Abstract
Purpose
To standardize upper abdominal normal organ contouring guidelines for Radiation Therapy Oncology Group (RTOG) trials.
Methods and Materials
Twelve expert radiation oncologists contoured the liver, esophagus, gastroesophageal junction (GEJ), stomach, duodenum, and common bile duct (CBD), and reviewed and edited 33 additional normal organ and blood vessel contours on an anonymized patient computed tomography (CT) dataset. Contours were overlaid and compared for agreement using MATLAB (MathWorks, Natick, MA). S95 contours, defined as the binomial distribution to generate 95% group consensus contours, and normal organ contouring definitions were generated and reviewed by the panel.
Results
There was excellent consistency and agreement of the liver, duodenal, and stomach contours, with substantial consistency for the esophagus contour, and moderate consistency for the GEJ and CBD contours using a Kappa statistic. Consensus definitions, detailed normal organ contouring recommendations and high-resolution images were developed.
Conclusions
Consensus contouring guidelines and a CT image atlas should improve contouring uniformity in radiation oncology clinical planning and RTOG trials.
Introduction
High precision radiation therapy, including intensity modulated radiation therapy (IMRT) and stereotactic body radiotherapy (SBRT), has increased in usage over the past decade, including in the upper abdomen.1 One of the main purposes of IMRT and SBRT is to reduce the dose to surrounding normal tissues. It is critical to accurately identify and contour the normal organ anatomy on computed tomography (CT)-based planning images to maintain appropriate radiation doses and minimize toxicity in critical normal tissues, for dose and volume reporting, and for investigations of dose–volume toxicity relationships. There has been little standardization to facilitate consistent and accurate normal organ contouring. Only recently, normal tissue contouring atlases for radiation planning (eg, lung, pelvis, postoperative pancreas) have been published.2–4
To establish consistency in contouring upper abdominal normal tissues, and in preparation for the Radiation Therapy Oncology Group (RTOG) 1112 trial, a randomized phase 3 study of sorafenib versus SBRT followed by sorafenib in hepatocellular carcinoma, the RTOG organized a consensus panel. Goals of the panel were to develop uniform definitions for upper abdominal normal tissue anatomy, to propose standards for contouring these organs, and to create an image-rich upper abdominal organ atlas. This effort was largely initiated for the purpose of education in a relatively novel site for radiation therapy, the liver, where there are many contouring challenges including mobile, deformable organs, “variant” anatomy, complex vasculature, and a requirement to understand the terminology used among other oncologic specialties (ie, liver segment definitions).
This report provides consensus panel recommendation and serves as a template for standard anatomic definitions for the contouring of upper abdominal normal organs for radiation therapy planning.
Methods and materials
A panel of 12 radiation oncologists experienced in upper abdominal radiation therapy (who had published and participated in prior studies of radiation therapy for upper abdominal malignancies) and 1 radiologist specializing in upper abdominal anatomy participated in this upper gastrointestinal contouring atlas project.
An anonymized patient CT dataset demonstrating typical normal anatomy was obtained for developing an educational atlas. The patient was scanned in a 64-slice multidetector CT scanner (Aquilion 64, Toshiba Medical Systems, Tustin, CA), in the supine position, during a breath hold, with 100 mL of intravenous contrast at an injection rate of 3 mL/s; 1000 mL of water was taken orally to delineate the intestinal tract. Two hundred fifty-eight axial CT slices with a 3-mm slice thickness and 1.5-mm interslice intervals were obtained in the portal venous phase.
Thirty-nine structures and vessels (right hepatic vein [RHV], middle hepatic vein [MHV], left hepatic vein [LHV], celiac artery [CA], common hepatic artery [CHA], splenic artery [SA], right anterior portal vein [RTAPV], superior mesenteric artery [SMA], portal vein [PV], splenic vein [SV], inferior vena cava [IVC], right posterior portal vein [RPPV], superior mesenteric vein [SMV], inferior mesenteric vein [IMV], and left portal vein [LPV]) were contoured by S.A.H. and edited by L.A.D., in collaboration with an expert abdominal radiologist (T.K.K.).
The patient dataset was exported to the Image Guided Therapy QA (quality assurance) Center (ITC) and distributed by the ITC web server. Participants downloaded and imported the data files into their respective contouring and treatment planning systems, where they reviewed the contoured structures and edited or re-contoured.
Each participant contoured the liver, esophagus, gastroesophageal junction (GEJ), stomach, duodenum, and common bile duct (CBD). The datasets were then electronically uploaded to the ITC for analysis.
Analysis
Panelists’ contours were imported into the Computerized Environment for Radiation Research, an open source MATLAB (The MathWorks, Natick, MA)-based radiation therapy planning analysis tool.5 Individual contours were overlaid and compared for agreement using an imputation method using an expectation-maximization algorithm for simultaneous truth and performance level estimation.6 Proposed consensus segmentation was computed from the observed contours as a maximum-likelihood estimate of the true segmentation.2 The S95 contour, defined as the simultaneous truth and performance level estimation algorithm consensus at the 95% confidence level, was created for the 6 organs.
The agreement among contours was assessed using the intersection and union of contour volume and Kappa statistic.7 Using Landis and Koch’s benchmarks for interpretation of strength of agreement, kappa < 0.00: poor; 0.00–0.20: slight; 0.21–0.40: fair; 0.41–0.60: moderate; 0.61–0.80: substantial; 0.81–1.00: almost perfect agreement.8 The MIM contouring software (MIMvista Corporation, Cleveland, OH) was used to review and render images.
Results
Good contouring consistency was found among most organs (Table 1), with the greatest consistency for liver, stomach, duodenum, and esophagus contours, and the least consistency for the CBD and GEJ contours.
Table 1.
Kappa measures for agreement among physician contours
| Structure measure | Stomach | CBD | Duodenum | Esophagus | GEJ | Liver |
|---|---|---|---|---|---|---|
| No. of experts | 12 | 9 | 12 | 12 | 11 | 11 |
| Vol. (cc) maximum | 288.9 | 3.2 | 158.7 | 15.7 | 29.8 | 1480.4 |
| Vol. (cc) minimum | 247.3 | 0.9 | 122.5 | 5.1 | 1.3 | 1380.0 |
| Vol. (cc) average | 268.3 | 2.2 | 143.5 | 10.3 | 11.6 | 1439.0 |
| Vol. (cc) SD | 13.0 | 0.6 | 10.6 | 3.2 | 8.9 | 33.0 |
| Vol. intersection | 191.1 | 0.01 | 94.4 | 1.8 | 0.04 | 1233.2 |
| Vol. union | 383.2 | 6.8 | 190.5 | 20.6 | 42.7 | 1634.8 |
| Kappa | 0.90 | 0.42 | 0.87 | 0.62 | 0.41 | 0.94 |
| Measure | Near perfect | Moderate | Near Perfect | Substantial | Moderate | Near perfect |
CBD, common bile duct; GEJ, gastroesophageal junction; Vol., volume.
Edits were made to all S95 contours for the final atlas contours. The largest edit was made to the CBD S95 contour to extend it to the level of the second portion of the duodenum. Liver S95 contour was edited at the medial portion of the liver near the portal hepatis to include segment I completely, which was missed (27%) or partially covered (36%) in the “caudate tail” region (Fig 1A;B). The duodenum S95 contour was edited to include the entire duodenum, including the fourth portion, which was often missed.
Figure 1.
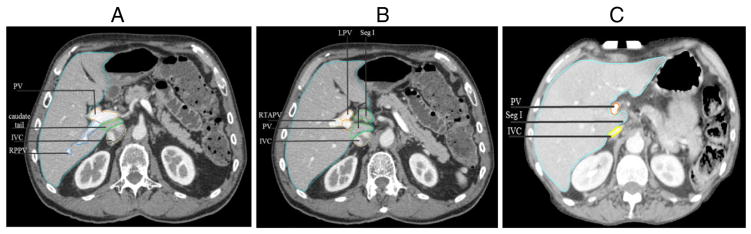
For purposes of contouring the liver, the portal vein should be included in the liver (light blue) contour when segment (Seg) I (caudate lobe) is left of the portal vein (PV). (A) Demonstrates segment I, “caudate tail,” posterior to the PV, so the liver contour should exclude the PV. (B) Demonstrates segment I to the left of the PV, so the liver contour should include segment I and the PV. Left portal vein (LPV), right anterior portal vein (RTAPV), inferior vena cava (IVC), Right posterior portal vein (RPPV) in (A). (C) Demonstrates a patient example in which segment I does not fully extend to the left of the PV, and the PV is excluded from the liver contour.
Details of the summary consensus contours follow.
Gastroesophageal junction
The GEJ, marked on the mucosal surface between the squamous esophageal mucosa and the gastric columnar mucosa at the “Z line”,9 is attached to the liver at the fissure for the ligamentum venosum by the gastrohepatic ligament. On axial imaging, the GEJ may appear thickened,9 at the level of the lower esophageal sphincter (Fig 2B;C). At the insertion of the phrenoesophageal ligament, the circular muscle layer of the esophageal wall becomes thicker than at higher levels, and the circular muscles of the esophagus transition to become more oblique. Three to 5 centimeters below the phrenoesophageal ligament insertion, the Z line is present.10
Figure 2.
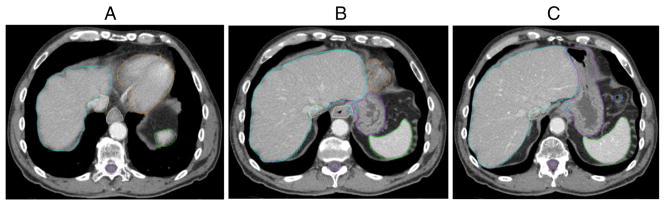
(A) Consensus contours of liver (light blue), inferior vena cava (IVC) (yellow), esophagus (white), heart (orange), spleen (green), stomach (purple in 2B and 2C), spinal canal (purple), top of gastroesophageal junction (GEJ) (light blue in 2B), and bottom of GEJ (light blue in 2C), large bowel (2C).
Although GEJ contouring is not routine practice, better understanding of the precise location of the GEJ may be important for dose escalation or combined modality studies. The wedge-shaped GEJ contour should include (and overlap with) the most distal esophagus and the cardia of the stomach (Fig 2).
Stomach
Oral contrast is recommended during imaging for optimal delineation of the gastric walls. The stomach, separated into cardia (near the heart), fundus, body, and antrum and pylorus, should be contoured as 1 organ. The cardia begins caudal to the GEJ.11 The gastric fundus, the most cephalad portion of the stomach, abuts the left hemidiaphragm, and is to the left and superior to the cardia. The body is the central, largest portion of the stomach and the antrum is the gateway into the pylorus, the sphincter opening into the duodenum9 (Figs 2C; 3A; 3D; 4B–E).
Figure 3.
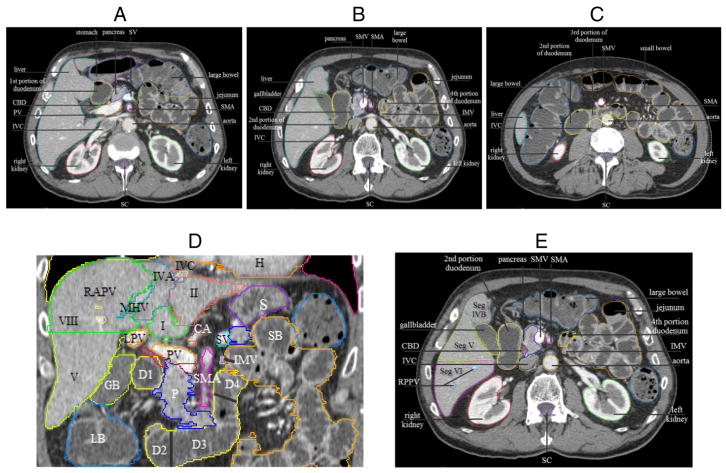
(A), (B), and (C) Axial images demonstrating 4 portions of the duodenum (shown in yellow). Splenic vein (SV), portal vein (PV), common bile duct (CBD), inferior vena cava (IVC), superior mesenteric artery (SMA), SMV, spinal canal (SC). (D) Coronal images illustrating the 4 portions of duodenum and liver segments. Liver segments I, II, IVA, V, VIII, and D1–D4: first to fourth portions of duodenum. IMV, inferior mesenteric vein, demarcates the duodenojejunal junction; RAPV, right anterior portal vein; MHV, middle hepatic vein; LPV, left portal vein; IVC (yellow) with adjacent left hepatic vein (blue, not labeled); H, heart; S, stomach; LB, large bowel; SB, small bowel; GB, gallbladder; P, pancreas; PV, portal vein, to the right of the PV is CBD (red), to the right and inferior of the PV is superior mesenteric vein (SMV, burgundy) (ie, SMV-PV confluence); CA, celiac artery, with splenic artery (orange) to the right and inferior of CA. (E) Axial image showing duodenojejunal junction demarcated by IMV.
Figure 4.
Liver segments (Seg), demonstrated on axial images, descending from superior to inferior slices (A)–(E). CA, celiac axis; LVH, left hepatic vein; MHV, middle hepatic vein; RHV, right hepatic vein; RPPV, right posterior portal vein; RTAPV, right anterior portal vein; LPV, left portal vein; SA, splenic artery; IVC, inferior vena cava; CHA, common hepatic artery; SV, splenic vein; SC, spinal canal.
Duodenum
The duodenum begins caudal to the pylorus and is retroperitoneal except for the first portion of the duodenum, which spans approximately 5 cm and is suspended by the hepatoduodenal ligament. The CBD, PV, and IVC are posterior to the first portion of the duodenum11 (Fig 3A). The second (descending) part of the duodenum is attached to the head of the pancreas, where the pancreatobiliary papilla enters medially through the ampulla of Vater. The second portion is located to the right of the L1 to L3 vertebral bodies, parallel to and right (lateral) of the IVC (Fig 3B;C), turning medially at L3, where it becomes the third (transverse) portion of the duodenum, crossing to the left, anterior to the aorta and IVC and posterior to the SMA and SMV,9 marking the end of the C-loop of the duodenum (Fig 3B;C). The fourth (ascending) duodenum travels superiorly, left of L3, to the inferior pancreatic body (Fig 3A). The ligament of Treitz suspends the duodenojejunal junction, marking the end of the duodenum and the start of the jejunum.9
Coronal views can aid in accurate contouring, specifically the third portion that may be under-distended (Fig 3D). An anatomic landmark that can help identify the transition from the duodenum to the jejunum is the IMV12 (Fig 3E).
Liver
The porta hepatis is the site of entry of the PV, CHA, hepatic ducts, the hepatic nerve plexus, and lymphatic vessels.9
The liver has a dual afferent blood supply consisting of the PV, the major tributary to the liver supplying 70%–80% of the liver’s blood supply, and the hepatic artery, which contributes to the balance. There are usually 3 hepatic veins (right, middle, left), which collect blood from the liver and return it to the IVC at the confluence of the hepatic veins9 (Fig 4B). Identification of the 3 hepatic veins as well as the main PV should be done routinely to gain proper orientation and understanding of the anatomic lobes of the liver.
There are 8 hepatic segments (often referred to as ‘sectors’ or ‘sections,’ labeled with Arabic or Roman numerals),13 beginning with the caudate lobe known as segment I, part of the left lobe, and moving clockwise on a coronal view. The left lobe of the liver consists of segment II (lateral superior), segment III (lateral inferior), segment IVA (medial superior), and segment IVB (medial inferior). The right lobe includes segment V (anterior inferior), segment VI (posterior inferior), segment VII (posterior superior), and segment VIII (anterior superior)9 (Fig 3D).
The segments of the liver follow multiple planes along vessels, ligaments, or organs.9 The right and left lobe are separated by a plane extending vertically through the gallbladder fossa and middle hepatic vein. The right anterior and posterior segments are divided by a vertical plane through the right hepatic vein (V and VIII anteriorly from VI and VII posteriorly). The left hepatic vein and falciform ligament separate the left lateral and medial segments (II and III from IV). A plane of the main right and left PV demarcates superior from inferior segments (VII and VIII from V and VI).9 Radiation oncologists should become familiar with this nomenclature (Fig 4) because it will aid in descriptions of multiple tumors and in communication with other specialists.
For liver contouring the gallbladder should be excluded. The IVC should be excluded from the liver contour when it is discrete and separate from the liver. On the right side of the liver, the caudate has a small process (“tail”’), which separates the portal vein from the IVC and forms a bridge between the caudate lobe and right lobe11 (Fig 1A). For “simple” contouring of the liver, rather than excluding all vessels from the liver contour, the consensus recommendation is that the PV should be excluded from the liver contour only when segment I is present posterior to the PV. The PV can be included in the liver contour when segment I is left of the PV (Fig 1B). Branches of the PV and other vessels should be included within the liver contour.
Common bile duct
The CBD (usually measuring 8–10 cm in length, 5–6 mm in diameter) is the common drainage pathway of the cystic duct of the gallbladder and common hepatic duct (about 4 cm in length). The CBD contour should start at the first bifurcation or at its entry to the portal triad inferiorly to the first portion of the duodenum. It passes posterior and medial to the duodenum and joins with the pancreatic duct to form the ampulla of Vater, draining into the second portion of the duodenum by the major duodenal papilla.9
Irradiation of caudate lobe liver tumors may lead to high radiation doses being received by the CBD. Although there are no clear dose constraints for avoiding CBD stricture, it is reasonable to avoid hotspots over the CBD.
Pancreas
The pancreas sits at the level of L1–L3. The pancreatic head is located to the right of the SMA. The uncinate process, an extension of the pancreatic head, is posterior to the SMV.11 The pancreatic body is between the celiac trunk and SMA, where it lies anterior to the aorta (Figs 3D; 4D;E).
Jejunum and ileum (“small bowel” a la RTOG nomenclature) and colon
The simplest path to achieving the correct small bowel and colon contours is to track the bowel slice by slice without the intertwining mesentery from proximal to distal bowel (or vice versa) (Figs 2; 3C–E). Another option is to contour the “bowel bag,” which incorporates all portions of the peritoneal cavity aside from non-bowel structures.2 The bowel bag technique may be useful in instances during which no small bowel oral contrast was used, there is a small proportion of large bowel in the upper abdomen, or to avoid hotspots during IMRT planning. The panel’s consensus is the bowel bag technique is not routinely recommended in place of small and large bowel contouring for upper abdomen treatment planning, as the maximal doses to the large and small bowel are clinically important.
Adrenals
Each adrenal gland sits superior and medial to the kidneys, appearing as inverted Ys or Vs on axial images. The right adrenal gland is nestled among the liver to the right, the crus to its left, and the IVC anteriorly. The left adrenal gland is bordered by the crus to the left, the pancreas anteriorly, and the kidney medially9 (Fig 4D;E).
Kidneys
The renal pelvis consists of a funnel-shaped expansion of the upper end of the ureter, which collects from the major calices. The renal cortex is the outer portion.9 The renal pelvis is generally included in kidney contours for upper abdominal malignancy radiation therapy (Figs 3A–C; 4D;E).
Chest wall
Consistent with chest wall contouring guidelines from the lung normal tissue atlas,3 the chest wall contour can be created by a 2 cm auto-expansion of the liver and lung in the anterior, posterior, and lateral directions to include the ribs as well as soft tissues of the chest wall, excluding the sternum and the vertebral bodies.3
Major vessels
Portal vein
The PV is formed behind the pancreatic neck by the intersection of the SMV and SV9 (Fig 3E). The PV is located posterior to the CBD and hepatic artery (Fig 4E). The PV bifurcates into the right posterior portal vein, right anterior PV, and left PV (Fig 4D; E). The PV divides the liver anatomy into superior and inferior segments. The bifurcation may be extrahepatic (26%), at the liver capsule (26%), or intrahepatic (48%).14
Superior mesenteric vein
The SMV lies to right of and slightly anterior to the SMA, unites with SV at the confluence of SV and SMV to form the PV behind the pancreatic neck (Fig 3B). SMV and SMA are located behind the pancreatic neck and anterior to the third part of the duodenum.9
Celiac artery
The CA has 3 branches consisting of the left gastric, splenic, and CHA. The CHA gives rise to the gastroduodenal and proper hepatic arteries.15
Superior mesenteric vein
The SMA lies 1 cm caudal to the celiac artery, posterior to the body of the pancreas and the splenic vein (Fig 3A–C). The SMA usually arises at the level of the lower portion of L1.
Common hepatic artery
The CHA arises from the CA, which branches into the proper hepatic artery followed by the right and left hepatic arteries, which supply the corresponding lobes of the liver9 (Fig 4E).
Hepatic vein
Hepatic veins, formed by the union of the central veins, drain blood from the liver into the IVC just below the diaphragm. Superior and inferior hepatic veins drain blood from the left and right lobes of the liver, respectively. The caudate lobe is usually supplied by the MHV. The IVC bifurcates into the RHV, MHV, and LHV9 (Fig 4B).
The axial slice images of the RTOG upper abdominal normal organ contouring atlas may be viewed at http://www.rtog.org/CoreLab/ContouringAtlases/UpperAbdominalNormalOrganContouringConsensusGuidelines.aspx.
Discussion
One of the hurdles facing the safe implementation of IMRT and SBRT is contouring of elective target volumes and normal surrounding critical organs. The standardization of normal organs and vessel contouring definitions provided in this manuscript and atlas afford a basis for prospective studies of conformal radiation for upper abdominal malignancies and establishes contouring guidelines for radiation oncologists. Uniform contouring of organs at risk may allow better quantification of dose–volume histogram-toxicity relationships that are important not only in radiation dose intensification, but also with intensification of other therapies, including targeted therapies.
The panel demonstrated excellent overall concordance in the liver, duodenum, and stomach contours and contouring definitions. However, liver segment I was often incompletely contoured. The fourth portion of the duodenum, GEJ, and CBD were less reproducibly contoured, highlighting that these regions may be more challenging to see and delineate on axial CT imaging and suggests the need for educational aids such as this one. Noncompletion of the CBD contours from all the experts (9 submitted) is probably related to inexperience and uncertainty in contouring as it is not routinely contoured in radiation planning. Training and peer review may aid in accuracy of contouring.
Inconsistency of dose–volume guidelines for toxicity can result from variable normal tissue contouring. For example, investigators from the William Beaumont Hospital (Royal Oak, MI) contoured the full duodenum with gemcitabine and radiation therapy (36–42 Gy/15 fractions), with or without erlotinib (30–38 Gy/2 Gy per fraction), for pancreatic cancer; the V25 and V35, respectively, predicted gastrointestinal bleeding in the setting of 2.4 or 2.8 Gy/day.16 Investigators from Stanford University (Stanford, CA) generated a dosimetric model of duodenal toxicity after pancreatic SBRT. In contrast, the duodenal contour was defined as 1.0 cm beyond the superior and inferior extent of the PTV (planning target volume), and 3 cm medial, lateral, anterior, and posterior to the PTV. Using a single fraction of 25 Gy, multiple dose–volume histogram endpoints, including a V15 of ≥9.1 cm3 versus a V15 of < 9.1 cm3, gave duodenal toxicity rates of 52% and 11%, respectively.17 We recommend contouring the entire duodenum so dose–volume limits may be more easily compared.
Many of the structures included in this atlas do not need to routinely be contoured in radiation oncology planning (eg, adrenal glands, vessels), but awareness of the location of these normal tissues is important.
Limitations of this work include that the CT data set is “ideal,” obtained in breath hold. The quality of imaging, use of contrast, and breath hold is unlikely to be as consistently good for all radiation planning CT scans. Variant anatomy and postoperative anatomy have not been addressed.
Conclusions
These consensus directives and atlas should serve as a template for normal organ contouring in radiation treatment planning for upper abdominal malignancies. This standardization should allow for normal organ uniformity among clinicians and treatment protocols such as the RTOG 1112 study, which should lead to a better understanding of the dose–volume toxicity relationships for upper abdominal tissues.
Acknowledgments
Sources of support: Supported by grants CA81647 and NCIU24 from the National Cancer Institute.
The authors are grateful to Laleh Melstrom, MD, hepatobiliary surgical oncologist, for input regarding the liver segmental anatomy.
Footnotes
Conflicts of interest: None.
References
- 1.Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1442–1457. doi: 10.1016/j.ijrobp.2010.07.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman KA, Regine WF, Dawson LA, et al. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int J Radiat Oncol Biol Phys. 2012;83:901–908. doi: 10.1016/j.ijrobp.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 6.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Lozi R, Li XA, White J, et al. Tools for consensus analysis of experts’ contours for radiotherapy structure definitions. Radiother Oncol. 2010;97:572–578. doi: 10.1016/j.radonc.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 9.Federle MP, Rosado-de-Christenson ML, Woodward PL, et al. Diagnostic and surgical imaging anatomy: chest, abdomen, pelvis. 1. Salt Lake City: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 10.Bombeck CT, Dillard DH, Nyhus LM. Muscular anatomy of the gastroesophageal junction and role of phrenoesophageal ligament; autopsy study of sphincter mechanism. Ann Surg. 1966;164:643–654. doi: 10.1097/00000658-196610000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore KA. Clinically oriented anatomy. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 12.Rossi P, Ricci P, Broglia L. Portal hypertension. Berlin, Germany: Springer; 2000. [Google Scholar]
- 13.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000;2:333–339. HPB (Oxford) 2002;4:99–100. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz SR, LaBerge JM, Gordon RL, Warren RS. Anatomy of the portal vein bifurcation: intra-versus extrahepatic location–implications for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1994;5:457–459. doi: 10.1016/s1051-0443(94)71529-3. [DOI] [PubMed] [Google Scholar]
- 15.Gray H. In: Anatomy of the human body. 20. Lewis WH, editor. New York: Bartleby.com; 2000. [Google Scholar]
- 16.Huang J, Robertson JM, Ye H, Margolis J, Nadeau L, Yan D. Dose-volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;83:1120–1125. doi: 10.1016/j.ijrobp.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]