacterium bifidum OLB6378 on expression of the human polymeric immunoglobulin receptor (pIgR) Digest. Org. Mucos. Immunol. 2005;42:53–56. (in Japanese with English abstract) [Google Scholar]
- 19.Nakamura Y, Terahara M, Iwamoto T, et al. Up-regulation of polymeric immunoglobulin receptor expression by the heat-inactivated potential probiotic Bifidobacterium bifidum OLB6378 in a mouse intestinal explant model. Scand. J. Immunol. 2011;75:176–183. doi: 10.1111/j.1365-3083.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayes JA, Adamson-Macedo EN, Perera S, et al. Detection of secretory immunoglobulin A (SIgA) in saliva of ventilated and non-ventilated preterm neonates. Neuroendocrinol. Lett. 1999;20:109–113. [PubMed] [Google Scholar]
- 21.Wan AK, Seow WK, Purdie DM, et al. Immunoglobulins in saliva of preterm and full-term infants. Oral Microbiol. Immunol. 2003;18:72–78. doi: 10.1034/j.1399-302x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujii T, Ohtsuka Y, Lee T, et al. Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating Smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2006;43:83–88. doi: 10.1097/01.mpg.0000228100.04702.f8. [DOI] [PubMed] [Google Scholar]
- 23.Campeotto F, Suau A, Kapel N, et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011;22:1–10. doi: 10.1017/S0007114510005702. [DOI] [PubMed] [Google Scholar]
- 24.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 2008;64:418–422. doi: 10.1203/PDR.0b013e318181b7fa. [DOI] [PubMed] [Google Scholar]


