Abstract
Insulin-producing cells are transplanted by portal vein injection as an alternative to pancreas transplantation in both clinical and preclinical trials. Two of the main limitations of portal vein transplantation are the prompt activation of the innate immunity and concomitant loss of islets and a small but significant risk of portal vein thrombosis. Furthermore, to mimic physiological release, the insulin-producing cells should instead be located in the pancreas. The trans-vessel wall approach is an endovascular method for penetrating the vessel wall from the inside. In essence, a working channel is established to the parenchyma of organs that are difficult to access by percutaneous technique. In this experiment, we accessed the extra-vascular pancreatic parenchyma in swine by microendovascular technique and injected methylene blue, contrast fluids and insulin-producing cells without acute adverse events. Further, we evaluated the procedure itself by a 1-year angiographical follow-up, without adverse events. This study shows that the novel approach utilizing endovascular minimal invasiveness coupled to accurate trans-vessel wall placement of an injection in the pancreatic parenchyma with insulin-producing cells is possible. In clinical practice, the potential benefits compared to portal vein cell transplantation should significantly improve endocrine function of the graft and potentially reduce adverse events.
This study presents one-year follow-up safety data on the microendovascular trans-vessel wall technique and shows that the technique can be used to transplant insulin-producing cells to the swine pancreas parenchyma.
Keywords: Endovascular, islet transplantation, minimal-invasive transplantation, SPECT/CT, swine
Significance Statement
In clinical trials, insulin-producing cells are today transplanted by injection into the portal vein with cell embolization to the liver. A minimal invasive method for direct transplantation to the pancreas parenchyma without causing pancreatitis could enable transplantation to the natural physiological niche in humans. Many preclinical trials support increased endocrine effect and the use of lower number of cells.
In this article, we show a feasible method for transplantation of insulin-producing cells to the pancreas parenchyma and track the cells with single photon emission computed tomography (SPECT) and computed tomography (CT). Further, we have performed a 1-year follow-up of the method itself without complications. This study, with clinical materials, in large animals, is a solid start for a first clinical trial of the trans-vessel wall method in type 1 diabetes mellitus.
Introduction
The development of procedures for intraportal transplantation of insulin-producing cells in type 1 diabetes patients has been performed for more than 20 years. These techniques have been thoroughly evaluated and have improved considerably 1–3. For example, protocols for immunosuppression 4,5 have been optimized and autoimmunity 6 is monitored. Refinements to reduce the risk of the actual transplantation procedure are also being made since both bleeding and portal vein thrombosis are potentially severe adverse events 7.
Although current portal vein embolizations do have significant disadvantages, they are still performed in preclinical as well as in clinical trials. In addition to the risks mentioned above, the actual site of the implantation is important for both the function of the transplanted cells as tested in both canine and rat where the pancreas was deemed as a superior site to liver and kidney 8 and in mice where both the function and the gene expression within the graft clearly showed the pancreas to be a superior site as compared to liver 9. The pancreas could be the preferential site of islet transplantation since this is their “natural” physiological niche 10.
For insulin to exert its effect in a biologically optimal way, the release should mimic the physiological response with release into the portal vein circulation. The natural islets in the pancreas also have a good vascular supply and high oxygen tension 11, which would require the transplanted cell to induce angiogenesis. However, in accordance with the “natural” physiological niche, all the prerequisites for high oxygen tension are there.
The pancreas is, however, due to safety concerns a hard to reach organ by either open surgical approach or percutaneous CT- or ultrasound-guided techniques.
Modern imaging-based interventional techniques now provide alternatives to open surgical access and arteries and veins can be regarded as “internal routes” to essentially anywhere in the body. An endovascular approach with intraluminal transplantation as suggested by Hirshberg et al 12, would be minimally invasive and still provide access to the pancreas. However, there are potential drawbacks from, for example exposing the cells to the blood stream and a lack of control over the actual site of engraftment. In general, results obtained so far have not been satisfactory 12. We here propose a trans-vessel wall access to the pancreas parenchyma based on the use of a prototype catheter system 13,14. A standard endovascular clinical catheter system, including an introducer, a guidecatheter and a microcatheter, is used to navigate within the vasculature to several vessels supplying the pancreas. Once the microcatheter is in the desired location within the microvasculature, the prototype system is advanced through the microcatheter. The prototype catheter (outer diameter [OD] 0.193 ± 0.0127 mm, inner diameter [ID] 0.104 ± 0.0127 mm and total length 1700 mm) then safely penetrates the arterial wall, as a “nano”catheter, to reach the extravascular space, for example the parenchyma of the pancreas, using the same principles as the introducer 15.
We tested the feasibility of using this minimally invasive endovascular trans-vessel wall technique for direct cell transplantation to the pancreas parenchyma in large animals with full integration with clinical materials. For all forms of endovascular implants the golden standard for long-term follow-ups is angiography and the follow-up time recommended by the Food and Drug Administration is 1 year in large animals 16. To address possible adverse events, we therefore performed 1-year follow-ups for the endovascular intervention itself within the target pancreas parenchyma. This study provides proof of concept for this novel way of transplanting insulin-producing cells and demonstrates the versatility of the trans-vessel wall working channel principle.
Materials and Methods
All animal studies were conducted according to Karolinska Institutet guidelines of animal experiments. The studies were approved by the Regional Ethics Committee for Animal Research in Stockholm, Sweden.
Overview
Four Swedish rural swine were included in the acute transplantation part of the study with a minimum of three interventions per animal. Interventions included transplantation of insulin-producing cells followed with SPECT/CT imaging (n = 2), methylene blue (n = 4) and contrast agent injection (n = 2) through the nitinol tube at a flow rate of 2–5 μm/s.
Further, 10 Göttingen mini swine were included in the long-term follow-up. They received one to two implants each (n = 17) and were monitored with blood samples up until follow-up angiography 1 year later.
The trans-vessel wall prototypes were all manufactured by hand as previously described 13.
Anesthesia
Induction of surgical anesthesia in the swine started with intramuscular injection of Domitor vet. (1 mg/mL; Orion BioPharma Animal Health, Sollentuna, Sweden) and atropine (0.5 mg/mL; Mylan AB, Stockholm, Sweden). A single dose of 250 mg Aspirin iv (Bayer Vital, Leverkusen, Germany) was given intravenously at the beginning of the procedure. Continuous infusion pentobarbital and fentanyl (50 µg/mL; B Braun Medical, Danderyd, Sweden) was used with a Siemens 900 servo-ventilator (Siemens Healthcare, Upplands Väsby, Sweden).
Angiography
All angiography was performed using a Philips XD20 angiographical system (Philips Medical Systems, Best, the Netherlands) and a Philips 3DRA workstation. Visipaque 270 contrast agent (GE Healthcare, Cleveland, OH) was used for all contrast-enhanced applications. XperCT images obtained were reviewed using the XD20 systems XperCT high dose program and soft tissue algorithms (Philips Medical Systems) 17.
We used a 5 French Introducer (Terumo, Somerset, NJ) with femoral cut down access. To each 1000 mL NaCl flush solution for the guide- and microcatheters, 5000 E Heparin LEO (Orifarm, Stockholm, Sweden) and 2 mg Nimotop (Bayer Schering Pharma, Berlin, Germany) was added. A 5 French Cobra guiding catheter with a Renegade microcatheter (Boston Scientific) and a Transend Platinum Tip guidewire (Boston Scientific, Boston, MA) were navigated to the microvasculature of the pancreas. The guidewire was withdrawn from the microcatheter and the prototype catheter was deployed through the arterial wall. Injections with methylene blue and contrast agent were performed to additionally prove the concept of substance administration. After the finished procedure, a 10-V current was applied thereby detaching the distal part and leaving it as an implant through the arterial wall to control possible bleedings from the perforation. Perforations were followed up to 3 h by high-resolution digital subtraction angiography (DSA). Following euthanasia with an overdose of pentobarbital, dissections were performed using an OPMI6-DF operating microscope (Carl Zeiss AB, Stockholm, Sweden) connected to a CCD camera.
Blood samples
Blood samples were obtained prior to surgical procedures and after 1 week, 1 month and prior to euthanasia. Samples were analyzed by the Karolinska University Hospital Clinical Chemistry Department with GCP accredited methods (Swedac, Stockholm, Sweden).
Cell culturing
The INS-1 cell line, derived from rat insulinoma, with positive GLP1-R expression was cultured in RPMI1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin/streptomycin (10 000 U/mL), 1 mM sodium pyruvate, 50 µM β-mercaptoethanol and 10 mM HEPES. Cells were incubated at 37°C, 90% humidity and 5% CO2.
For tracking of transferred cells, insulin-producing cells were incubated with 2 µM CellTracker CM-DiI (Invitrogen, Stockholm, Sweden) for 5 min at 37°C, then quickly transferred to 4°C for 15 min. After incubation, the excess CM-DiI was washed away with sterile phosphate-buffered saline (PBS). Cells were detached with 2 mM ethylenediaminetetraacetic acid (EDTA) (Sigma, Stockholm, Sweden) for 30 min at 37°C. The detached cells were centrifuged and re-suspended in RPMI 1640 medium (Invitrogen) for further Indium-111 incorporation.
Labeling cells with Indium-111
Labeling was performed essentially according to the instructions for cell labeling provided by the indium-oxinate supplier (Mallinckrodt Medical B.V., Le Petten, Holland). INS-1 cells, 1.6–2 × 106 in buffer, 90% viable according to the trypan blue exclusion test, were centrifuged and resuspended in 1 mL physiological saline (9 mg/mL). 111In-oxinate (2.5–3.5 MBq) in tris buffer (0.15–0.2 mL) was added and allowed to react for 30 min at room temperature with occasional swirling to maintain suspension. The tubes were centrifuged, the unreacted radioactivity in the supernatant removed, 1 mL physiological saline was added and the cells resuspended with swirling. The centrifugation was repeated one more time and the radioactivity in the cells and supernatants was measured. Labeling efficiency was 50–60%. The labeled cells were resuspended in 0.125 mL physiological saline for the transplantation procedure and tested for a >0.90 viability ratio with trypan blue.
The SPECT/CT system (Symbia T; Siemens GmbH, Erlangen, Germany) consisted of a dual-head variable-angle γ-camera equipped with low-energy high-resolution collimators and a multislice spiral CT component optimized for rapid rotation. The SPECT acquisition (128 × 128 matrix, 81 frames, 45 s/frame) was performed using 4.5° angular steps in a 50-s time frame. For CT (158 kV, 210 mAs, B50s kernel, 512 × 512 matrix), 0.75-mm slices were obtained. After reconstruction, SPECT images were corrected for attenuation and scatter. Both SPECT and CT axial 5-mm slices were generated using Hermes Gold 450 (Hermes Medical Solution, Stockholm, Sweden). Images were then analyzed with OsiriX imaging software (OsiriX Foundation, Geneva, Switzerland).
Immunohistochemical staining and tissue analysis
After gross examination, individual pieces of the pancreas were quickly frozen in isopentane-dry ice before cryosectioning with a Leica cryostat (CM 3000; Leica Instruments GmbH, Nussloch, Germany). The sections were thaw mounted onto Super Frost/Plus object glasses (Menzel-Gläzer, Braunschweig, Germany) and stored at −20°C prior to use. Frozen sections were air-dried, rehydrated in 1× PBS, fixated in formalin and methanol, stained with a primary insulin antibody (Guinea Pig; Abcam, Cambridge, UK) with a secondary Alexa 488 anti Guinea Pig (Abcam) mounted and examined with microscope.
Results
We first evaluated the trans-vessel wall approach in several acute settings in Swedish rural swine (n = 4). We could preoperatively determine the abdominal organ anatomy and vascular supply with contrast-enhanced XperCT scans 18 (Figure 1). The vascular supply of the pancreas was successfully visualized by high-resolution DSA for navigation to the posterior pancreatic artery (PPA) (Figure 1). Both vessel trunks were deemed suitable for trans-vessel wall intervention. The basic anatomy corresponded well with the literature 19.
Figure 1.
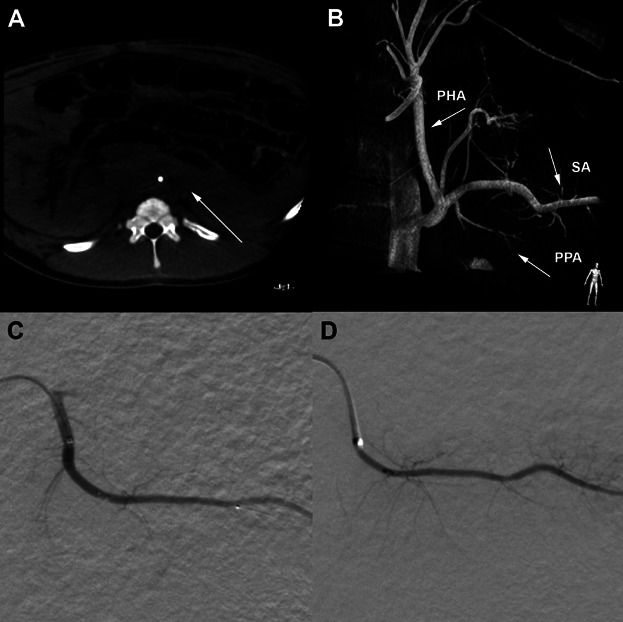
Radiological images from the trans-vessel wall intervention in the pancreas and an angiographic follow-up 1 year later. (A) An example of a CT reconstruction with axial orientation from the XperCT algorithm reconstructed from raw data of the C-arm. (B) A 3D reconstruction of the aorta, celiac trunk and the posterior pancreatic artery (PPA), marked with arrow. Also marked are the proper hepatic artery (PHA) and splenic artery (SA). (C) Primary intervention within the PPA segment followed with (D), the same vessel 1 year later.
We used DSA series as road maps (Smart Mask™; Philips Medical Systems) to navigate the microcatheter to the chosen point of intervention. The microguidewire was then exchanged for a prototype catheter that was subsequently forwarded through the system. When reaching tip to tip, the prototype was gently pushed out through the vessel wall thereby creating a working channel between the proximal end of the catheter and the pancreas parenchyma. No hemorrhage or embolic complications were observed when creating access to the parenchyma. Vasospasm was observed, a well-described phenomena more easily occurring in swine than in humans 20. We chose to administer local intra-arterial spasmolytic drugs with the guide catheter rinsing solution, when appropriate, to relieve the spasm with good effect.
Through the working channel of the prototype system, we performed injections of a blue colored dye to mark the parenchymal access (Figure 2). Furthermore, contrast agent was injected to demonstrate that the deposited suspension was not washed out with the blood stream, thus showing second-order evidence by angiographical techniques that trans-vessel wall parenchymal access in the pancreas is feasible. After injection of fluids through the system, we detached the distal part of the prototype. The distal portion acts as a plug in the vessel wall to secure the vessel wall defect, thereby creating a safe exit strategy for the procedure. Neither bleeding in the interventional area nor distal emboli was detected at follow-up angiographies obtained up to 3 h after intervention.
Figure 2.
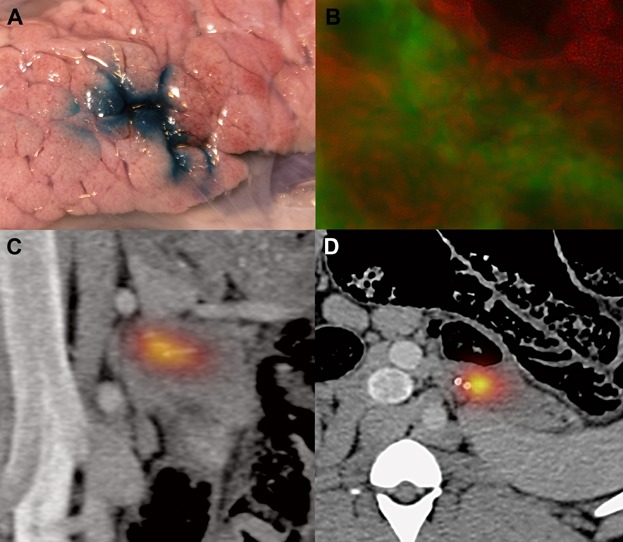
Access to the pancreas for injection of dye and insulin-producing cells. (A) Methylene blue injected through the working channel. (B) DiI (red)-marked insulin-producing cells within the pancreas parenchyma counter stained for insulin (green). (C) Frontal projection and (D) axial projection of SPECT/CT fusion images showing Indium-111-labeled insulin-producing cells within the pancreas parenchyma.
For practical reasons related to the gain of weight during the follow-up time, we performed the long-term study in Göttingen mini swine instead of Swedish rural swine. We performed 17 successful interventions in 10 swine and subsequently followed them for 1 year. The primary outcome was survival of the swine. None died or showed any signs of morbidity. No stenosis or dysfunction of the vessel subject to intervention was found in the 1-year follow-up angiographies (Figure 1). The implanted distal parts of the catheter could be visualized separate from the contrast stream. This indicates that the catheter part is safely and actively pushed out through the vessel wall to the periadventitial space over a long period of time, which confirms indications from previous studies 14. We also examined the pancreas parenchyma with high-resolution XperCT. The entire parenchyma showed normal attenuation, with no cysts or other pathology.
Plasma pancreatic amylase was measured prior to intervention, at 1 week, 4 weeks and in conjunction with the follow-up angiography and euthanasia. One animal had a transient elevation of pancreas amylase after 7 days by a clinically insignificant factor of 2.8 from baseline (Table 1). On the average, the baseline elevation in the first week was 1.3 (SD 0.6) thereafter returning to baseline at the 1-month sampling. Further, clinical chemistry monitoring included lipase, hematocrit, white blood cell count and C-reactive peptide. No deviations were observed.
Table 1.
Plasma biochemical analysis
| Subjet no. | Pancreatic amylase | Lipase | CRP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Preimplantation (µkat/L) | One week (µkat/L) | One month (µkat/L) | One year (µkat/L) | One year (µkat/L) | Preimplant (mg/L) | One week (mg/L) | One month (mg/L) | One year (mg/L) | |
| 01 | 31.9 | 34.73 | 35.96 | 31.29 | 0.17 | <1 | <1 | <1 | <1 |
| 02 | 37.18 | 104.12 | 38.15 | 33.35 | 0.17 | <1 | <1 | <1 | <1 |
| 03 | 40.87 | 64.46 | 40.66 | 41.62 | 0.3 | <1 | <1 | <1 | <1 |
| 04 | 40.65 | 58.59 | 37.8 | 35.17 | 0.19 | <1 | <1 | <1 | <1 |
| 05 | 31.38 | 33.01 | 31.99 | 32.67 | 0.23 | <1 | <1 | <1 | <1 |
| 06 | 34.32 | 50.66 | 39.76 | 38.54 | 0.23 | <1 | <1 | <1 | <1 |
| 07 | 52.26 | 34.38 | 32.87 | 45.4 | 0.22 | <1 | <1 | <1 | <1 |
| 08 | 30.88 | 39.86 | 33.29 | 27.48 | 0.31 | <1 | <1 | <1 | <1 |
| 09 | 32.99 | 25.86 | 34.52 | 39.32 | 0.26 | <1 | <1 | <1 | <1 |
| 10 | 41.36 | 47.66 | 47.23 | 41.45 | 0.29 | <1 | <1 | <1 | <1 |
After the successful 1-year follow-up, we performed two new acute phase experiments with transplantation of insulin-producing cells in Swedish rural swine. These cells were labeled with CellTracker CM-DiI (a fat soluble indocarbocyanine flurochrome) and with Indium-111, the latter making it possible to perform SPECT/CT of the cell localizations.
Following deposition of cells, we transferred the anesthetized animals to the SPECT/CT suite. The images clearly showed that the 111In-labeled cells were located within the pancreas parenchyma (Figure 2). Furthermore, no obvious parenchymal damage could be seen on the CT scans (Figure 2). After euthanasizing the animals, we dissected the organs and used radioactivity measurements to guide the excision of representative samples for microscopical evaluation. In the subsequently cryosectioned slides, we confirmed that CM-DiI labeled cells, counterstained for insulin (Figure 2), were within the pancreas.
Discussion
In this study, we have shown that it is feasible to reach the pancreas parenchyma of swine with a trans-vessel wall approach utilizing a prototype catheter inside standard clinical endovascular microcatheters. The accurate location of the cells was confirmed by SPECT/CT and histological techniques. The lack of procedure-related adverse effects was confirmed by 1-year follow-up angiography and clinical biochemistry sampling.
The endovascular approach is a minimally invasive method to reach organs. As such, we conclude that the limitations commonly associated with the portal vein approach to transplantation—not the natural physiological niche and suboptimal insulin secretion 10 or percutaneous ultrasound/CT-guided injections to the pancreas—could be avoided by the use of endovascular techniques. Unfortunately, attempts with intraluminal cell delivery methods have had limited success 1. The intraluminal transplantation technique exposes the cells to the blood stream triggering instant blood-mediated inflammatory reaction, resulting in early opsonization/necrosis in a substantial fraction of the cells. A method for safe trans-vessel wall access to the pancreas parenchyma could improve the method for transplantation of insulin-producing cells by in essence placing the cells accurately in the pancreas parenchyma by a minimally invasive technique.
The main limitation to accessing the pancreas is the risk of triggering an acute pancreatitis that is a potentially lethal condition and a clear indication for intensive care. To manipulate the pancreas in any way, the gentlest handling possible is indicated. The trans-vessel wall method uses the vessels to navigate into the pancreas parenchyma thereby avoiding manipulating the parenchyma and causing the spiral of events with enzymatic leakage that is the pathology behind pancreatitis. When we then manipulate the pancreas, we are already within the parenchyma and the diameter of the catheter is sufficiently small to establish a working channel without causing pancreatitis.
Though this study has primarily focused on developing and validating the technique for successfully performing the transplantations, the endocrine function of the cells after transplantation still needs to be confirmed. The trans-vessel wall approach shields the cells from the blood stream and probable early opsonization/necrosis during passage through the working channel to the pancreas parenchyma. We have transplanted single cell suspensions and not islets. In this article, we have focused on single cells due to stability and availability. However, with an ID of 104 µm and the clinical possibility to sort out sufficiently small islets, we see no problems for islet transplantations through the system. Further, it is no problem to increase the ID to approximately 200 µm and still keep the physiological properties of the system with respect to bleeding and other acute complications.
In summary, we have shown here that it is possible to create a working channel, deposit cells in the pancreas and that the trans-vessel wall procedure in itself does not cause adverse events during a 1-year follow-up. The integration of the prototype systems with commercially available catheters should make translation into clinical practice straightforward. The techniques developed here could potentially be useful in many other applications such as local administrations of pharmaceutical agents, radioactive substances or for cytological sampling in different organ systems.
Acknowledgments
The authors wish to thank Johanna Doshé, Pellina Jansson, Oscar Ardenfors and Ulrika Svanholm for excellent technical assistance.
Glossary
- CT
computed tomography
- DSA
digital subtraction angiography
- FDA
Food and Drug Administration
- IBMIR
instant blood-mediated inflammatory reaction
- ID
inner diameter
- OD
outer diameter
- PPA
posterior pancreatic artery
- SPECT
single photon emission computed tomography
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. The medical device/catheter system described in the paper is subject to US and PCT patent application by SH, JL and SJ as inventors. The patent application is still in process. The entire system/device has been manufactured by hand and is not part of any currently available commercial products or modified commercial products. None of the authors are shareholders in any company associated with the patent nor has any of the work with the device/catheter system been part of an employment or consultancy from any catheter developing company. The authors will freely make available any materials and information associated with their publication that are reasonably requested by others for the purpose of academic, noncommercial research. This study was supported by grants from the Swedish Research Council (K2006-73X-20139-01-3), Karolinska Institutet funds, Karolinska Institutet Innovations AB, the Swedish Society of Medicine, Vinnova Vinn Verifiering, Söderbergska Stiftelsen, The Swedish Brain Fund and Strokefonden. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39:515–518. doi: 10.2337/diab.39.4.515. ,Et al. [DOI] [PubMed] [Google Scholar]
- 2.Scharp DW, Lacy PE, Santiago JV, et al. Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients. Transplantation. 1991;51:76–85. doi: 10.1097/00007890-199101000-00012. , [DOI] [PubMed] [Google Scholar]
- 3.Kemp CB, Knight MJ, Scharp DW, Ballinger WF, Lacy PE. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9:486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. , [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. , [DOI] [PubMed] [Google Scholar]
- 6.Roep BO, Stobbe I, Duinkerken G, et al. Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes. 1999;48:484–490. doi: 10.2337/diabetes.48.3.484. , [DOI] [PubMed] [Google Scholar]
- 7.Villiger P, Ryan EA, Owen R, et al. Prevention of bleeding after islet transplantation: Lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5:2992–2998. doi: 10.1111/j.1600-6143.2005.01108.x. , [DOI] [PubMed] [Google Scholar]
- 8.Stagner JI, Rilo HL, White KK. The pancreas as an islet transplantation site. Confirmation in a syngeneic rodent and canine autotransplant model. JOP. 2007;8:628–636. [PubMed] [Google Scholar]
- 9.Lau J, Mattsson G, Carlsson C, et al. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes. 2007;56:1544–1550. doi: 10.2337/db06-1258. , [DOI] [PubMed] [Google Scholar]
- 10.Merani S, Toso C, Emamaullee J, Shapiro AMJ. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95:1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 11.Papas KK, Long RC, Sambanis A, Constantinidis I. Development of a bioartificial pancreas: II. Effects of oxygen on long-term entrapped betaTC3 cell cultures. Biotechnol Bioeng. 1999;66:231–237. [PubMed] [Google Scholar]
- 12.Hirshberg B, Montgomery S, Wysoki MG, et al. Pancreatic islet transplantation using the nonhuman primate (rhesus) model predicts that the portal vein is superior to the celiac artery as the islet infusion site. Diabetes. 2002;51:2135–2140. doi: 10.2337/diabetes.51.7.2135. , [DOI] [PubMed] [Google Scholar]
- 13.Lundberg J, Jonsson S, Holmin S. New endovascular method for transvascular exit of arteries and veins: Developed in simulator, in rat and in rabbit with full clinical integration. PLoS ONE. 2010;5:e10449. doi: 10.1371/journal.pone.0010449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg J, Jonsson S, Holmin S. Long term follow-up of the endovascular trans-vessel wall technique for parenchymal access in rabbit with full clinical integration. PLoS ONE. 2011;6:e23328. doi: 10.1371/journal.pone.0023328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: A new technique. Acta Radiol. 1953;39:368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 16.Abel DB, Dehdashtian MM, Rodger ST, Smith AC, Smith LJ, Waninger MS. Evolution and future of preclinical testing for endovascular grafts. J Endovasc Ther. 2006;13:649–659. doi: 10.1583/06-1872.1. [DOI] [PubMed] [Google Scholar]
- 17.Söderman M, Babic D, Homan R, Andersson T. 3D roadmap in neuroangiography: Technique and clinical interest. Neuroradiology. 2005;47:735–740. doi: 10.1007/s00234-005-1417-1. [DOI] [PubMed] [Google Scholar]
- 18.Söderman M, Babic D, Holmin S, Andersson T. Brain imaging with a flat detector C-arm. Neuroradiology. 2008;50:863–868. doi: 10.1007/s00234-008-0419-1. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann FA, Pistorius G, Grabowsky K, Motsch J, Marzi I. Pancreatic autotransplantation in the pig: Variations in epigastric arterial blood supply. Transpl Int. 1989;2:193–198. doi: 10.1007/BF02414533. [DOI] [PubMed] [Google Scholar]
- 20.Smith AC, Spinale FG, Carabello BA, Swindle MM. Technical aspects of cardiac catheterization of swine. J Invest Surg. 1989;2:187–194. doi: 10.3109/08941938909015350. [DOI] [PubMed] [Google Scholar]


