Abstract
Dual-systems theories explain lapses in self-control in terms of a conflict between automatic and deliberative modes of behavioral control. Numerous studies have now tested whether the brain areas that control behavior are organized in a manner consistent with dual-systems models. Brain regions directly associated with the mesolimbic dopamine system, the nucleus accumbens (NAcc) and ventromedial prefrontal cortex (vmPFC) in particular, capture some of the features assumed by automatic processing. Regions in the lateral prefrontal cortex (lPFC) are more closely linked to deliberative processing and the exertion of self-control in the suppression of impulses. While identifying these regions crudely supports dual-system theories, important modifications to what constitutes automatic and deliberative behavioral control are also suggested. Experiments have identified various means by which automatic processes may be sculpted. Additional work decomposes deliberative processes into component functions such as generalized working memory, reappraisal of emotional stimuli, and prospection. The importance of deconstructing dual-systems models into specific cognitive processes is clear for understanding and treating addiction. We discuss intervention possibilities suggested by recent research, and focus in particular on cognitive training approaches to bolster deliberative control processes that may aid quit attempts.
Keywords: dual systems, fMRI, cognitive training, dopamine system, prefrontal cortex, executive function
Introduction
Addiction is at its core a problem of self-control.1 The compulsion to take drugs often overwhelms the intention and desire to improve health and life circumstances. Treatment is sought or quitting is initiated when significant negative life events arise (e.g., poor health, employment loss, criminal charges, financial difficulties, significant health outcomes) from that compulsion to use drugs.2 Drug abstinence is also associated with a clear sense of conflict, particularly during attempts at quitting, such that a persistent, instinctual compulsion to use impedes the desire to abstain from drugs.
Conflict between compulsions and longer-term interests is not unique to drug abuse. Instead, this conflict is a component of daily life and accompanies everything from mundane decisions (e.g., “Should I have dessert?”) to more profound choices (e.g., “Should I cheat on my spouse?”). Individual differences in the ability to overcome temptations has likewise been shown to predict a number of life outcomes, ranging from success in school to rates of obesity and divorce.3,4 For addiction, the hope is that understanding the psychological basis of conflict between immediate temptations and longer-term goals will give rise to novel and more effective interventions and treatments.
Investigating self-control in the laboratory was famously first accomplished by Walter Mischel and colleagues, who carried out studies of young children during the early 1970s.5 But for adults, Mischel’s experiments requiring patience to obtain additional desserts are trivial, and the current standard procedure in self-control research is to measure time preference using psychometric methods.6 Participants are asked to serially indicate their preference between pairs of rewards of different amounts available at different time delays. Thus, a person may be asked whether they would prefer to be paid $10 today or $12 in one week’s time. By varying the amount of the rewards, an indifference point can be identified or inferred. The percent difference in the reward amounts at the indifference point indicates the amount that the future reward is discounted at the studied time delay. Varying time delays establishes an individual discount function over a range of delays, and the associated discount rate. The discount rate has proven to be an extremely informative measure from a clinical perspective.7
Discount rates are elevated in a number of psychiatric disorders associated with deficits in self-control, ranging from attention deficit hyperactivity disorder (ADHD)8,9 to schizophrenia.10 This association has led to the suggestion that intertemporal choice paradigms assess a core process fundamental to mental health.11 For addiction, delay discounting and drugs of abuse are so intertwined that we have argued that discount rates ought to be thought of as a behavioral marker of addiction.7
Given the importance of discount rates in addiction research, understanding how delay discounting arises from cognitive and/or brain function is paramount. Investigations into the neurocognitive basis of intertemporal preferences began with the conceptualization of self-control that we started with above. Namely, the apparent conflict between immediate temptation and long-term goals suggests a dichotomy in underlying cognitive and neural processes.12,13 We refer to the systems that underlie these processes as automatic and deliberative herein, although others have used different labels, as discussed briefly in the next section.
We review progress in understanding self-control through the lens of dual-systems models of impulse control and addiction. Our goal is to show how the broad concepts subsumed by the crude automatic and deliberative distinction has given way to a progressively more nuanced understanding of the cognitive processes at play in self-control. Advances in the study of self-control are closely linked to existing and developing approaches to addiction treatment. We therefore discuss studies of self-control with a particular eye toward treatment.
Dual-systems models
Dual-systems models date back millennia. Plato used the term akrasia to refer to the tendency to do things that we know to be against our own better judgment. The famous analogy relating the intrapsychic conflict that accompanies temptation to a rider (deliberative) trying to control a horse (automatic) was developed by Freud into the concepts of the ego and id. In economics, Adam Smith differentiated reason (“impartial spectator”) and the passions.14 In short, the automatic/deliberative dichotomy has been a timeless component of thinking about the human condition.
In psychology, dual-systems models have been distinguished based on their presumed cognitive basis. Mischel and colleagues therefore refer to “hot” and “cold” processes to highlight the common link between automatic control and hot emotions versus the more cool rational basis of deliberation.12 Emotions seem to be a critical feature of temptations and compulsions. Loewenstein argued that self-control challenges only arise when there is a visceral need and an immediately available quencher of that need.13 One consequence of Loewenstein’s insight is that not all immediately available rewards produce impulsivity. Instead, a reward must satisfy some fundamental need state. Not surprisingly, many drugs of abuse target the midbrain dopamine system, an evolutionarily conserved brain network that underlies basic motivational processes activated by survival- and reproduction-related stimuli.15 Likewise, emotion has been an important component of several dual system models explicitly developed to address addiction.16,17 One such model explicitly refers to the competition between what that model calls the impulsive decision system and the executive decision system.18,19
A second line of inquiry suggests a possible normative reason why automatic and deliberative modes of thought may coexist. The critical notion here is that deliberatively reasoning through every decision that we face would be prohibitively burdensome. Instead, automatic processes may enable rapid judgments and decisions that are accurate most of the time.20,21 Automatic judgments can only be adaptive in this sense insofar as they accurately capture important statistical regularities in the world. Deliberation, in turn, should only be necessary when contingencies change in such a way that automatic responses lead to errors. A cost–benefit analysis is implicit in the elicitation of deliberative control; errors must be significant enough to warrant the extra time and effort to slow down and reason through the problem.22 The terms system 1 (automatic) and system 2 (deliberative) capture the hierarchy suggested by this correction-based structure of behavioral control.20
The true value of dual-systems models extends beyond their intuitive appeal. They are also extremely useful as conceptual models to guide investigation into the psychological processes that underlie self-control and healthy, far-sighted behavior. Dual-systems models make several predictions about how to modulate impulsivity. Anything that increases reliance on automatic processing should be associated with increased impulsivity. The classic manipulations along these lines either prime emotional responsivity23,24 to increase automaticity or tax executive functioning25,26 to reduce deliberative control. Extending this line of reasoning is easy: consider how changes in body states such as hunger may render you more emotionally labile and, simultaneously, cognitively depleted,27,28 with consequent effects on self-control. Finally, chronic dysregulation in favor of either the automatic or deliberative systems should be evident in distinct psychiatric disorders. Indeed, psychiatric disorders associated with increased executive (deliberative) control manifest as excessive restraint (e.g., anorexia29) or excessive focus on future outcomes (e.g., obsessive–compulsive disorder30). Similarly, addiction7 and ADHD31 are associated with decreased cognitive resources relative to automatic control.
Insofar as dual system models make predictions about factors that contribute to the waxing and waning of self-control, they should also indicate potential means by which to remedy related problem behaviors. Interventions that train executive functions underlying the deliberative control of behavior should, in principle, restore self-regulatory control to some degree, likely proportional to the extent of dysfunction (e.g., rate dependence).32,33 Distinguishing between hot/automatic and cool/reflective processes suggests interesting targets for interventions to enhance self-control.
Challenges from testing behavior alone
Automatic and deliberative processes are extremely challenging to measure behaviorally. Delay discounting has become a standard measure of self-control, as noted above. Importantly, discount rates only indicate the relative contributions of the dual systems and not their absolute strength.7 More problematic is the fact that manipulating automatic or deliberative processing is not clear-cut. The most highly cited paper taxing working memory with a dual-task design did indeed increase choices for immediate rewards.26 However, the data are equally explainable by positing that performing the secondary task simply increased response errors on the delay-discounting task.34 Similarly, affective priming does increase discount rates,23 but the effect is notoriously fickle35 —and this fickleness should be expected. If people attend to the fact that their emotional state is attributable to a specific cue, then that attribution can eliminate any effect on delay discounting. Schwartz and Clore36 famously demonstrated such effects: in one of their studies, asking people what the weather is like was sufficient to eliminate a clear impact of weather on perceived well-being.
We are wary of being overly dismissive of behavioral studies. In the case of addiction, withdrawal is a potent cause of increased affective responsivity. Giordano et al.37 showed increased discount rates for money during states of opiate deprivation. Field et al.38 showed a similar effect with cigarette smokers. Similarly, deliberative control decreases with repeated exertion, a phenomenon known as ego depletion,28 with significant behavioral consequences. We expect that the persistent demand to suppress the desire to use drugs could significantly impair deliberative control. However, again, awareness of ego-depletion effects can mitigate or eliminate the behavioral consequences of depletion manipulations.39,40 Note that these concerns do not present fundamental challenges to the suppositions of dual-systems models; they just imply that elucidating the mechanisms governing behavior in any circumstance may be challenging from studying the behavior alone. In such cases, identifying the neural substrates of automatic and deliberative behavioral control may clarify mechanisms, since this would potentially allow for direct measurement of the underlying processes. Neuroimaging could thereby provide an empirical basis by which to more precisely define the functions subsumed by automatic and deliberative reasoning and their interaction—a persistent challenge in dual-systems research to date.21 Behavioral studies clearly indicate that there are two broad pathways for influencing behavior.41 However, as we come to better understand the mechanisms that govern the neural systems associated with each of these pathways, then interventions are bound to become ever more sophisticated.
Contributions from neuroimaging
A long-standing hypothesis about the neural basis of automatic and deliberative control dates back to early conceptions of brain organization. Paul MacLean’s42 “triune brain” distinguished between the paleomammalian limbic system, a potential locus for emotions, and the neocortex, which supports higher-level cognition. The classical functions associated with the limbic system and the neocortex (particularly the frontal cortex) suggest a clear mapping onto automatic and deliberative behavioral control, respectively. This mapping has been appealing enough to leak into economic theory.43 Of course, neither the brain nor behavior is simple enough to succumb to such a simple mapping. The limbic system, in particular, has been criticized as a useless categorical term for describing brain function, and the link to emotions is certainly strained.44 The association between the prefrontal cortex and cognitive control/executive function is more clear.45 Damage to prefrontal cortex impedes the ability to maintain and guide behavior based on goals.46 The result of such prefrontal dysfunction is overreliance on automatic responses to environmental stimuli, producing behavior termed “environmental dependency syndrome.”47
For rewards like drugs of abuse, the automatic–limbic mapping has given way to a focus on the midbrain dopamine system. Early brain-stimulation studies identified dopamine in mediating reward-seeking behaviors that bared similarity to those observed with addiction.15 Direct recordings from dopaminergic neurons in awake behaving primates led to a refined theory about how dopamine function is connected to behavior.48 Dopamine release is thought to signal a prediction error that communicates differences between expected and received rewards.49 In the striatum, dopamine is therefore believed to function in part as a teaching signal, biasing synaptic changes to strengthen cortical inputs that trigger actions leading to reward.50 With sufficient experience, dopamine-mediated striatal learning gives rise to stereotyped or habitual behaviors51 like those associated with automatic behavioral control.
McClure et al.52,53 provided the first evidence that activity in brain regions associated with the dopamine system biases behavior toward more impulsive responding in delay discounting. Numerous subsequent studies have improved on these findings. For example, one might expect that individual differences in dopamine system function predict differences in discount rates. Indeed, responses to the unexpected receipt of reward in the nucleus accumbens (NAcc; in the ventral striatum) correlates with differences in impulsivity across people.54 Pharmacological manipulation of the dopamine system, through administration of dopamine precursor l-DOPA, also increases impulsivity.55 These findings, by themselves, are informative for addiction research. Addiction is associated with depressed striatal dopamine function,56 which may be related to increased reward-seeking behaviors.
A similarly positive line of research links lateral prefrontal cortex function to more patient behavior in delay discounting. McClure et al.53,57 showed that greater prefrontal cortex activity predicts a higher percentage of choices for delayed rewards under conditions of temptation. The role of the lateral prefrontal cortex (lPFC) in self-control was further refined with the finding that the lPFC appears to modulate behavior by downregulating value-related responses in the ventromedial prefrontal cortex (vmPFC)58,59 —a cortical region including the orbitofrontal, ventral anterior cingulate, and subgenual cingulate cortices that receives dense inputs from the midbrain dopamine system. Instructing participants to increase control enhances lPFC activity,60 and individual differences in prefrontal cortex function predict differences in discount rates.61 The lPFC implements control in part by biasing processing in other areas of the cortex, principally through connections with the posterior parietal cortex (pPC).45 The lPFC–pPC network has been directly related to impulsivity in both controls and alcoholics.62
While links between the NAcc/vmPFC and automatic behavioral control and the lPFC/pPC and deliberative control have largely been supported, important discrepancies have also arisen. In our opinion, discrepancies were inevitable. Precise definitions of automatic and deliberative behavioral control are difficult to develop, for the reasons outlined in the previous section. Linking fuzzy concepts to specific brain regions is likewise necessarily problematic. Nonetheless, we believe that even the crude connection has tremendous theoretical and translational power. Establishing an initial link between automatic and deliberative control and the brain opens the door to studies that directly measure from these brain systems to refine what properties automatic and deliberative control have, at least in the case of delay discounting. This approach has already been fruitful, and the remainder of this article elaborates on the progress that has been made along these lines. Specifically, unexpected features of automatic behavioral control have been identified that are reflected in NAcc/vmPFC function and have led to novel manipulations that alter automatic behaviors and reduce impulsivity. Intervention domains are summarized in Figure 1 and are addressed sequentially in the sections that follow. Similarly, deliberative control has been decomposed into component processes, which likewise may be independently targeted by interventions (Fig. 1).
Figure 1.
Dual-systems models of delay discounting posit that cognitive processes can be distinguished by automatic and deliberative modes of behavioral control. Neuroimaging research has linked automatic and deliberative processes to the NAcc/vmPFC and the lPFC/pPC, respectively. However, this research has also shown that automatic valuation processes can be modified by choice framing, temporal attention, and the perceptual fluency of the prospective rewards. Deliberative processing is dependent on overall capacity constraints that govern the extent to which prospection and flexible appraisal of rewards can be used to guide behavior.
Reward framing
A fundamental notion in automatic behavioral control is its stereotypy. Automatic behaviors are thought to develop slowly through the regular co-occurrence of stimuli and reinforcers. The link between automatic processes and the dopamine system bolsters this associative learning foundation; dopaminergic neurons respond to reward-predicting cues through a process of conditioning49 that occurs outside of awareness.63 The notion of acquired stereotypy is fundamental to drug addiction as well. Environmental cues associated with drug use trigger activation of the dopamine reward system and craving.64,65 Similar effects are evident in problem gamblers.66 The stereotypy of the cue–response link has been a fundamental component of dual-systems models. Kahneman20 used an analogy with visual illusions that illustrate its power. Even when we know cognitively that two tables are the same size (as in the Shepard illusion), we cannot help but see the vertically oriented table as elongated. Specific environmental conditions must be met (table orientation) to give rise to an unavoidable behavioral effect (elongated table appearance).
Stereotypy is strained when applied to delay discounting. Consider the importance of immediacy, for example. Immediate rewards have been presumed to have special provenance because learning mechanisms underlying conditioning function best over short time delays. The fact that discount rates are greater over near-term delays than over longer-term delays supports the importance of immediacy.52 Yet many behaviors associated with near-term impulsivity can be expressed for monetary rewards at delays of several months.67 The critical manipulation is to frame the rewards so that a payment in several months is perceived as the soonest possible. Once this manipulation is pointed out, the ubiquity of the concept becomes clearly apparent. In almost all studies of monetary intertemporal choice, immediate rewards are truly only available after quite some delay—at least as long as it takes to complete the experiment and fill out the form to obtain payment. When shopping, people pay a premium for overnight delivery of purchases. Similarly, drug cues elicit craving and behaviors to obtain drugs that play out over many minutes to hours. Clinically, drug addicts are often characterized by persistent and prolonged demands to satisfy visceral desires.
The fundamental insight from this line of discussion is the surprising flexibility in the drivers of automatic responses. Choice framing can therefore have a potent effect on determining whether automatic compulsions are triggered. This framing effect has not yet been exploited in addiction interventions, but we believe that building off this line of work will eventually enable creative language choices that frame rewards in a way that helps stave off compulsions.
A second key feature of framing research is that the flexibility of behavioral impulsivity translates to activation of brain areas associated with the dopamine system as well. Responses in the NAcc and vmPFC both track the soonest possible reward.67 Not only does this correspondence bolster the link between the dopamine system and automatic behavioral control, but also it suggests the tantalizing possibility that the stimulus attributes that predicted dopaminergic activation may be isomorphic with those that predict impulsivity.
Fluency
The fact that future rewards can be reframed to be more viscerally appealing should not be taken to mean that slowly acquired automaticity is not critical. Experience with stimuli as abstract as particular typefaces produces perceptual fluency and a sense of familiarity that has direct impact on affect and judgments.68 We have recently shown that similar effects of stimulus fluency are evident in delay discounting as well.69
Perceptual fluency with monetary rewards differs between amounts that we consider commonly (e.g., $25) versus those that are somewhat obfuscated (e.g., $25.18). To investigate the consequences of this, we first asked people to indicate how positively aroused they would feel after winning a rounded dollar amount (e.g., $25) and after winning a decimal value (e.g., $25.18). Even though the decimal values were greater, people expressed stronger positive emotions for the rounded values.69
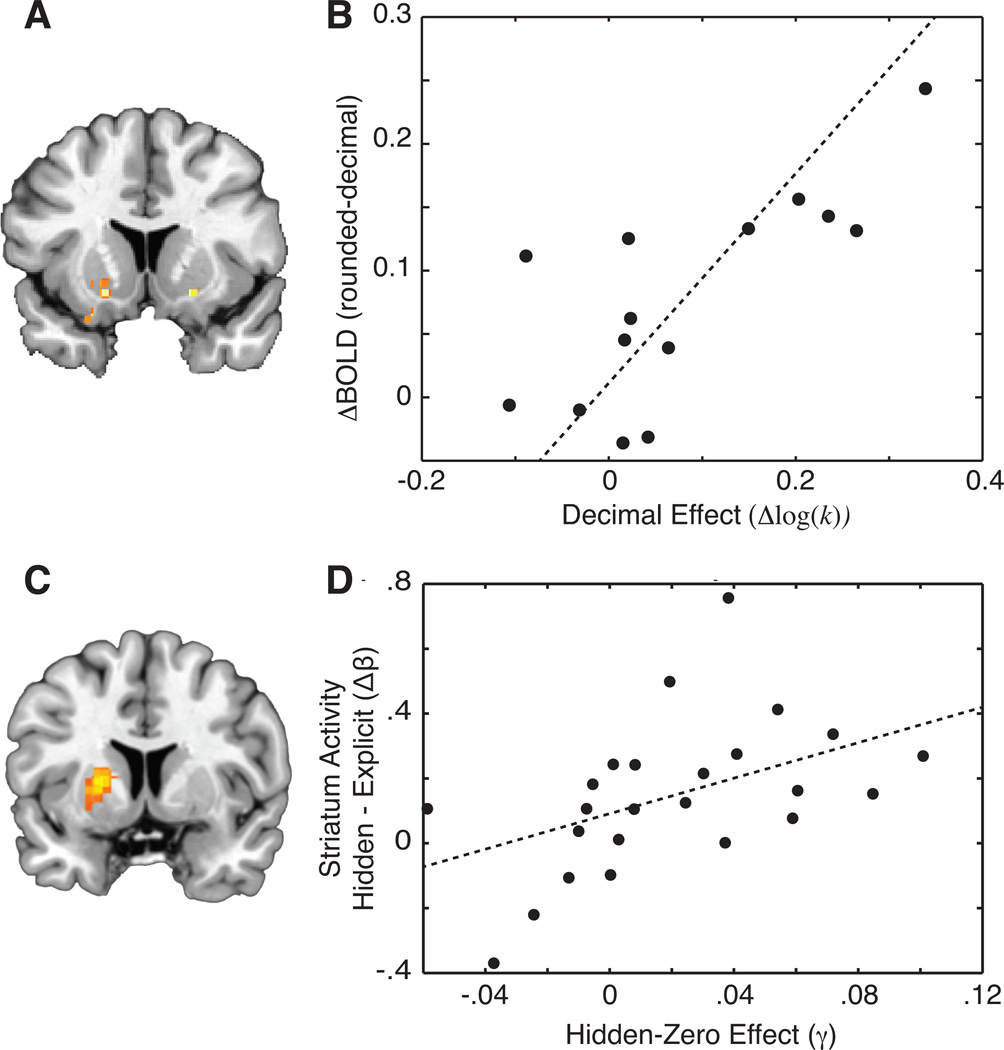
We interpret this decimal effect to indicate the importance of reward fluency on affective and behavioral responses. We found that discount rates were greater when choices were made between rounded-value rewards than payments with non-zero decimal values; this behavioral effect translated to brain activation as well. Obfuscating the amounts of rewards reduced responses in the NAcc (Fig. 2A) in a manner that predicted a shift to more patient behavior across participants (Fig. 2B).69
Figure 2.
Flexibility of automatic valuation processes. (A) Perceptual fluency affects the degree to which the striatum responds to immediate rewards. By changing rewards to include non-zero decimal values (e.g., $10.00 vs. $10.21), discount rates are decreased. (B) The impact of this change on discount rates is correlated with the size of the change in measured NAcc activity across subjects. (C) Contrary to the assumed stereotypy of automatic processes, choice framing can also be used to decrease striatal responses to immediate rewards. In this case, rewards were reframed to include zero-valued rewards so that “$10 now” becomes “$10 now and $0 in a week,” for example. (D) The degree to which dorsal and ventral striatum responses are altered by the inclusion of null outcomes predicts the effect of framing on delay discounting. (A) and (B) were adapted from Ref. 69; (C) and (D) were adapted from Ref. 78.
Clinically, making use of the impact of reward fluency could prove productive. We tested how the decimal effect on discount rates translates to ADHD. Indeed, using non-zero decimal values reduced measured discount rates in ADHD individuals, bringing them closer to the baseline discount rates measured in controls.69 Conceivably, obfuscating the perception of drugs by some means may, in turn, lessen their visceral impact and facilitate abstinence.
Attention
One reason why immediate rewards produce impulsivity may be their ubiquity.70 The commonality of rewards in the present may support acquisition of learned affective responses, as suggested previously, but may also increase the availability of immediate rewards in memory, supporting automatic evaluations.70 Insofar as our daily behavior is directed towards near-term goals and objectives, a natural bias of attending to the present may develop naturally. The observation that people have a default present bias is well recognized.71
Chronic drug use implies prolonged attention to immediate visceral needs at the expense of attention to longer-term goals. Therefore, addiction may be expected to increase present bias. A study testing this hypothesis asked control and heroin-dependent subjects to complete narratives such as, “After awakening, Bill began to think about his future. In general, he expected to…”72 Responses were coded for how long in the future the completed narratives were stated to occur. Control subjects generated stories that occurred an average of 4.7 years in the future. In heroin addicts, the mean considered future was only 9 days from the present. We have argued that one important component of addiction is therefore a narrowing of temporal attention so that future outcomes are often excluded from consideration.73
The clinical relevance of temporal attention is potentially profound. Treatments appealing to long-term consequences of addiction may feel impossibly remote to effectively influence addicts. Instead, treatments must acknowledge and incorporate present focus to be beneficial. One example of a treatment program that embraces the present is contingency management (CM).74 CM operates by working in the short time frame that addicts and individuals at risk for addiction can handle.75 If addicts are drug free for two days, they get reinforced. Therefore, CM may establish a proximal reference that progressively renders drug use somewhat delayed and makes abstinence easier to maintain. CM’s effectiveness may be consistent with a report looking at cross-commodity discounting, where cocaine-dependent individuals showed the greatest amount of discounting when money was offered now and cocaine was offered later.76
Manipulating how attention is distributed across time may have consequences for how choices are made in the present moment as well. One manipulation that appears to work this way is known as the hidden-zero effect.77 The idea behind the hidden-zero manipulation is that asking people whether they prefer, for example, $10 now or $12 later invites consideration of the immediate payment in isolation. This isolated consideration of immediate reward likely interacts with present bias and acquired affective responses to promote impulsivity. Reducing the ability to focus on an immediate reward alone may promote improved future-oriented decision making. The hidden-zero effect therefore presents rewards so that immediate outcomes are always associated with delayed outcomes. Choices are constructed to ask for preferences between “$10 now and nothing later or nothing now and $12 later.”77 Note that this manipulation does not change the prospective payments but instead links a null future outcome to immediate consumption. People are significantly more patient when choices include such “explicit zeros.”71,77,78
We have argued that the hidden-zero effect derives from the fact that forcing people to consider future outcomes reduces present bias and increases attention to the future.71 A natural extension to this argument is to ask whether immediate bias in brain reward responses are similarly influenced by the explicit zero presentation; we recently found this to be the case. Explicitly stating zero-valued outcomes reduced activity in the striatum to immediate rewards78 (Fig. 2C), and the degree to which striatum activity was reduced predicted the size of the behavioral effect across subjects (Fig. 2D). Perhaps more critically, we found that reducing reward responsivity using explicit zero framing was able to promote self-control without requiring additional cognitive effort (assayed behaviorally and through lPFC activity). This latter finding suggests that temporal attention may be particularly useful for addiction interventions owing to the well-documented fatigue effects on willpower and the deliberative control of behavior.79 Moreover, drug taking can be conceptualized as “drug now with negative effects later” instead of dissociating the immediate rewarding and later punishing consequences of the choice. If addicts can learn to bundle outcomes in this manner, then our data suggest that this would ameliorate the heightened present bias evident with addiction.72
Automatization
A clear distinction exists in behavioral control between actions generated for the sake of obtaining desired outcomes (deliberative) and actions generated out of habit (automatic). In the first case, decision making is more flexible, depends on making use of known causal structure in the environment,51 and can be quickly extinguished when outcomes are not desired.80 In the latter case, habitual behaviors are evoked by environmental stimuli directly and (at least at the present moment) independent of the desirability of the outcome. Examples of both forms of behavioral control are easy to introspect from daily life, as is the annoyance of habitually responding only to have to deliberatively correct the undesired outcome.
Neuroscience has long been interested in understanding which brain systems underlie habitual and goal-directed behavioral control as well as the mechanisms governing which systems underlie behavior at any given time.81 Some general principles are clear. First, goal-directed behavior depends on a network of brain regions including the lateral PFC, the medial temporal lobe (MTL), and regions of the default mode network. The MTL and the default model network are thought to be critical for constructing potential future scenarios,82 which can then be evaluated in comparison to one’s goal. The lateral prefrontal cortex is also implicated in this process.83 The role of the lateral PFC is probably to direct retrieval from the MTL, so as to strategically generate and navigate possible future outcomes. Second, habitual control depends on parts of dorsal striatum.81 Third, transfer of motor control from goal-directed to habitual control occurs reliably after extensive training.84 One hypothesis for what governs this transfer relates to how reliable each control system is.51 After extensive training, habits are expected to produce desirable outcomes most of the time because the behaviors that underlie the habits have proven reliable in acquiring reward.
The literature on habit acquisition is critical to consider with regard to delay discounting. Consider again the type of question posed as part of a delay-discounting experiment: participants are asked variants on whether they prefer $10 today or $12 next week. Real-life choices rarely if ever are so discrete, and instead are significantly more complicated, variable, and probabilistic in nature. Given the novelty of this laboratory task, we expect that most experiment-naive participants will approach such decisions using goal-directed deliberative mechanisms to try to arrive at a reasonable choice strategy. Experienced subjects may be expected to have adopted a more efficient habit-like strategy or decision heuristic.
No direct evidence exists for the transfer from deliberative to automatic/habitual strategies in delay discounting. Clear evidence of the consequences of habitization exists in the literature. To see this, we first note that producing habitual control of behavior can be accomplished by having participants complete the same laboratory task in a relatively small number of laboratory sessions, where each session includes many individual trials.85 A well-known study by Kable and Glimcher86 used such a paradigm to study delay discounting: their participants completed multiple laboratory sessions of a delay-discounting task before undergoing functional brain imaging to study brain responses associated with delay discounting. Moreover, Kable and Glimcher explicitly excluded subjects who did not show consistent behavior across sessions, potentially increasing the proportion of participants under automatic behavioral control. Previous research on automatic and habitual behavioral control suggests that habitization should lead behavior to be controlled principally by brain reward systems (the NAcc and vmPFC) and other circuits linked to the basal ganglia. Kable and Glimcher’s study found precisely these brain areas.86 Behavior in their task could be fully accounted for by the pattern of responses measured in the NAcc and vmPFC.
As with temporal attention, the importance of habitization for addiction treatment is potentially high. Many treatment approaches, such as cognitive behavioral therapy (CBT), introduce future-oriented behavioral strategies. However, in our view, CBT appeals to deliberative behavioral control. Insofar as addiction involves choices that have become habitized, behavior may be generated independent of deliberative control—as potentially found in the Kable and Glimcher study.86 Not surprisingly, CBT, although more effective than control conditions, has limited efficacy.87 Instead, as automatic control correlates with increased discount rates, delay discounting at the beginning of treatment ought to be predictive of therapeutic outcomes—as we have found.88–90 Those people who discount the most and may exhibit habitized behavior respond the least to treatment.
Cognitive capacity and cognitive training
Dual-systems models posit that impulsivity is counteracted by deliberative behavioral control that is able to formulate long-term goals and bias behavior toward accomplishing these goals. Goal-related capacities fall under the title of executive function or cognitive control, have long been associated with function of the lPFC, and, as mentioned earlier, consist of several component processes.19 Goals must be selected, maintained through potential distractions, and used to influence ongoing behavior, while behavior must be evaluated with respect to accomplishing the selected goals. The lPFC has been associated with all of these component processes.45 One view of the lPFC and associated cortical regions (i.e., the pPC) is that it is a large, general-purpose region of cortex that may be flexibly used to accomplish any of a myriad of control functions.91
The generality of the lPFC in supporting executive functions suggests that, if cognitive control is strengthened in one domain, then the effects may generalize to other domains, since there is a shared neural substrate.92,93 This generalization effect was famously demonstrated by Jaeggi and colleagues.94 In this study, participants performed an increasingly challenging dual n-back task in numerous sessions spanning 1–2 weeks. The investigators showed that this working-memory training improved fluid intelligence as measured by Raven’s progressive matrices.
Subsequent work on cognitive training has highlighted the difficulty inherent in generalizing the effect of training to other domains. The most common finding in the literature is that cognitive training improves performance on the trained task but does not transfer to other cognitive domains.95,96 These findings do not mean that transfer is purely wishful thinking. In many cases, transfer effects or the lack thereof may be related to the degree of executive dysfunction. The effects of transfer have been documented among schizophrenics and children with ADHD.97,98 Collectively, these findings may indicate that generalization does not occur because of ceiling effects among otherwise healthy individuals, while transfer may only be large enough to be reliable when executive functions are initially dysfunctional—as may frequently be the case with addiction.19
Despite the mixed behavioral effects related to cognitive training, transfer effects continue to be reported alongside associated changes in brain function.99 Changes in cortical white and gray matter densities have been identified following working-memory training,100,101 as have increases in prefrontal and parietal cortex activity.102 Concomitant increases have also been found for striatal dopamine release.103 This may be important in addiction, given that several studies have documented decreased dopamine concentration and function among those with addiction.56
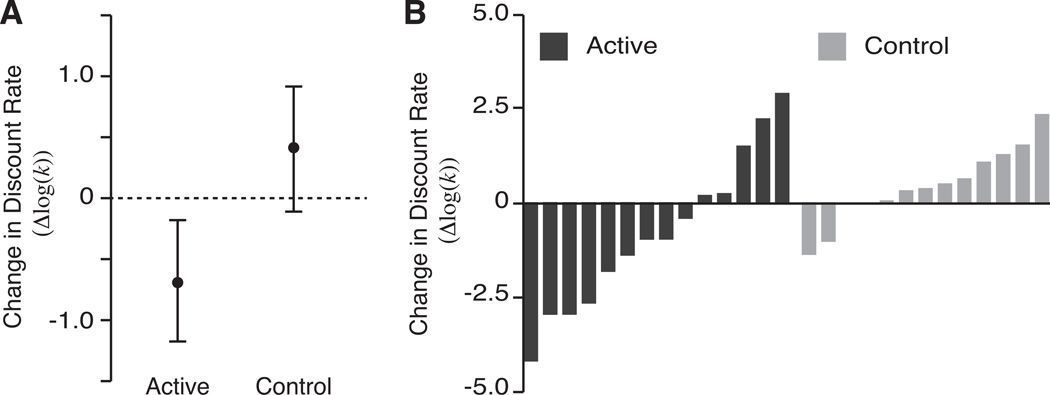
The prevalence of drug addiction is inversely associated with educational attainment and socioeconomic status (SES). Lower educational attainment and lower SES are, in turn, associated with executive dysfunction, and those with drug dependence generally exhibit reduced executive function.104 Thus, efforts to improve executive control may be a useful adjunct to treatment.87 We have shown that a progressive and more challenging working-memory task reduces discount rates among patients in treatment for stimulant use.105 Moreover, the neural correlates associated with working memory and delay discounting overlap.106 The use of working-memory training among problem drinkers has also been shown to reduce their alcohol consumption relative to a control group.107 The long-term success of such efforts in enhancing therapeutic outcomes remains to be determined. However, improving executive-control abilities show promise as a useful adjunct to treatment.
Reappraisal
With improved capacity for cognitive control, attention naturally shifts to cognitive strategies that may be particularly effective at promoting self-control. One set of such strategies falls in the general category of emotion regulation (ER). ER refers to the various strategies that may be used to change how rewards are construed to support behavioral goals.108 We employ numerous ER strategies in daily life. These range from deliberatively avoiding situations where undesired temptations may be high to explicitly suppressing attention to distracting or tempting stimuli.109
One ER strategy that has recently been investigated with respect to self-control is reappraisal.60 Reappraisal refers to explicitly changing the way a situation is perceived. So, viewing an image of a person in pain may be reappraised more positively as an intermediate stage in a process of recovery from a disease or injury. Unsurprisingly, reappraisal depends on the lPFC110 and may be thought of as a particular form of cognitive control. More importantly, increased lPFC activity during reappraisal correlates with reduced activity in brain regions associated with emotional responding such as the amygdala and the vmPFC.110 In choice domains related to self-control, such as choosing between tasty or healthy foods, reappraisal is associated with increased lPFC activity and improved self-regulation.60
For addiction, reappraisal may be thought of as a cognitive control strategy that would promote far-sighted behavior. We suspect that therapies aiming to improve reappraisal would be selectively effective for those individuals with high executive function. Regardless, a critical future direction for research will be to decompose deliberative behavioral control into component cognitive processes and strategies that may support self-control. Emotion regulation captures one class of such processes that demonstrably rely on lPFC and pPC function. Brain imaging has proven effective for establishing this common neural link, and may be an effective tool for assaying who may benefit from therapies aiming to improve ER (i.e., those with high lPFC/pPC function) and who may not.
Prospection
Deliberative control of behavior has been used almost synonymously with the generation, implementation, and maintenance of long-term behavioral goals. However, the temporal distance implicit with long-term goals presents another fundamental obstacle to healthy behavior. When people consider near-term rewards, the benefits are construed in definitive, concrete terms that facilitate rich emotional responses.111 By contrast, future outcomes are generally construed more abstractly and absent the elaboration that seems essential to make them viscerally compelling.111 This distinction in how rewards are construed is easy to intuit. If you are told that you are to be given $20, then your mind easily generates visions of the fancy coffee drink and desert that you can buy with the money. If instead you are told that you will be given $20 in a month, then the uses of the money tend to be more general (e.g., “I’ll buy some food”) and lacking in specific detail.
Elaboration of future outcomes falls in the domain of prospection.112 As discussed above in the section on habitization, generation of plausible future scenarios depends on interactions between the MTL and the default mode network under the control of the lPFC.82,83 Like emotion regulation, we consider prospection to be a special case of cognitive control that is particularly important for self-control. Establishing the link between prospection, the lPFC, and deliberative behavioral control is therefore another instance in which neuroscience can contribute to decomposing deliberative control into component processes.
There have been numerous recent studies investigating the utility of prospection in curbing impulsive behavior. The studies take the general approach of having participants generate specific episodes associated with future dates. Delay discounting is then compared for rewards available at elaborated future times versus those available after other arbitrary delays. Peters and Büchel113 showed that such a manipulation increased MTL and lPFC activity for dates associated with episodic future thinking. Delay discounting was reduced for these choices as well. Moreover, increased MTL–lPFC activity was associated with moderated time dependence in brain reward areas (the NAcc and vmPFC). Lebreton et al.114 extended these findings to show that the manner in which rewards are presented can invite greater elaboration. Showing participants’ rewards as pictures increased hippocampus recruitment and reduced discount rates.
The clinical implications of the role of the MTL in the valuation of future rewards have recently begun to be investigated. Daniel and Epstein115 asked people to consider events in the future that they were looking forward to. They then established tags for these events and showed the tags during a delay-discounting task. As may be expected given the discussion so far, this manipulation reduced discount rates. The exciting extension to this manipulation targeted obesity. Among the obese, similar findings were observed.116 However, when provided access to food, cues of future events reduced food consumption (caloric intake) relative to control cues. Similar episodic tags may help drug addicts by providing an additional means to overcome a restricted window of temporal attention.
Future directions relevant for addiction
One clear benefit of understanding the neurocognitive basis of self-control is in enabling evidence-based individualized treatment. Knowing the source of self-control problems promises to enable targeted interventions. If this seems like too strong of a promise, then, at least, this line of research can help constrain the types of interventions used on different patient groups. There is a flip side to this line of thought as well. Specifically, the efficacy of treatments is predicted to be highly variable across patients. Cognitive behavioral therapy (CBT) or contingency management (CM) may be effective to the degree to which a patient has the requisite cognitive capacity or attention to future consequences of behavior. Clearly, an important line of future research will be in applying recent findings emerging from cognitive neuroscience to the clinic.
A variety of manipulations are known to influence delay discounting that have not yet been studied using functional neuroimaging. For example, social context influences discount rates such that people tend to be more impatient when choosing rewards for themselves than for others.117 This social context effect differs between smokers and problem drinkers,118 indicating that important individual differences may exist with clinical relevance. The importance of social context may further connect with some aspects of group therapy. We feel that there are bound to be numerous routes to influencing automatic and/or deliberative behavioral control that have yet to be discovered or fully explored. Many therapies are likely to touch on a variety of these routes to modifying behavior. Our hope is that more fully understanding the mechanisms that underlie these different treatments will enable more potent novel interventions. This may be a very distant vision, but one that we believe is worth striving for.
Conceptual gaps
The link between automatic and deliberative processes and mesolimbic reward and cognitive-control neural networks is imperfect at best. While many of the influences on self-control have been associated with differential NAcc/vmPFC or lPFC/pPC function, many aspects of automatic functions also are likely to be associated with other neural systems. The dopamine system is closely associated with reward seeking. However, arousal (valence independent) is also closely related to impulsivity.37 The neural substrates of arousal remain somewhat nebulous at this time, with different accounts pointing to the norepinephrine system119 or the amygdala,120 among other candidate brain systems. Similarly, stress depends on the release of glucocorticoids and is an important risk factor for addiction and relapse,121 indicating an alternate pathway by which emotions can affect self-control.
We would be remiss if we did not mention the exciting recent work on the anterior insula with respect to addiction. A recent publication by Naqvi and colleagues122 showed that smokers with acquired lesions to the anterior insula had a very easy time quitting. These findings dovetail with recent work relating the anterior insula to introspection and awareness of body state.123 Perhaps the anterior insula interacts with brain reward systems to translate internal drives to motivated behavior. This may be wishful thinking, but the point we hope to make is that the brain is complex. Many neural systems contribute to the myriad motivations that underlie drug abuse. We have chosen to simplify our discussion for the sake of clear presentation and to reflect what we consider to be the current state of knowledge. Understanding and relating brain and behavior are necessarily going to be more complicated than our crude automatic–NAcc/vmPFC and deliberative–lPFC/pPC scheme indicates. Nonetheless, we believe that this initial dichotomy has proven useful to date.
Two additional caveats need to be stated. First, the role of the vmPFC in behavioral control remains an active area of research. We have grouped the vmPFC with the NAcc as a component of automatic behavioral control. However, the dominant theory of the vmPFC is that it is a convergence point where various factors that determine the value underlying behavior meet.124 Consistent with this, greater vmPFC activity is associated with greater impulsivity,52,58 but there is evidence that the lPFC and pPC function by downmodulating vmPFC responses.58,59 However, recent work argues that the vmPFC functions to extract higher-order statistical relationship between stimuli, behavior, and eventual reward.125 In this view, the role of the vmPFC in intertemporal decision making may be highly dependent on task structure. This might explain why some vmPFC lesions lead to increased impulsivity126–128 and others to decreased impulsivity.129–131 Overall, we suspect that the role of the vmPFC in intertemporal decision making will be significantly revised from the hypothesis we put forth herein.
Second, we have knowingly overstated the dichotomy indicated by the dual-systems approach. The brain is a continuous, parallel information-processing system. Automatic responses may be linked to the mesolimbic dopamine system, but the dopamine system influences behavior by altering cortical activity and is engaged by higher-order cortical regions (i.e., through nigrostiatal and mesocortical pathways). Likewise, the lPFC is believed to be the seat of goal representations that bias other neural processes.45 However, the prefrontal cortex depends on reward responses to determine which goals are relevant and hence which to maintain.132 Like the dopamine system, the lPFC is, therefore, just one stage of a continuous neural system. Thus, not only do two-systems models not capture the full range of each processing stream, but they also suggest an independence of function that simply is not accurate.59
We do not wish to overstate the interrelationship of the dopamine system and the lPFC, however. Dual-systems models are a powerful heuristic for decomposing neural function and linking it to behavior. Moreover, interconnected but functionally distinct pathways are the norm in the brain. The visual system has the magno and parvo pathways (among many). These visual pathways are not entirely independent, and pointing to one set of cognitive processes or one set of neurons and associating them with one system or the other is, at best, difficult. Instead the magno and parvo systems are highly interconnected, like the lPFC–NAcc/vmPFC system. However, the magno/parvo distinction is still extremely useful because it highlights an important contrast in their contributions to perception (e.g., motion versus form). Dual-systems models offer a clear means of communicating an important functional distinction between different neural networks. These models also provide a clear set of hypotheses to test—many of which have now been validated. Finally, these models indicate clear paths for progress. Understanding how automatic or deliberative processes relate to neural systems will almost necessarily force us to refine our theories of what “automatic” and “deliberative” truly mean. In the end, we may end up with functions that are well defined only in neural terms. However, we suspect that these neural terms will preserve the titles “automatic” and “deliberative” or some substitute with the same high-order, vague imprecisions for which “automatic” and “deliberative” may be rightly criticized.
Conclusions
Dual-systems models have proven highly influential in the cognitive neuroscience studies of self-control that have emerged over the past decade. Steady progress has been made along two parallel fronts on the basic science of self-control. First, many studies have now tested the predictions of dual-systems models and have shown a consistent association between automatic behavioral control and brain regions associated with the mesolimbic dopamine system and between deliberative control and the lPFC/pPC. Second, these same studies have required revising what is meant by “dual systems” at the functional level. Specifically, automatic processes are much more flexible than their presumed stereotypy would have predicted, and deliberative control is a multi-component collection of distinct executive processes. We believe that progress along these parallel fronts will continue through a progressive process of bootstrapping. Imperfections both in dual-systems theories and understanding of brain function will continue to be identified, but these imperfections will lead to refinements of theory and more detailed understanding of the mechanisms of self-control. The benefit is bound to be a refined understanding that will benefit development of novel, improved treatments for addiction.
Figure 3.
Working-memory training reduced discount rates among participants being treated for stimulant use. (A) Subjects were included in an active, adaptive working-memory training condition or a control condition. Discount rates decreased with active working-memory training, but not the yoked control over the (on average) 25-day experimental treatment. (B) The change in discounting is shown for each subject in the active and control conditions. Discount rates were decreased in the majority of participants after active working-memory training. Adapted from Ref. 105.
Acknowledgments
This article was supported by NAtional Institute of Health (NIH).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Bickel WK, Mueller ET, Jarmolowicz DP. What is addiction? In: McCrady B, Epstein E, editors. Addictions: a Comprehensive Guidebook, Second Edition. 2nd ed. New York: Oxford University Press; 2013. pp. 3–16. [Google Scholar]
- 2.Bickel WK, Madden GJ, Petry NM. The price of change: The behavioral economics of drug dependence. Behavior Therapy. 1998;29:545–565. [Google Scholar]
- 3.Mischel W, Ayduk O, Berman MG, et al. “Willpower” over the life span: decomposing self-regulation. Social Cognitive and Affective Neuroscience. 2011;6:252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duckworth AL, Seligman MEP. Self-Discipline Outdoes IQ in Predicting Academic Performance of Adolescents. Psychological Science. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- 5.Mischel W. Processes in Delay of Gratification. In: Berkowitz L, editor. Advances in Experimental Social Psychology. Vol. 7. Advances in Experimental Social Psychology. Academic Press; 1974. pp. 249–292. [Google Scholar]
- 6.Mazur JE. In: An Adjusting Procedure for Studying Delayed Reinforcement. Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Hillsdale, NJ: Quantitative Analysis of Behavior: Vol 5. The Effect of Delay and Intervening Events on Reinforcement Value; 1987. pp. 55–73. [Google Scholar]
- 7.Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweitzer JB, Sulzer-Azaroff B. Self-control in boys with attention deficit hyperactivity disorder: effects of added stimulation and time. J Child Psychol Psychiatry. 1995;36:671–686. doi: 10.1111/j.1469-7610.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 9.Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biological Psychiatry. 2010;67:641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cognitive neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bickel WK, Mueller ET. Toward the Study of Trans-Disease Processes: A Novel Approach With Special Reference to the Study of Co-morbidity. Journal of Dual Diagnosis. 2009;5:131–138. doi: 10.1080/15504260902869147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Loewenstein G. Out of control: Visceral influences on behavior. Organizational behavior and human decision processes. 1996;65:272–292. [Google Scholar]
- 14.Ashraf N, Camerer CF, Loewenstein G. Adam Smith, Behavioral Economist. The Journal of Economic Perspectives. 2005;19:131–145. [Google Scholar]
- 15.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 16.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 17.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 18.Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl) 2012;221:361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahneman D. A perspective on judgment and choice: Mapping bounded rationality. American psychologist. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- 21.Evans JSB, Stanovich KE. Dual-process theories of higher cognition advancing the debate. Perspectives on Psychological Science. 2013;8:223–241. doi: 10.1177/1745691612460685. [DOI] [PubMed] [Google Scholar]
- 22.Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergh BVD, Dewitte S, Warlop L. Bikinis Instigate Generalized Impatience in Intertemporal Choice. Journal of Consumer Research. 2008;35:85–97. [Google Scholar]
- 24.Li X. The Effects of Appetitive Stimuli on Out of Domain Consumption Impatience. Journal of Consumer Research. 2008;34:649–656. [Google Scholar]
- 25.Shiv B, Fedorikhin A. Heart and Mind in Conflict: the Interplay of Affect and Cognition in Consumer Decision Making. Journal of Consumer Research. 1999;26:278–292. [Google Scholar]
- 26.Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- 27.Wang XT, Dvorak RD. Sweet Future: Fluctuating Blood Glucose Levels Affect Future Discounting. Psychological Science. 2010;21:183–188. doi: 10.1177/0956797609358096. [DOI] [PubMed] [Google Scholar]
- 28.Danziger S, Levav J, Avnaim-Pesso L. Extraneous factors in judicial decisions. Proceedings of the National Academy of Sciences. 2011;108:6889–6892. doi: 10.1073/pnas.1018033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc. 2012;18:773–780. doi: 10.1017/S1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto A, Steinglass JE, Greene AL, Weber EU, Simpson HB. Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biological Psychiatry. 2014;75:653–659. doi: 10.1016/j.biopsych.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333:959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bickel WK, Landes RD, Kurth-Nelson Z, Redish AD. A quantitative signature of self-control repair: Rade-dependent effects on successful addiction. Clinical Psychological Science. doi: 10.1177/2167702614528162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franco-Watkins AM, Pashler H, Rickard TC. Does working memory load lead to greater impulsivity? Commentary on Hinson, Jameson, and Whitney (2003) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:443–447. doi: 10.1037/0278-7393.32.2.443. [DOI] [PubMed] [Google Scholar]
- 35.Gross JJ. The Future’s So Bright, I Gotta Wear Shades. Emotion Review. 2010;2:212–216. [Google Scholar]
- 36.Schwartz N, Clore GL. Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology. 1983;45:513–523. [Google Scholar]
- 37.Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology (Berl) 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- 38.Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology (Berl) 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- 39.Job V, Dweck CS, Walton GM. Ego Depletion--Is It All in Your Head?: Implicit Theories About Willpower Affect Self-Regulation. Psychol Sci. 2010;21:1686–1693. doi: 10.1177/0956797610384745. [DOI] [PubMed] [Google Scholar]
- 40.Miller EM, Walton GM, Dweck CS, Job V, Trzesniewski KH, McClure SM. Theories of willpower affect sustained learning. PLoS ONE. 2012;7:e38680. doi: 10.1371/journal.pone.0038680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99:32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacLean PD. The Triune Brain in Evolution. Springer; 1990. [Google Scholar]
- 43.Shefrin H, Thaler RH. The behavioral life-cycle hypothesis. Economic Inquiry. 1988;26:609–643. [Google Scholar]
- 44.LeDoux JE. Emotional memory systems in the brain. Behavioural Brain Research. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 45.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. d. [DOI] [PubMed] [Google Scholar]
- 46.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 47.Lhermitte F. Human autonomy and the frontal lobes. Part II: Patient behavior in complex and social situations: the "environmental dependency syndrome". Ann Neurol. 1986;19:335–343. doi: 10.1002/ana.410190405. [DOI] [PubMed] [Google Scholar]
- 48.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 49.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- 51.Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 52.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 53.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 58.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 59.Monterosso JR, Luo S. An Argument Against Dual Valuation System Competition: Cognitive Capacities Supporting Future Orientation Mediate Rather Than Compete With Visceral Motivations. J Neurosci Psychol Econ. 2010;3:1–14. doi: 10.1037/a0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci. 2012;32:13543–13554. doi: 10.1523/JNEUROSCI.6387-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shamosh NA, Deyoung CG, Green AE, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 62.Boettiger CA, Mitchell JM, Tavares VC, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pessiglione M, Petrovic P, Daunizeau J, Palminteri S, Dolan RJ, Frith CD. Subliminal instrumental conditioning demonstrated in the human brain. Neuron. 2008;59:561–567. doi: 10.1016/j.neuron.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garavan H, Pankiewicz J, Bloom A, et al. Cue-Induced Cocaine Craving: Neuroanatomical Specificity for Drug Users and Drug Stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND. Cocaine Cues and Dopamine in Dorsal Striatum: Mechanism of Craving in Cocaine Addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miedl SF, Büchel C, Peters J. Cue-induced craving increases impulsivity via changes in striatal value signals in problem gamblers. J Neurosci. 2014;34:4750–4755. doi: 10.1523/JNEUROSCI.5020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kable JW, Glimcher PW. An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. J Neurophysiol. 2010;103:2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oppenheimer DM, Frank MC. A rose in any other font would not smell as sweet: Effects of perceptual fluency on categorization. Cognition. 2008;106:1178–1194. doi: 10.1016/j.cognition.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Fassbender C, Houde S, Silver-Balbus S, et al. The Decimal Effect: Behavioral and Neural Bases for a Novel Influence on Intertemporal Choice in Healthy Individuals and in ADHD. J Cogn Neurosci. 2014 doi: 10.1162/jocn_a_00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart N, Chater N, Brown G. Decision by sampling. Cognitive Psychology. 2006;53:1–26. doi: 10.1016/j.cogpsych.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Radu PT, Yi R, Bickel WK, Gross JJ, McClure SM. A mechanism for reducing delay discounting by altering temporal attention. J Exp Anal Behav. 2011;96:363–385. doi: 10.1901/jeab.2011.96-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- 73.Bickel WK, Kowal BP, Gatchalian KM. Understanding addiction as a pathology of temporal horizon. Behavior Analyst Today. 2006;7:32–47. [Google Scholar]
- 74.Higgins ST, Silverman K, Heil SH. Contingency Management in Substance Abuse Treatment. Guilford Press; 2007. [Google Scholar]
- 75.Stanger C, Elton A, Ryan SR, James GA, Budney AJ, Kilts CD. Neuroeconomics and adolescent substance abuse: individual differences in neural networks and delay discounting. J Am Acad Child Adolesc Psychiatry. 2013;52:747–755. doi: 10.1016/j.jaac.2013.04.013. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bickel WK, Landes RD, Christensen DR, et al. Single- and cross-commodity discounting among cocaine addicts: the commodity and its temporal location determine discounting rate. Psychopharmacology (Berl) 2011;217:177–187. doi: 10.1007/s00213-011-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magen E, Dweck C, Gross JJ. The hidden-zero effect: Representing a single choice as an extended sequence reduces impulsive choice. Psychological Science. 2008;19:648–649. doi: 10.1111/j.1467-9280.2008.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magen E, Kim B, Dweck CS, Gross JJ, McClure SM. Behavioral and neural correlates of increased self-control in the absence of increased willpower. Proceedings of the National Academy of Sciences. :1–12. doi: 10.1073/pnas.1408991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- 80.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 81.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 82.Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 84.Moors A, De Houwer J. Automaticity: A Theoretical and Conceptual Analysis. Psychological Bulletin. 2006;132:297. doi: 10.1037/0033-2909.132.2.297. [DOI] [PubMed] [Google Scholar]
- 85.Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bickel WK, Quisenberry AJ, Moody L, Wilson AG. Therapeutic opportunities for self-control repair in addiction and related disorders: Change and the limits of changes in trans-disease process. Clinical Psychological Science. doi: 10.1177/2167702614541260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sheffer C, Mackillop J, McGeary J, et al. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2012;21:221–232. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: A strong prognostic indicator of smoking relapse. Addict Behav. 2014 doi: 10.1016/j.addbeh.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stanger C, Ryan SR, Fu H, et al. Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 2012;20:205–212. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 92.Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR. Cognitive Bias Modification and Cognitive Control Training in Addiction and Related Psychopathology: Mechanisms, Clinical Perspectives, and Ways Forward. Clinical Psychological Science. 2013;1:192–212. [Google Scholar]
- 93.Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171:510–522. doi: 10.1176/appi.ajp.2013.13081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychonomic Bulletin & Review. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- 96.Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychological Bulletin. 2012;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- 97.Chacko A, Feirsen N, Bedard A-C, Marks D, Uderman JZ, Chimiklis A. Cogmed Working Memory Training for youth with ADHD: a closer examination of efficacy utilizing evidence-based criteria. J Clin Child Adolesc Psychol. 2013;42:769–783. doi: 10.1080/15374416.2013.787622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biological Psychiatry. 2014;75:361–370. doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 99.Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Developmental Cognitive Neuroscience. 2012;2:S167–S179. doi: 10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeuchi H, Kawashima R. Effects of processing speed training on cognitive functions and neural systems. Rev Neurosci. 2012;23:289–301. doi: 10.1515/revneuro-2012-0035. [DOI] [PubMed] [Google Scholar]
- 102.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 103.Bäckman L, Nyberg L, Soveri A, et al. Effects of working-memory training on striatal dopamine release. Science. 2011;333:718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- 104.Bickel WK, Moody L, Quisenberry AJ, Ramey CT, Sheffer CE. A competing neurobehavioral decision systems model of SES-related health and behavioral disparities. doi: 10.1016/j.ypmed.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biological Psychiatry. 2014;75:435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychol Sci. 2011;22:968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- 108.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 109.Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 111.Trope Y, Liberman N. Temporal construal. Psychological Review. 2003;110:403–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- 112.Seligman MEP, Railton P, Baumeister RF, Sripada C. Navigating Into the Future or Driven by the Past. Perspectives on Psychological Science. 2013;8:119–141. doi: 10.1177/1745691612474317. [DOI] [PubMed] [Google Scholar]
- 113.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 114.Lebreton M, Bertoux M, Boutet C, et al. A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol. 2013;11:e1001684. doi: 10.1371/journal.pbio.1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin H, Epstein LH. Living in the moment: effects of time perspective and emotional valence of episodic thinking on delay discounting. Behav Neurosci. 2014;128:12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daniel TO, Stanton CM, Epstein LH. The future is now: comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite. 2013;71:120–125. doi: 10.1016/j.appet.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Charlton SR, Yi R, Porter C, Carter AE, Bickel W, Rachlin H. Now for Me, Later for Us? Effects of Group Context on Temporal Discounting. Journal of Behavioral Decision Making. 2013;26:118–127. doi: 10.1002/bdm.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bickel WK, Pitcock JA, Yi R, Angtuaco EJC. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 120.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 121.Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol. 2011;19:53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- 122.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Craig ADB. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 124.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci. 2010;30:16429–16436. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mobini S, Body S, Ho M-Y, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 128.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 129.Kheramin S, Body S, Mobini S, et al. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- 130.Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.D'Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences. 2012;109:19900–19909. doi: 10.1073/pnas.1116727109. [DOI] [PMC free article] [PubMed] [Google Scholar]