Abstract
Polymeric nanoparticles are promising delivery platforms for various biomedical applications. One of the main challenges toward the development of therapeutic nanoparticles is the premature disassembly and release of the encapsulated drug. Among the different strategies to enhance the kinetic stability of polymeric nanoparticles, shell- and core-crosslinking have been shown to provide robust character, while creating a suitable environment for encapsulation of a wide range of therapeutics, including hydrophilic, hydrophobic, metallic, and small and large biomolecules, with gating of their release as well. The versatility of shell- and core-crosslinked nanoparticles is driven from the ease by which the structures of the shell- and core-forming polymers and crosslinkers can be modified. In addition, postmodification with cell-recognition moieties, grafting of antibiofouling polymers, or chemical degradation of the core to yield nanocages allow the use of these robust nanostructures as “smart” nanocarriers. The building principles of these multifunctional nanoparticles borrow analogy from the synthesis, supramolecular assembly, stabilization, and dynamic activity of the naturally driven biological nanoparticles such as proteins, lipoproteins, and viruses. In this review, the chemistry involved during the buildup from small molecules to polymers to covalently stabilized nanoscopic objects is detailed, with contrast of the strategies of the supramolecular assembly of polymer building blocks followed by intramicellar stabilization into shell-, core-, or core–shell-crosslinked knedel-like nanoparticles versus polymerization of polymers into nanoscopic molecular brushes followed by further intramolecular covalent stabilization events. The rational design of shell-crosslinked knedel-like nanoparticles is then elaborated for therapeutic packaging and delivery, with emphasis on the polymer chemistry aspects to accomplish the synthesis of such nanoparticulate systems.
Keywords: biomimetic, biomimetic nanoparticles, block copolymers, nanocages, nanoparticles, polymer brushes, shell-crosslinked knedel-like nanoparticles
INTRODUCTION
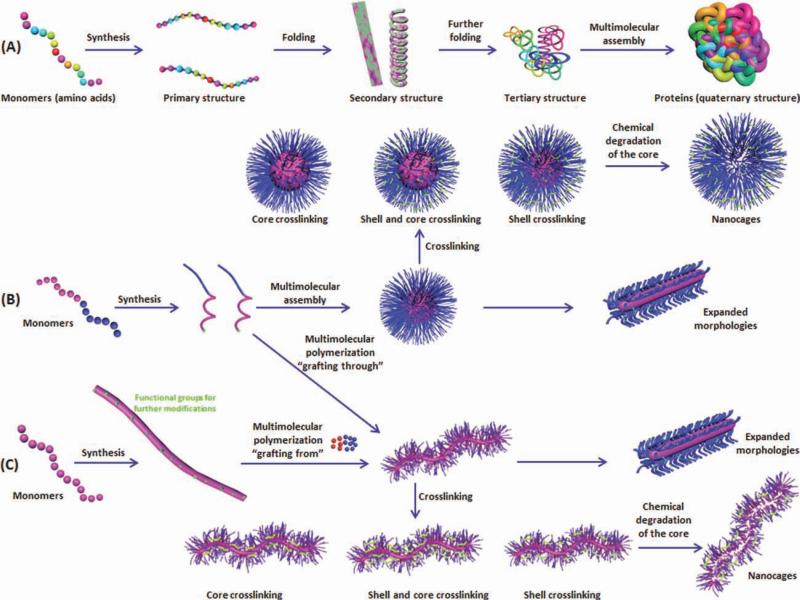
Intensive efforts have been directed to biomimicry approaches to design synthetic nanostructures that resemble natural nanosystems (e.g., viruses, lipoproteins, and proteins), which are endogenously used for transportation of biomolecules in the blood, organs, and on the cellular and subcellular levels, or for other physiological functions to emulate their general structural and functional features.1–7 As natural nanobiosystems are primarily organic, mainly composed of polypeptides, poly(nucleic acid)s, or polysaccha-rides, each of which is based on the covalent linking of small molecules that program supramolecular (intramolecular and intermolecular) interactions to guide the assembly into nanoscopic functional entities, synthetic analogs should follow the same general scheme for their construction (Fig. 1). Although the diversity and control over the sequence of the small molecule (monomer) units in biological systems are great, advances in synthetic polymer chemistry are rapidly allowing for increasing degrees of control over the placement of functionalities into specific regions of synthetic polymers so that different interactions can be “programmed” into the chain ter-mini and along the backbone (illustrated for a linear polymer in Figure 1, with other topologies also possible). The use of advanced controlled polymer chemistry, therefore, combined with supramolecular assembly and postmodification of the assembled nanostructures has enabled the building of synthetic nanomaterials of similar characteristics and controlled features to the natural systems (Fig. 2 and Table 1).
FIGURE 1.
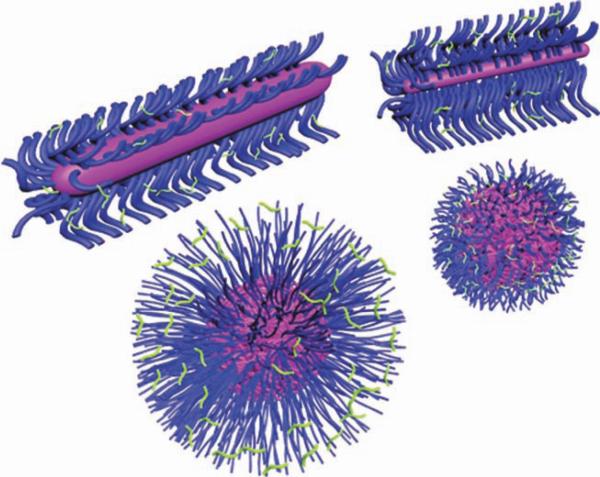
Two strategies (B and C) are being used to produce nanostructures that have elements resembling features of biosystems (A). The first strategy (B) depends on the supramolecular assembly of linear polymer chains, having the assembly “programmed” by differentiation of composition along the polymer structure, followed by crosslinking stabilization. The second strategy (C) forms polymer brushes by either “grafting-through” polymerization of a reactive chain terminus on the linear polymers of (B) or by “grafting-from” a polymer backbone having functional groups for initiation of the polymerization of added monomers (C). These polymer brushes can exist as unimolecular micelles or be converted to covalently linked nanostructures (other topologies are also possible). Shell- and/or core-crosslinking are used to enhance the kinetic stability of the nanoparticulates. Transformation from the SCKs or shell-crosslinked polymer brushes to nanocages is possible via chemical degradation of the core domains.
FIGURE 2.
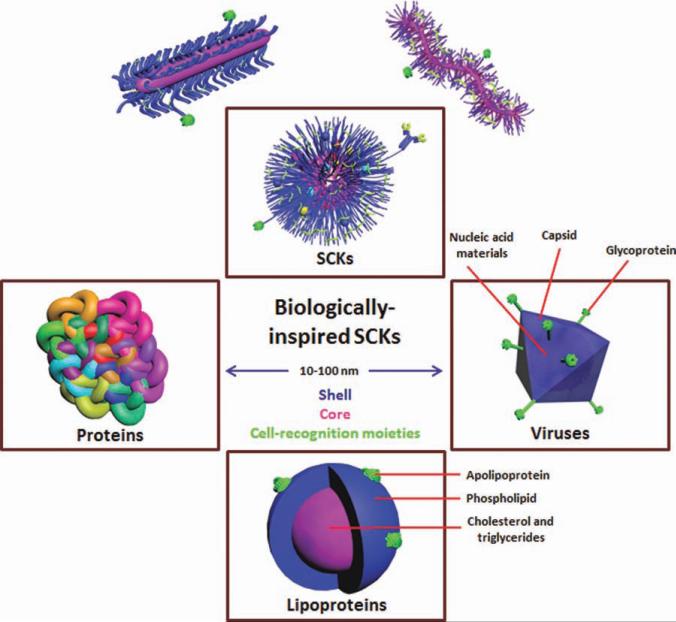
Biomimicry approaches used for the synthesis, supramolecular assembly, stabilization, and further functionalization of “smart, biomimic” SCKs, in which the synthetic SCKs are of similar dimensions (10–100 nm), can be made to present surface features resembling those of proteins, for example, cationic moieties of the histone core proteins of nucleosomes for packaging of nucleic acids, hydrophilic surfaces and hydrophobic cores like lipoproteins for the function of packaging water-insoluble guests, or internal sites for loading of the nucleic acids in a manner analogous to the behavior of viral capsids. SCKs, viruses, and lipoproteins share the features of a nanosized (10–100 nm) core (in red)/shell (in blue) morphology with surface-decorating moieties (in green).
TABLE 1.
Key Properties of SCKs, Proteins, Lipoproteins, and Viruses
| Feature | SCKs | Proteins (e.g., histone) | Lipoproteins | Viruses |
|---|---|---|---|---|
| Nanosize scale | Yes | Yes | Yes | Yes |
| Covalent stabilization | Yes | Yes | Yes | Yes |
| Stimuli responsiveness | Yes | Yes | Yes | Yes |
| Selective cellular uptake | Yes (when functionalized with targeting ligands) | Yes | Yes | Yes |
| Core/shell architecture | Yes | – | Yes | Yes |
| Condensation and packaging of nucleic acids | Yes | Yes | – | Yes |
| Solubilization of hydrophobic cargoes | Yes | – | Yes | – |
| Formed via synthesis and self-assembly | Yes | Yes | Yes | Yes |
The Wooley laboratory, along with other research groups, has focused on the design of synthetic nanomaterials that resemble histones or viral capsids and lipoproteins for the packaging and delivery of nucleic acids and hydrophobic therapeutics, respectively. Access to synthetic analogs of each of these quite different biological nanostructures has been based, largely, on amphiphilic core–shell polymer micelles derived from the assembly of well-defined amphiphilic block copolymers. In addition, special efforts have been focused on increasing the kinetic stability of these nanoassemblies to enable them to withstand the harsh biological barriers that they experience during circulation in the blood and down to the cellular and subcellular levels until they deliver their guest molecules. Among the different strategies to enhance the kinetic stability of polymer micelles, shell- and core-crosslinking have been shown to limit the premature disassembly and slow down the release of the encapsulated drug. More recently, the Wooley laboratory has extended to polymer brushes, which are also composed of amphiphilic block copolymers, in which those polymer chains are connected covalently through a polymer backbone into a stable, unimolecular micellar architecture.8 Core- and shell-crosslinking and core degradation processes have been performed for each of the block copolymer micelles and the polymer brushes to afford a library of complex nanoscopic objects (Fig. 1). Shell-crosslinked knedel-like nanoparticles (SCKs) have been studied to the greatest extent and are being explored as a platform technology in this review, although nanostructures of similar topologies have also been investigated by other groups.9–12 The sizes of SCKs could be controlled to produce nanostructures ranging from 10 to 100 nm of various morphologies and dimensions that are capable of encapsulating and delivering a wide range of therapeutics such as nucleic acids, chemotherapeutics, and antimicrobials to the tumor sites, lung, and bladder. In addition, multifunctional SCKs have been equipped with additional functionalities to deliver more than one cargo (e.g., more than one type of therapeutics and/or diagnostics) or to combine more than one targeting mechanism (i.e., passive and active targeting). Passive targeting allows the accumulation of the drug-loaded nanoparticles into pathological sites with leaky vasculature, such as the tumor tissues, via the enhanced permeability and retention effect. Active targeting can be achieved by decorating the surface of nanoparticles with cell-recognition moieties and/or the use of stimuli-responsive polymeric constituents. In this review, the chemistry and biomimetic strategies involved in the construction of covalently stabilized nanoscopic objects from both block copolymers and polymer brushes are highlighted, with emphasis on the rational design of SCKs for therapeutic packaging and delivery.
RATIONAL DESIGN OF “SMART, BIOMIMETIC” SCKs
Biomimicry Approaches
Understanding the different steps involved in drug and nucleic acids delivery by natural systems can lead to improvement in the design of polymers for encapsulating and complexing these cargoes, which in turn will increase the therapeutic efficiency of polymeric nanoparticles. Understanding natural processes such as viral transduction, DNA condensation by histones, and packaging and delivery of lipids by lipoproteins has provided the opportunity to specifically engineer molecular structures that could function like these natural nanodevices, yet avoid the disadvantages that come along with these systems and provide versatility for various cargoes and biomedical applications.13
Nucleosome core particles are composed of eight histone protein molecules that are assembled into octameric cores of ~11 nm in diameter and 5.7 nm in height (i.e., short cylinder or disc), around which 146 base pairs of DNA organize into a superhelix with assistance of cationic arginine groups.14–17 DNA duplexes form stable complexes with histone clusters to condense and store DNA within the cells. In addition, one more histone molecule fastens the DNA and assists a DNA linker in higher order packaging into long chromatin filaments.16 The degree of DNA condensation by histones and other proteins plays an important role in the regulation of gene expression in the nucleus of eukaryotic cells by controlling the accessibility of DNA-binding proteins to the various DNA sequences.15 Although there are significant differences between synthetic polymers or polymer particles and histones, it can be seen how the ratio of the polymer-to-nucleic acid and the net surface charge of the complexes can affect the properties of the polymer/nucleic acid complexes and the resulting gene expression/knockdown efficiency. Hence, the complexation via electrostatic and many other types of interactions, condensation, and stabilization of nucleic acids by the natural nanoparticulate systems and probably the organization through the covalent and noncovalent supramolecular assembly into different shapes (e.g., spheres, discs, and cylinders) are all necessary steps for the packaging of nucleic acids into functional forms.
Lipoproteins are endogenous biocompatible and biodegradable nanoparticles used naturally for transporting hydrophobic molecules (e.g., cholesterol and triglycerides) in the body (Fig. 2). These lipoproteins have core/shell architecture with a hydrophobic microenvironment composed of triglycerides, cholesteryl esters, and/or other poorly water-soluble molecules and a shell composed of a monolayer of phospholipids in which cholesterol and apolipoproteins are embedded. The phospholipid monolayer maintains the aqueous solubility of the lipoproteins. There are various types of lipoproteins depending on the size of the particles and the type of apolipoprotein bound on their surfaces. These lipid–protein complexes have the ability to avoid recognition by the mononu-clear phagocytic system and, thus, can circulate for long periods of time in the blood until delivering their guest bio-molecules to the target sites. Lipoproteins are selectively taken up by cells and importantly can redirect the biodistribution of the conjugated or encapsulated molecules to various organs depending on the type of the lipoprotein.18 Because of all these characteristic features, lipoprotein-inspired nanostructures have been designed and equipped with additional features to improve the delivery of hydro-phobic drugs (e.g., chemotherapeutics) and/or imaging probes to various target sites in the body.1 Chemical modifications of these lipoproteins have also been used to modify their characteristics or to impart additional functionalities.19
Viruses are core/shell nanoparticles on the order of 100 nm that composed of a protein coat (capsid) and nucleic acid core of either DNA or RNA and may have a lipid envelope decorated with glycoprotein surface moieties (Fig. 2). A variety of these viruses have been converted to vectors to deliver genes into the host cells (e.g., adenoviruses, retroviruses, and adenoassociated viruses). Viruses are able to condense nucleic acids and protect them against enzymatic degradation. They have developed molecular mechanisms that allow them to bind to the receptors of the host cell facilitated by their cell-recognition moieties. Fusion with cell membrane occurs, followed by release into the cytoplasm and translocation to the nucleus effectively delivering their genetic material. All these steps occur with assistance of various components of the viral nano-particles, for example, envelop proteins for attachment to the cells, peptides for fusion with the cell membrane and release into the cytoplasm, nuclear localization sequences for entering the nucleus, and enzymes that aid in the unpackaging and chromosomal integration of the nucleic acids.20–22 Despite the effectiveness and efficiency of viral vectors, drawbacks of such systems cannot be ignored, such as the possibility of insertional mutagenesis, limited size of the nucleic acid that can be loaded, and difficulty of storage and scalability.21,23,24 Interestingly, research on viral vectors is being shifted to the synthetic mimicry approaches via the covalent conjugation of antibio-fouling polymers onto the viral surface to reduce the immunogenicity, prolong the blood circulation time, and block the non-specific interactions with the nontargeted cells.25,26 The viral capsid can also be modified with targeting ligands to enhance the selective uptake of the viral particles at the target sites.27 The conjugation chemistry usually uses the free functional groups on the viral capsid; for instance, there are about 1800 free lysine groups on the capsid of adenovirus.28,29 These free groups give the opportunity for further functionalization with polymers, imaging probes, and so forth. Modification of the viral surface, although significantly reducing the immunogenicity of the viral particles, it partially abolishes the high transfection efficiency of viral vectors.
Chemistry versus Versatility
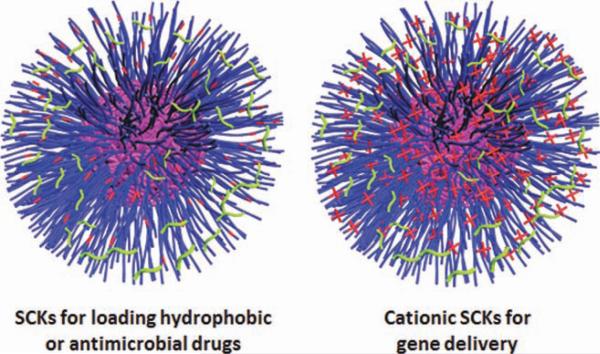
SCKs are a member of a large family of crosslinked block copolymer micelles that have shown great potential and versatility for biotechnology and medical applications.9 The assembly and construction of SCKs of various compositions and morphologies draw analogy from the fundamental construction of quaternary protein structures, lipoproteins, and viruses (Figs. 1 and 2).2 SCKs, proteins, and lipoproteins are formed via covalent polymerization of various monomers that dictate the properties and features of the final products. These polymeric units then undergo supramolecular assembly via weak intramolecular and intermolecular interactions, necessary for folding and assembly of linear, amphiphilic polypeptide or copolymer chains. Further stabilization, for instance, via crosslinking the active functional groups on the polymeric side chains (e.g., analogous to disulfide bridges in proteins) provides high kinetic stability and may be important for maintaining the particles in the functionally active form. Decoration of these diverse nanostructures with cell-recognition moieties, as in viruses, enhances their cellular uptake and imparts selectivity to their delivery. Furthermore, additional features have been built into the SCKs to improve their performance and selectivity, such as the incorporation of stimuli-responsive polymers that can respond to environmental or physiological triggers (e.g., pH, temperature, and cytosolic reduction), and thus, keeping the integrity of SCKs under specific environment while triggering the destabilization of the nanostructure and releasing the encapsulated drug at another environment into the target sites (Fig. 3 and Table 2). The continuous development of SCKs depends on understanding the various steps involved in packaging and delivery of biomolecules by the natural nanocarriers and applying these principles to the synthesis and constructions of the “smart, biomimic” SCKs. To prepare these nanostructures, a combination of controlled radical and ring-opening polymerizations, chemical transformation, and supramolecular assembly is used.30–33,40 In the following sections, detailed examples of the role of chemistry in designing and stabilizing these nanoparticles for drug and nucleic acids delivery will be illustrated.
FIGURE 3.
SCKs of versatile structures. The hydrophobic core can solubilize various hydrophobic drugs, whereas the hydrophilic shell can be converted to be anionic or cationic layer to complex positively or negatively charged (bio)molecules, respectively.
TABLE 2.
SCKs as Versatile Polymeric Nanostructures for Various Biomedical Applications
| SCKs | Composition | Cargo | Applications | Remarks (Stimuli Responsiveness) | References |
|---|---|---|---|---|---|
| Gene vectors | PAEA128-b-PS40: | 1. pDNA | 1. Cancer | 1. Bioreductively cleavable disulfide linkage between cSCKs and PNA: higher efficiency and lower toxicity than Lipofectamine or Arg9-PNA conjugates in Hela cells. | 34 – 37 |
| 1. cSCKs-S-S-PNA | 2. PNA | 2. Gene therapy (modulate incorrect splicing) | 2. cSCKs/oligonucleotide, PNA, or pDNA complexes: | ||
| 2. cSCKs complexes: phosphorothioate-2′-O-methyl oligonucleotides, PNA or pDNA | 3. Phosphorothioate, 2′-O-methyl oligonucleotides | a. N/P ratio of 6:1 gave the highest transfection. | |||
| 3. cSCKs complexes: The shell composition (primary amine, tertiary amine, and carboxylic acid group ratios): | b. Higher transfection, lower cytotoxicity, and lower materials used than most common transfection reagents. | ||||
| PAA- co-PAEDMA-b-PS | 3. Increasing the proportion of tertiary relative to primary amine reduced the cytotoxicity and increased transfection efficiency. | ||||
| PAA- co-PAEA-b-PS | |||||
| PAEA-co-PAEDMA-b-PS | |||||
| Chemotherapeutics | Thermosensitive: | Doxorubicin | Cancer | Tm of the core could be tuned (temperature) | 38,39 |
| PAA-b-P(ODA-co-DA) | |||||
| pH-responsive: | Doxorubicin | Cancer | 1. Higher Tg and Tm and aromatic styrene core (π–π-stacking): higher loading capacity and slower release rate. | 38,39,48 | |
| PAA-b-PS | 2. Increasing PAA/PS ratio: smaller core volumes, lower loading capacity, and lower release rate and extent. | ||||
| 3. Crosslinking: lower release rate. | |||||
| 4. The release rate was higher at pH 5 vs. pH 7.4. | |||||
| Antimicrobial | PAA-b-PS | Antimicrobial agents (Ag+ and SCCs) | Treatment of pulmonary and urinary tract infections | 1. Drugs could be loaded into the shell or the core of the nanoparticles. | 49 |
| 2. Sustained release over several days. | |||||
| 3. High antimicrobial activities against Gram-negative bacteria. | |||||
| Templates for multifunctional SCKs | PAA104-b-PpHS41 | Amino-terminated pyrazine (bifunctional fluorophore and crosslinker) | 1. pH-sensors | 1. Fluoresce and swell at high pH. | 50 |
| 2. In vivo imaging | 2. Shrink and quenched at low pH. | ||||
| 3. Fluorescence or confocal microscopy | |||||
| Multifunctional | Folate-SCKs based on PAA93-b-PMA164 | 1. 64Cu (PET and radiotherapy) | 1. In vivo tracking and imaging | 1. Multifunctional nanotheranostics. | 62 |
| 2. Fluorescein thiosemicarbazide (fluorophore) | 2. Cancer | 2. Tumor accumulation (EPR). | |||
| 3. Theranostic applications | 3. Specific uptake in vitro and in vivo (active targeting). |
CATIONIC SCKs FOR NUCLEIC ACID DELIVERY—HISTONE BIOMIMICRY
The delivery of nucleic acids and other negatively charged bio-macromolecules is usually achieved via electrostatic complexation and condensation with positively charged lipids and/or polymers. This condensation protects them from enzymatic degradation and allows their cellular and subcellular delivery in a compact form. Usually, excess positive charges or decoration with targeting ligands is required for high cellular uptake. Nucleic acids can also be delivered via conjugation to polymers or nanoparticles.36,41 Chemical modifications of nucleic acids are often used as a complementary strategy to enhance their stability and affinity toward the target sequences.42 Examples of these modifications include phosphorothioate, 2′-O-methylation, peptide nucleic acid (PNA), locked nucleic acid (LNA), and 2’-deoxy-2’-fluoro-β-D-arabinonucleic acid (2′F-ANA). Modifying the structure of nucleic acids imparts specific characteristics suitable for various biomedical applications. For instance, PNA, LNA, and 2′-O-methyl modifications do not induce RNAse H endonuclease activity, whereas phosphorothioate and 2′F-ANA oligonucleotides induce RNAse H. Induction of RNAse H is appropriate for gene knockdown applications (i.e., antisense), but not for modulating incorrect splicing of an aberrant gene, to avoid the possible degradation of the mRNA.43 Chemically modified nucleic acids are still subject to rapid renal clearance and have limited cellular uptake and transfection efficiency and require nanocarriers for their delivery.
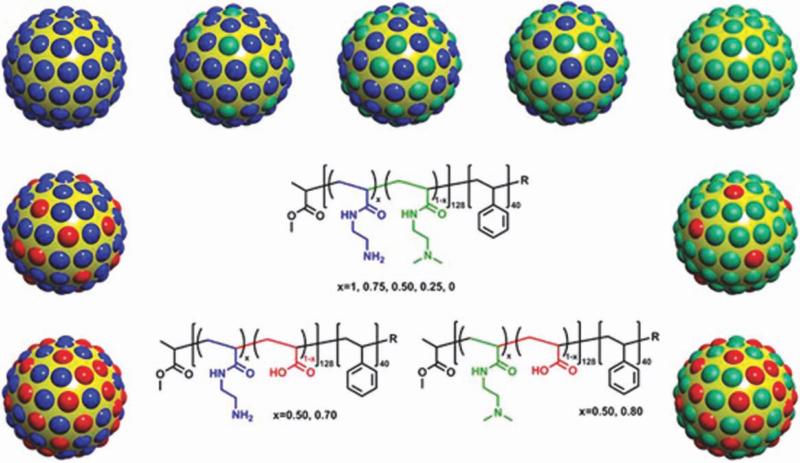
Over the last decade, Wooley and coworkers35 have exploited several approaches for the design of efficient nanoscopic gene delivery carriers. Cationic SCKs (cSCKs) are nanoparticles consisting of a hydrophobic core and a positively charged, highly functionalizable crosslinked shell (Fig. 3). The hydrophobic core has often consisted of nondegradable polystyrene (PS) that is linked to an aminofunctionalized derivative of poly(acrylic acid) (PAA), which establishes the hydrophilic nanoparticle shell, as a robust material to fully probe the nanoparticle characteristics, although degradable analogs are now being explored aggressively.44 The PAA-b-PS copolymer is synthesized using controlled radical polymerization techniques, for example, atom transfer radical polymerization, of the protected monomer precursors followed by deprotection.45 The carboxylic acid groups of PAA are then completely or partially converted into primary and/or tertiary amines to invert the surface charge of SCKs from negative to positive (Fig. 4). The nanoparticle is usually stabilized via covalent crosslinking (amidation) of some of the primary amine groups of the shell polymeric chain segments by reaction with an activated diester. The nucleic acids are then complexed to the cSCK or covalently conjugated onto their surfaces. Complexation and transfection efficiency of cSCKs with plasmid DNA (pDNA) and chemically modified oligonucleotides were studied using various cell lines.34–37 These nanoparticles were shown to condense nucleic acid materials of different structures, protect them against enzymatic digestion, and to afford high transfection efficiency.
FIGURE 4.
cSCKs with various shell compositions, including mixtures of primary/tertiary amines, mixtures of primary amines/carboxylic acids, and mixtures of tertiary amines/carboxylic acids. Reprinted from ref. 34, with permission from Elsevier.
Poly(acrylamidoethylamine)128-b-PS40 (PAEA128-b-PS40) was transformed from PAA128-b-PS40 and used for the creation of cSCKs (~10 nm) with high content of primary amines.36 The presence of excess positive charge could facilitate the cellular binding, association, and subsequent uptake and release of the nucleic acid cargo into the cytoplasm. cSCKs were used to complex (electrostatic) or conjugate (bioreductive disulfide linkage) and deliver PNA (~18 base pairs). Hybridization of PNA (neutral) with oligonucleotide is necessary to impart negative charge to the PNA before complexation with cationic agents. These complexes or conjugates showed higher transfection efficiency and lower toxicity than Lipofectamine complexes or Arg9 (cell-penetrating peptide)–PNA conjugates in HeLa cells. The ability of the same cSCKs to deliver other chemically modified oligonucleotides (phosphorothioate and 2′-O-methyl oligonucleotides) or DNA of larger size (pDNA, 4733 base pairs) were tested by preparing these complexes at various nitrogen-to-phosphate (N/P) ratios.37 The N/P ratio represents the molar ratio of the nitrogen and phosphate groups of the cationic vector and nucleic acids, respectively. A N/P ratio of 6:1 gave the highest transfection. Higher transfection was also observed for the cSCK/pDNA or oligonucleotide complexes when compared with Polyfect® commercial transfection agent in HeLa cells.
Because of the promising results obtained, two classes of modified cSCKs were prepared to study the effect of the shell composition [i.e., primary amine (PAEA), tertiary amine (acrylamidoethyldimethylamine, PAEDMA), and carboxylic acid groups (PAA)] on the toxicity and transfection efficiency of the complexes (Fig. 4).34 One class consisted of cSCKs with differing ratios of primary and tertiary amines to not only maintain the same charge and buffering capacity in the pH range 5.5–7 but also change the phosphate binding properties of the amines. The second class consisted of cSCKs with differing ratios of amines and carboxylic acids to change the net charge of the cSCK as well as its buffering capacity. The optimal composition was found with cSCKs of tertiary and primary amine ratios of 50:50 and 75:25, in which the highest transfection was achieved for both small oligonucleotides and pDNA, respectively, when compared with the commercial transfection reagents, namely, Oligofectamie, Polyfect, and Lipofectamine. The general lessons learned from this study were that the incorporation of tertiary amines into the polymer structure reduced the cytotoxicity and increased the transfection efficiency of the complexes and that the introduction of carboxylates into the shell greatly reduced bioactivity probably due to the lower buffering capacity and binding affinity.
The principles of the cSCKs are based on the characteristic features of the natural nanoparticles used for nucleic acids packaging and delivery. Synthesis of the amphiphilic copolymers, supramolecular assembly, covalent stabilization into nanosized cSCKs, and the cationic character of the shell are all common features between cSCK and histones, which are required for packaging and delivery of DNA/RNA. The future work of this promising category of nanoparticles for nucleic acids delivery will include the use of cSCKs with optimal composition (degradable polymer components, optimal N/P ratio, amine/carboxylic acid contents, or other functionalities), decoration with targeting ligands, and stabilization by using biodegradable crosslinkers. Chemical modifications of DNA/RNA may also be used to enhance the transfection efficiency of these nanocarriers and to impart greater stability to their nucleic acid cargoes. Combination of these strategies aims at designing cSCKs with comparable efficiency to the natural proteins and viral nanoparticles, but with lower toxicity and immunogenicity and higher selectivity and ease of production and scalability.
CHEMOTHERAPEUTIC SCKs—LIPOPROTEIN BIOMIMICRY
Controlling the polymer chemistry and composition can tune the size, dimension, loading capacity, release kinetics, cellular uptake, and pharmacokinetics of nanoparticles.46,47 For instance, nanoparticles of various dimensions have been shown to exhibit different pharmacokinetics in vivo, with longer filomicelle structures circulating for longer time periods in the blood, because of lower rates of phagocytosis and cellular uptake.46 In addition, the nanoparticles can be remotely controlled to respond to either pH or temperature variation. The effect of chemical composition and crosslinking of different block copolymers and the relative copolymer block length on the size, core/shell dimensions, loading capacity, release kinetics, and pH responsiveness and thermoresponsiveness of SCKs were studied for the delivery of doxorubicin, a widely used anti-cancer drug.38,39,48 First, PAA-b-PS copolymers of different relative block lengths were synthesized via sequential reversible addition-fragmentation chain transfer (RAFT) polymerization reactions of tert-butyl acrylate (tBA) and styrene, followed by removal of the t-butyl ester-protecting groups. PAA-b-poly(octadecyl acrylate-co-decyl acrylate) (PAA-b-PODA-co-PDA) copolymer was also synthesized using the RAFT polymerization reactions through the statistical copolymerization of ODA and DA from a tBA macrochain transfer agent. The use of controlled radical polymerization techniques (i.e., RAFT) allowed the synthesis of block copolymers of predetermined molecular weights and low polydispersities at intermediate temperatures without the addition of inorganic catalyst.39 It was hypothesized that packaging of drug molecules within a glassy or crystalline core domain would reduce their mobilities and slow their release rates, and that having a thermal transition between room temperature and physiological temperature could accelerate the drug release on administration. Therefore, PAA-b-PS and PAA-b-P(ODA-co-DA) were used to prepare SCKs, presenting cores of high and low glass transition temperatures (Tg), respectively.38,39 In addition, the melting temperature (Tm) of the P(ODA-co-DA) core could be tuned to be either below or above physiological temperature (i.e., thermoresponsive SCKs) by tuning the feed ratio of ODA to DA. The nanoparticles with the higher Tg and Tm values had higher loading capacities and slower release rates. In addition to the thermal effects, the SCKs having PS core domains might also be involved in π–π-stacking between the aromatic ring moieties of styrene and the doxorubicin drug molecules. Then, the effect of crosslinking and relative block length of PAA-b-PS copolymer on the characteristics of the SCKs was studied.48 SCKs of different core volumes were formed depending on the ratio of acrylic acid to styrene block lengths (Fig. 5). The nanoparticles with larger core volume (lower relative proportion of PAA to PS) were associated with higher loading capacity and higher rate and extent of drug release. Crosslinking could reduce the release rate of doxorubicin. The release rate was higher at pH 5 vs. pH 7.4 for both crosslinked and noncrosslinked nanoparticles (i.e., pH-responsive SCKs). These nanostructures borrow the size, core/shell architecture, and core hydrophobicity for drug solubilization from the structure of lipoproteins and have additional features to control the selective release of the drug at the desired target sites (e.g., the acidic pH of the tumor and endosomes or in response to temperature variations).
FIGURE 5.
SCKs of various sizes, morphologies, and dimensions usually exhibit different characteristics such as solubilization capacity, loading and release kinetics, in vivo pharmacokinetics, cellular uptake, and intracellular trafficking pathways.
ANTIMICROBIAL SCKs
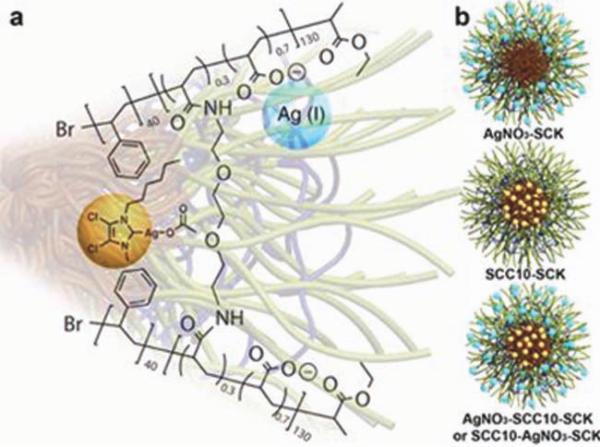
PAA-b-PS SCKs have been used to encapsulate, protect, and deliver silver-based antimicrobial agents [e.g., Ag+, silver– carbene complexes (SCCs), or both].49 SCCs act as a Ag+-pro-drug by decomposing in the presence of saline solution to release the active Ag+. Ag+ and SCCs were loaded into the hydrophilic PAA shell and the hydrophobic PS core, respectively, with the possibility of localizing at the core/shell interface of the SCKs (Fig. 6). SCKs loaded with both compounds could also be prepared. Sustained release of the encapsulated drug from the drug-loaded SCKs was observed over several days and was associated with high antimicrobial activities against Gram-negative bacteria (e.g., urinary isolates of Escherichia coli and respiratory isolates of Pseudo-monas aeruginosa). These antimicrobial SCKs could have potential applications for the treatment of urinary tract and pulmonary infections. Pulmonary delivery via inhalation can be convenient for the treatment of various types of lung diseases. It is particularly useful for the treatment of respiratory tract infection by concentrating the administered dose at the site of infection while minimizing the undesired systemic effects. It can also be used for systemic delivery because of the large available surface area. Exploiting SCKs decorated with specific targeting ligands and controlling the route of administration are currently under investigation to treat pulmonary and urinary tract infections. These SCKs are designed to selectively bind to specific receptors expressed on the bladder or pulmonary epithelial surfaces. Subsequent release of the antimicrobial payloads in the epithelial cells is then expected to eradicate the bacteria and to treat the infections.
FIGURE 6.
Loading of SSC (yellow ball) and Ag+ (blue ball) into the core and/or shell of PAA130-b-PS40 SCKs. Reproduced from ref. 49, with permission from the Royal Society of Chemistry.
MULTIFUNCTIONAL SCKs
Photonic nanoparticles are nanocarriers loaded with fluorescent dyes, either to improve their in vivo pharmacokinetics (e.g., prolong the circulation time or accumulate in specific tissues) or to enhance their photophysical properties (e.g., brightness). Wooley and coworkers50–52 have developed SCKs to show different fluorescent patterns at different pH values or to enhance the photophysical properties of various fluorescent dyes. For instance, photonic SCKs were constructed via the supramolecular assembly of PAA104-b-poly(p-hydroxystyrene)41 (PAA-b-PpHS) copolymer in water, followed by diaminoterminated pyrazine shell crosslinking.50 Pyrazine imparts both fluorescence (photonic) and stability (crosslinking) to the nanoparticles. These nanocarriers showed enhancement of photophysical properties as a result of changing the pH across the physiological range (i.e., pH-responsive photonic SCKs). In this system, high fluorescence is emitted when the shell is swollen at elevated pH. On the contrary, the fluorescence is quenched when the shell shrinks at low pH. As the pH increases, two factors play major roles in expansion of the nanoparticles: (1) as more PAA blocks become deprotonated, negatively charged carboxylates repel PAA chains from one another within the confined SCK structure; and (2) as the PpHS blocks become deprotonated at high pH, the hydrophilicity of the PpHS core increases, allowing water molecules to enter the nanoparticles (i.e., fluorescing on loss of self-attractive interactions at high pH and quenched as self-associations re-establish at low pH). The expansion and shrinkage of these entities were also confirmed by measuring the hydrodynamic diameter at different pH values. As long as the degree of crosslinking was limited to maintain the integrity while allowing for expansion/contraction of the nanostructures, approximately a twofold increase in hydrodynamic diameter of the SCKs was observed to give a fourfold increase in fluorescence emission intensity when the pH of the solution was increased from 6 to 8. Highly interesting differences were observed for photonic SCKs having spherical versus cylindrical morphologies.53 Although the spherical nanostructures composed of PAA-b-PpHS and crosslinked by a diamino pyrazine exhibited an increase in the fluorescence emission intensity with increase of pH, the cylindrical analogs gave a blue shift in the fluorescence emission wavelength. This difference in behavior was explained by higher densities of PAA chain packing along the backbone of the cylindrical nanoassembly, which promoted amidation of the aromatic amines of the pyrazine crosslinker, in addition to the expected aliphatic amino groups, as occurred for the spherical morphology. These photonic SCKs, or other types,51,52 can serve as a basic mold for loading various therapeutics and surface decoration with functional groups for targeting or diagnostic purposes, that is, converted to multifunctional SCKs.54
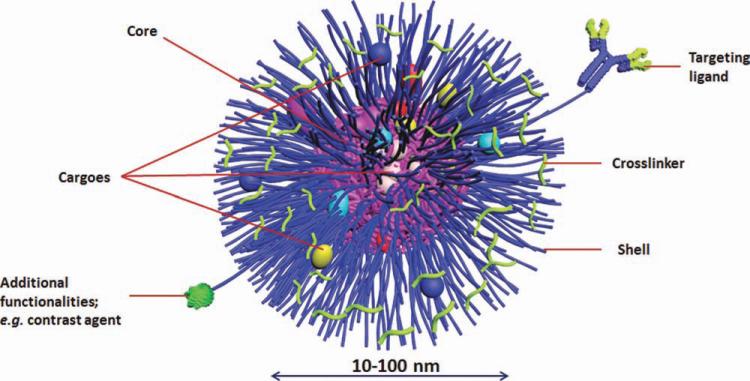
The term “multifunctional” means to incorporate more than one cargo or smart feature into the same nanocarrier to maximize the therapeutic benefits, minimize the toxicity, and enhance the efficiency of the treatment and diagnosis of diseases.55 Many guest molecules, more than one type of therapeutics and/or diagnostics, can be loaded into the same nanocarrier. For example, theranostic nanoparticles “nanotheranostics” involve the use of multifunctional nanoparticles loaded with therapeutic drugs and imaging probes for the combined therapy and diagnosis. Moreover, passive and active targeting features can also be built into the same nanostructures (Fig. 7). In this case, the synthetic procedures become more complicated to synthesize polymeric units that are capable of binding more than one drug and carry other functionalities for targeting and/or imaging.
FIGURE 7.
Multifunctional SCKs.
SCK TRANSFORMATION TO NANOCAGES—VIRAL CAPSID MIMICRY
Nanocages have been prepared from SCKs that are built from block copolymers56–58 or polymer brushes59 as analogs of viral capsids. Nanocages can have potential for encapsulating large quantities or sizes of cargoes within the water-filled core domain. Crosslinking of the shell of the micellar or polymer brush structures, for instance, via using diamino crosslinkers to form bridges between some of the carboxylic acid groups along the PAA segments, has been used to form crosslinked polyacryl-amide shells. Then, chemical degradation of the core domain (e.g., ozonolysis of polyisoprene56–59 or hydrolysis of polyesters44,60) yields a hollow nanostructure. Optimization of the reaction conditions58 and control of the placement of the various functional groups within the internal or external sites of the core/shell architecture (regiochemistry)56 are critical to allow for removal of the core while maintaining the shell integrity. By constructing a well-defined polymer precursor, highly complex nanocages can result, in which a unique internal chemistry for differentiation of the external and internal nanocage surfaces is created,56,61 approaching more closely the structural features of viral capsids. Theoretically, nanocages can be loaded with nucleic acid materials into their aqueous cores and decorated with targeting ligands onto their surfaces to facilitate their cellular uptake. In addition, the composition of the polymer constituents may be modified to impart endosomolytic or nuclear localizing features that could enhance their transfection efficiency “viral mimicry,” while avoiding the immunogenic adverse reactions induced by viruses. Studying and comparing these nano-cages, side by side, with viral vectors will also help in the further understanding of the natural systems and aid in the design of safe yet efficient nucleic acid delivery nanovectors.
CONCLUSIONS
Advances in polymer chemistry have allowed the preparation of crosslinked nanoparticles of various sizes, morphologies, shell and core domains (including nanocages), surface chemistries, and targeting properties. In the Wooley laboratory, two distinct approaches are taken, each of which uses multiple types of controlled polymerizations and chemical modifications: in one case, block copolymers are assembled in solution followed by cross-linking stabilization reactions, and in the other case, polymer brushes are constructed as unimolecular nanostructures that then undergo further chemical transformation reactions. Such well-defined nanomaterials have potential for application in a number of diverse areas; however, this highlight has focused on their design for biomedical applications, with emphasis on SCKs, as they have been advanced most significantly. Control over the polymer properties (e.g., Tm, Tg, and pKa) imparts selectivity to the nanosystems, which is expected to allow the release of their payloads at the target biological sites while minimizing toxicity to healthy tissues. Transformation to degradable nanoparticle structures,44 including the polymer and crosslinker components, and precise selection of the polymeric precursors are the main clues to control and dictate the type and quantity of diagnostics and/or therapeutics to be loaded into SCKs. Synthesis, assembly, stabilization, and further functionalization of SCKs are similar to the natural processes involved in the production of various biological nanodevices such as proteins and viruses. In addition, they mimic these natural biological particles in their ability to selectively bind to specific cell types and to deliver their cargoes down to the subcellular and molecular levels. There are two main advantages of using the synthetic nanoparticles over the natural ones. First, they can be designed to be less immunogenic and thus can be injected over several times, on the contrary to other viral or protein particles, which can usually be injected once or few times. Second, they have more flexibility in modifying their structures to comply with various biomedical applications and can be equipped with many “smart” features such as decoration with targeting ligands, inclusion of stimuli-responsive polymers, and together with incorporation of imaging probes for the simultaneous diagnosis and therapy. However, the main limitation of these synthetic nanoparticles is that their efficiency is still behind that of the natural systems. Current efforts focus on further understanding of the structure, interactions, and intracellular trafficking pathways of the natural nano-particles and to apply this knowledge for the design of the synthetic nanoparticles.
Acknowledgments
The authors gratefully acknowledge financial support from the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01-DK082546), and Covidien Pharmaceuticals, Inc. The Welch Foundation is gratefully acknowledged for support through the W. T. Doherty-Welch Chair in Chemistry (grant no. A-0001).
Biography
 Mahmoud Elsabahy is co-assistant director of the Laboratory for Synthetic-Biologic Interactions at Texas A&M University. He is also a senior research scientist in collaboration with Professor Karen Wooley in the Department of Chemistry, Texas A&M University, and a lecturer at the Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Assiut, Egypt. He received a B.S. in Pharmaceutical Sciences from the Assiut University, and then he finished his M.Sc. and Ph.D. degrees (Polymeric micelles as versatile carriers for targeted drugs and nucleic acids delivery) from the Faculty of Pharmacy, University of Montreal, under supervision of Professor Jean-Christophe Leroux (currently at the Institute of Pharmaceutical Sciences, Drug Formulation and Delivery, ETH, Zürich). He spent 1 year as a postdoctoral fellow and another year as a research associate at the School of Pharmacy, University of Waterloo (Ontario, Canada), studying the application of nonviral vectors for dermal and transdermal gene therapy. His research interests include the rational design of nanomedicines for various biomedical applications. He has obtained the Academic merit award (J. A. DeSève). In addition, he has been nominated as a meritorious candidate for both a Natural Sciences and Engineering Research Council of Canada (NSERC) Visiting Fellowship and a NSERC Industrial R&D Fellowship in the cellular and molecular biology areas of expertise.
Mahmoud Elsabahy is co-assistant director of the Laboratory for Synthetic-Biologic Interactions at Texas A&M University. He is also a senior research scientist in collaboration with Professor Karen Wooley in the Department of Chemistry, Texas A&M University, and a lecturer at the Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Assiut, Egypt. He received a B.S. in Pharmaceutical Sciences from the Assiut University, and then he finished his M.Sc. and Ph.D. degrees (Polymeric micelles as versatile carriers for targeted drugs and nucleic acids delivery) from the Faculty of Pharmacy, University of Montreal, under supervision of Professor Jean-Christophe Leroux (currently at the Institute of Pharmaceutical Sciences, Drug Formulation and Delivery, ETH, Zürich). He spent 1 year as a postdoctoral fellow and another year as a research associate at the School of Pharmacy, University of Waterloo (Ontario, Canada), studying the application of nonviral vectors for dermal and transdermal gene therapy. His research interests include the rational design of nanomedicines for various biomedical applications. He has obtained the Academic merit award (J. A. DeSève). In addition, he has been nominated as a meritorious candidate for both a Natural Sciences and Engineering Research Council of Canada (NSERC) Visiting Fellowship and a NSERC Industrial R&D Fellowship in the cellular and molecular biology areas of expertise.
 Karen L. Wooley holds the W. T. Doherty-Welch Chair in the Department of Chemistry at Texas A&M University, with a joint appointment in the Department of Chemical Engineering. She received a B.S. in chemistry from the Oregon State University in 1988 and then studied under the direction of Professor Jean M. J. Frechet at the Cornell University, obtaining a Ph.D. in polymer/organic chemistry in 1993. She began an academic career as an assistant professor of chemistry at the Washington University in St. Louis, Missouri, was promoted in 1999 to full professor with tenure, and was installed as a James S. McDonnell Distinguished University Professor in Arts and Sciences in 2006. In 2009, she relocated to Texas A&M University. Her research areas include the synthesis and characterization of degradable polymers, unique macromolecular architectures and complex polymer assemblies, and the design and development of well-defined nanostructured materials, for which she has received several awards, including an Arthur C. Cope Scholar Award, a Herman F. Mark Scholar Award, and awards from the National Science Foundation, the Office of Naval Research, and the Army Research Office. Karen serves as an Editor for the Journal of Polymer Science, Part A: Polymer Chemistry. She directs a National Heart Lung and Blood Institute-supported Program of Excellence in Nanotechnology, serves on the National Institutes of Health NANO study section, and also serves on the Scientific Advisory Panel for the National Institutes of Health Nanomedicine Development Centers and on the International Scientific Advisory Board for the Dutch BioMedical Materials Program.
Karen L. Wooley holds the W. T. Doherty-Welch Chair in the Department of Chemistry at Texas A&M University, with a joint appointment in the Department of Chemical Engineering. She received a B.S. in chemistry from the Oregon State University in 1988 and then studied under the direction of Professor Jean M. J. Frechet at the Cornell University, obtaining a Ph.D. in polymer/organic chemistry in 1993. She began an academic career as an assistant professor of chemistry at the Washington University in St. Louis, Missouri, was promoted in 1999 to full professor with tenure, and was installed as a James S. McDonnell Distinguished University Professor in Arts and Sciences in 2006. In 2009, she relocated to Texas A&M University. Her research areas include the synthesis and characterization of degradable polymers, unique macromolecular architectures and complex polymer assemblies, and the design and development of well-defined nanostructured materials, for which she has received several awards, including an Arthur C. Cope Scholar Award, a Herman F. Mark Scholar Award, and awards from the National Science Foundation, the Office of Naval Research, and the Army Research Office. Karen serves as an Editor for the Journal of Polymer Science, Part A: Polymer Chemistry. She directs a National Heart Lung and Blood Institute-supported Program of Excellence in Nanotechnology, serves on the National Institutes of Health NANO study section, and also serves on the Scientific Advisory Panel for the National Institutes of Health Nanomedicine Development Centers and on the International Scientific Advisory Board for the Dutch BioMedical Materials Program.
REFERENCES AND NOTES
- 1.Ng KK, Lovell JF, Zheng G. Acc. Chem. Res. 2011;44:1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wooley KL. J. Polym. Sci. Part A: Polym. Chem. 2000;38:1397–1407. [Google Scholar]
- 3.Ayres N, Holt DJ, Jones CF, Corum LE, Grainger DW. J. Polym. Sci. Part A: Polym. Chem. 2008;46:7713–7724. doi: 10.1002/pola.23075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, Zeng X, Zhang Y, Feliu N, Lundberg P, Fadeel B, Malkoch M, Nyström AM. J. Polym. Sci. Part A: Polym. Chem. 2012;50:217–226. [Google Scholar]
- 5.Zeng X, Zhang Y, Wu Z, Lundberg P, Malkoch M, Nyström AM. J. Polym. Sci. Part. A: Polym. Chem. 2012;50:280–288. [Google Scholar]
- 6.Nottelet B, Tommaso CD, Mondon K, Gurny R, Möller M. J. Polym. Sci. Part. A: Polym. Chem. 2010;48:3244–3254. [Google Scholar]
- 7.Kaur G, Chang SLY, Bell TDM, Hearn MTW, Saito K. J. Polym. Sci. Part. A: Polym. Chem. 2011;49:4121–4128. [Google Scholar]
- 8.Li Z, Zhang K, Ma J, Cheng C, Wooley KL. J. Polym. Sci. Part. A: Polym. Chem. 2009;47:5557–5563. doi: 10.1002/pola.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Nostrum CF. Soft Matter. 2011;7:3246–3259. [Google Scholar]
- 10.Read ES, Armes SP. Chem. Commun. 2007:3021–3035. doi: 10.1039/b701217a. [DOI] [PubMed] [Google Scholar]
- 11.Fujii S, Cai Y, Weaver JV, Armes SP. J. Am. Chem. Soc. 2005;127:7304–7305. doi: 10.1021/ja050049a. [DOI] [PubMed] [Google Scholar]
- 12.Moughton AO, O'Reilly RK. J. Am. Chem. Soc. 2008;130:8714–8725. doi: 10.1021/ja800230k. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Nat. Rev. Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 14.Richmond TJ, Davey CA. Nature. 2003;423:145–150. doi: 10.1038/nature01595. [DOI] [PubMed] [Google Scholar]
- 15.Segal E, Fondufe-Mittendorf Y, Chen L, Thastrom A, Field Y, Moore IK, Wang JP, Widom J. Nature. 2006;442:772–778. doi: 10.1038/nature04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daban JR. Micron. 2011;42:733–750. doi: 10.1016/j.micron.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Finch JT, Lutter LC, Rhodes D, Brown RS, Rushton B, Levitt M, Klug A. Nature. 1977;269:29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- 18.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Nat. Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Jarzyna PA, van Tilborg GA, Nguyen VA, Cormode DP, Klink A, Griffioen AW, Randolph GJ, Fisher EA, Mulder WJ, Fayad ZA. FASEB J. 2010;24:1689–1699. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas KL. Biotechnol. Prog. 2008;24:871–883. doi: 10.1021/bp070319o. [DOI] [PubMed] [Google Scholar]
- 21.Waehler R, Russell SJ, Curiel DT. Nat. Rev. Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth CM, Sundaram S. Annu. Rev. Biomed. Eng. 2004;6:397–426. doi: 10.1146/annurev.bioeng.6.040803.140203. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CE, Ehrhardt A, Kay MA. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 24.Lehrman S. Nature. 1999;401:517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee D, Liu AP, Voss NR, Schmid SL, Finn MG. ChemBioChem. 2010;11:1273–1279. doi: 10.1002/cbic.201000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn MG. Biomacromolecules. 2003;4:472–476. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- 27.Singh R, Kostarelos K. Trends Biotechnol. 2009;27:220–229. doi: 10.1016/j.tibtech.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Pearce OM, Fisher KD, Humphries J, Seymour LW, Smith A, Davis BG. Angew. Chem. Int. Ed. Engl. 2005;44:1057–1061. doi: 10.1002/anie.200461832. [DOI] [PubMed] [Google Scholar]
- 29.Tong GJ, Hsiao SC, Carrico ZM, Francis MB. J. Am. Chem. Soc. 2009;131:11174–11178. doi: 10.1021/ja903857f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Chem. Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales K, Chen Z, Wooley KL, Pochan DJ. Nano Lett. 2008;8:2023–2026. doi: 10.1021/nl8013082. [DOI] [PubMed] [Google Scholar]
- 32.O'Reilly RK, Hawker CJ, Wooley KL. Chem. Soc. Rev. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]
- 33.Hawker CJ, Wooley KL. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 34.Zhang K, Fang H, Wang Z, Li Z, Taylor JS, Wooley KL. Biomaterials. 2010;31:1805–1813. doi: 10.1016/j.biomaterials.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang K, Fang H, Shen G, Taylor JS, Wooley KL. Proc. Am. Thorac. Soc. 2009;6:450–457. doi: 10.1513/pats.200902-010AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang H, Zhang K, Shen G, Wooley KL, Taylor JS. Mol. Pharm. 2009;6:615–626. doi: 10.1021/mp800199w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K, Fang H, Wang Z, Taylor JS, Wooley KL. Biomaterials. 2009;30:968–977. doi: 10.1016/j.biomaterials.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 38.Nystrom AM, Xu Z, Xu J, Taylor S, Nittis T, Stewart SA, Leonard J, Wooley KL. Chem. Commun. 2008:3579–3581. doi: 10.1039/b805428b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nystrom AM, Wooley KL. Soft Matter. 2008;4:849–858. doi: 10.1039/b719314a. [DOI] [PubMed] [Google Scholar]
- 40.Cui H, Chen Z, Zhong S, Wooley KL, Pochan DJ. Science. 2007;317:647–650. doi: 10.1126/science.1141768. [DOI] [PubMed] [Google Scholar]
- 41.Elsabahy M, Zhang M, Gan SM, Waldron KC, Leroux JC. Soft Matter. 2008;4:294–302. doi: 10.1039/b714221h. [DOI] [PubMed] [Google Scholar]
- 42.Watts JK, Deleavey GF, Damha MJ. Drug Discov. Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Sazani P, Kole R. J. Clin. Invest. 2003;112:481–486. doi: 10.1172/JCI19547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samarajeewa S, Shrestha R, Li Y, Wooley KL. J. Am. Chem. Soc. 2012;134:1235–1242. doi: 10.1021/ja2095602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Q, Wooley KL. J. Polym. Sci. Part. A: Polym. Chem. 2000;38:4805–4820. [Google Scholar]
- 46.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nat. Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K, Rossin R, Hagooly A, Chen Z, Welch MJ, Wooley KL. J. Polym. Sci. Part. A: Polym. Chem. 2008;46:7578–7583. doi: 10.1002/pola.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin LY, Lee NS, Zhu J, Nystrom AM, Pochan DJ, Dorshow RB, Wooley KL. J. Controlled Release. 2011;152:37–48. doi: 10.1016/j.jconrel.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Hindi K, Watts KM, Taylor JB, Zhang K, Li Z, Hunstad DA, Cannon CL, Youngs WJ, Wooley KL. Chem. Commun. 2010;46:121–123. doi: 10.1039/b916559b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee NS, Sun G, Neumann WL, Freskos JN, Shieh JJ, Dorshow RB, Wooley KL. Adv. Mater. 2009;21:1344–1348. doi: 10.1002/adma.200803053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun G, Berezin MY, Fan J, Lee H, Ma J, Zhang K, Wooley KL, Achilefu S. Nanoscale. 2010;2:548–558. doi: 10.1039/b9nr00304e. [DOI] [PubMed] [Google Scholar]
- 52.Sun G, Cui H, Lin LY, Lee NS, Yang C, Neumann WL, Freskos JN, Shieh JJ, Dorshow RB, Wooley KL. J. Am. Chem. Soc. 2011;133:8534–8543. doi: 10.1021/ja200182t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee NS, Sun G, Lin LY, Neumann WL, Freskos JN, Karwa A, Shieh JJ, Dorshow RB, Wooley KL. J. Mater. Chem. 2011;21:14193–14202. doi: 10.1039/C1JM11854D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann WL, Dorshow RB, Freskos JN, Wooley KL, Lee NS, Lin LY, Sun G. 2011 May 15; U.S. Patent11/36,392.
- 55.Lammers T, Kiessling F, Hennink WE, Storm G. Mol. Pharm. 2010;7:1899–1912. doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- 56.Turner JL, Chen Z, Wooley KL. J. Controlled Release. 2005;109:189–202. doi: 10.1016/j.jconrel.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 57.Huang H, Remsen EE, Kowalewski T, Wooley KL. J. Am. Chem. Soc. 1999;121:3805–3806. [Google Scholar]
- 58.Turner JL, Wooley KL. Nano Lett. 2004;4:683–688. [Google Scholar]
- 59.Cheng C, Qi K, Khoshdel E, Wooley KL. J. Am. Chem. Soc. 2006;128:6808–6809. doi: 10.1021/ja061892r. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, Remsen EE, Wooley KL. J. Am. Chem. Soc. 2000;122:3642–3651. [Google Scholar]
- 61.Moughton AO, Patterson JP, O'Reilly RK. Chem. Commun. 2011;47:355–357. doi: 10.1039/c0cc02160a. [DOI] [PubMed] [Google Scholar]
- 62.Rossin R, Pan D, Qi K, Turner JL, Sun X, Wooley KL, Welch MJ. J. Nucl. Med. 2005;46:1210–1218. [PubMed] [Google Scholar]