Abstract
The purpose of this research was to evaluate the factor structure of the Pittsburgh Sleep Quality Index (PSQI) in rheumatoid arthritis (RA). The sample included 107 patients with RA, 88 females and seven males, with an average age of 56.09 years, recruited from the greater Southern California area. Confirmatory factor analysis evaluated single, two- and three-factor models. The single factor solution yielded a poor fit to the data. While the three-factor solution had the best fit, the two-factor solution, comprised of sleep efficiency and perceived sleep quality factors, was optimal because it had very good fit, and acceptable reliability for its individual factors. Clinical indices were consistently correlated with the sleep quality factor, but not with the sleep efficiency factor.
Keywords: sleep quality, rheumatoid arthritis, confirmatory factor analysis, PSQI
Rheumatoid arthritis (RA) is an inflammatory, autoimmune disease characterized by joint pain, joint swelling, and fatigue that can lead to significant disability and impairments in role functioning (Kirwan et al., 2003; Verbrugge & Juarez, 2006). In addition, studies have shown a very high prevalence of sleep disturbance in RA patients including difficulties with initiating and maintaining sleep, poor sleep quality, and sleep fragmentation (Hirsch et al., 1994; Wolfe, Michaud, & Li, 2006). Importantly, poor sleep in RA may contribute to increased pain and disability in some patients (Luyster, Chasens, Wasko, & Dunbar-Jacob, 2011). The etiology of poor sleep in RA is not well understood, although previous research has shown that pain and depression contribute to greater self-reported sleep disturbance (Nicassio et al., 2012; Nicassio & Wallston, 1992).
While a limited amount of research has used polysomnographic assessment to identify objective features of sleep (Drewes et al., 1998; Mahowald, Mahowald, Bundlie, & Ytterberg, 1989), most studies of sleep disturbance in RA have relied on self-report instruments that provide a global estimate of patients’ sleep quality. The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is a well-established, commonly used measure of sleep disturbance that has been applied across a range of adult populations, including those with RA and other medical conditions (Burkhalter et al., 2010; Carpenter & Andrykowski, 1998). The PSQI has also been used as an outcome measure in clinical trials evaluating interventions for both pain and sleep (Cole, Dubois, & Kosinski, 2007). The PSQI is comprised of 19 items, yielding a total score of sleep disturbance. While the scale has acceptable internal consistency employing the total score, recent studies have raised questions about the unidimensionality of the scale, and have suggested that a two- or three-factor solution may provide a better fit and increase our understanding of the complexity of patients’ self-reported sleep problems (Cole et al., 2006; Skouteris, Wertheim, Germano, Paxton, & Milgrom, 2009). For example, Cole et al. (Cole et al., 2006) reported that a three-factor solution, comprised of sleep efficiency, perceived sleep quality, and daily disturbances provided optimal fit in a sample of community dwelling depressed and non-depressed adults 60 years of age and older.
Other studies have supported the multidimensional nature of the PSQI with somewhat varying results. Burkhalter et al. (Burkhalter et al., 2010) replicated the Cole et al. (Cole et al., 2006) three-factor solution with slight modifications in a sample of renal transplant patients, while Skouteris et al. (Skouteris et al., 2009) found that two factors, sleep efficiency and night and daytime disturbances, and an overall sleep quality factor, provided the best fit in a sample of pregnant women. In an Australian adult sample, Magee et al. (Magee, Caputi, Iverson, & Huang, 2008) provided additional evidence confirming the superiority of the two-(perceived sleep quality and sleep efficiency) and three-factor solutions reported by Cole et al. (Cole et al., 2006). Collectively, these studies suggest that a single score of overall sleep quality from the PSQI underestimates the complexity of self-reported sleep problems, and that the measure may not perform uniformly in different clinical populations.
Although the PSQI has been used in research with RA (Nicassio et al., 2012), its factor structure has not been explored in patients with this medical condition. Given previous evidence that the PSQI may be comprised of multiple dimensions, the further use of the PSQI would benefit significantly from an understanding of its scale structure in RA. For example, the relationship between different components of sleep disturbance and clinical functioning could be determined, which could affect our understanding of the importance of sleep in patients’ health outcomes and inform treatment decisions. The major objective of this research was to examine the factor structure of the PSQI in an outpatient RA population recruited from the greater Southern California area. We adopted confirmatory factor analytic (CFA) techniques to evaluate single, two, and three factor solutions of the PSQI. A secondary objective was to provide evidence of the validity of the PSQI factor solutions by examining the relationship between factors and measures of psychological functioning, pain, fatigue, disease activity, disability, and health-related quality of life (HRQOL) in RA patients.
Method
Participants
Patients were recruited through newspaper advertisements and flyers posted in clinic offices in the divisions of rheumatology at UCLA and Cedars Sinai Medical Center (CSMC), Los Angeles, to participate in a psychosocial research program designed to help them manage their RA. After a telephone screening at UCLA, patients participated in a physical examination at CSMC during which the diagnosis of RA was confirmed, and an evaluation of disease activity was carried out. Patients were then referred to the UCLA Cousins Center for an evaluation of their sleep, clinical functioning, and psychiatric status. Details on the recruitment and evaluation process have been reported previously (Nicassio et al., 2011).
Eligible patients (a) were 18 years of age or older, (b) met American College of Rheumatology (ACR) revised criteria for RA, (c) were stable on disease-modifying drug regimen for three months prior to study entry, (d) had a stable disease course for three months (no major changes requiring medication changes or administration of injected or pulse corticosteroids), (e) were free of serious co-morbid medical conditions such as diabetes, congestive heart failure, renal failure, or cancer that would confound interpretations of health status, (f) were not pregnant, (g) did not have a serious psychiatric condition such as bipolar disorder, psychosis, or post-traumatic stress disorder, and (h) were not suicidal. All patients underwent a psychiatric evaluation using the Structured Clinical Interview for DSM-IV Disorders (Spitzer, Williams, & Gibbon, 1987), under the direction of the project psychologist (PN) and psychiatrist (MRI).
Measures
The data in this report were obtained from a baseline evaluation of patients prior to their participation in a clinical trial evaluating behavioral treatments for RA. We report findings from self-report measures of sleep, fatigue, pain, disease activity, illness beliefs, depression, disability, and health-related quality of life.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI (Buysse et al., 1989) measured patients’ reported sleep quality over the past month. The PSQI is comprised of 19 items measuring the following seven components: (a) subjective sleep quality, (b) sleep latency, (c) sleep duration, (d) habitual sleep efficiency, (e) sleep disturbances, (f) use of sleep medications, and (g) daytime dysfunction. While two and three factor solutions have been proposed (Cole et al., 2006; Skouteris et al., 2009), a PSQI score of five or greater, based on the total scale score, is recommended as the cutoff for detecting sleep impairment in a range of populations (Buysse et al., 1989).
RA disease activity/pain
The DAS-28 (Fransen, Creemers, & Van Riel, 2004) and the Rapid Assessment of Disease Activity in Rheumatology (RADAR; Mason et al., 1992) measured patients’ disease activity. The DAS-28 is a physician-based measure consisting of the number of tender and swollen joint counts (0 to 28), erythrocyte sedimentation rate (ESR), and patient global score (0 to 100). The RADAR is a self-report measure, consisting of questions about past and current disease activity, pain, morning stiffness, and the degree of pain/tenderness in 10 joints on the right and left sides of the body. Items are rated on a 4-point Likert scale; the sum score may range from 0 to 60, with higher scores indicating more severe joint pain/tenderness. The RADAR has been shown to be a valid proxy for physician assessments of disease activity and joint pain (Wong et al., 1999).
Depression
The Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) measured the severity of depressive symptoms over the past month. The CES-D has been used extensively in studies with patients with arthritis (Nicassio et al., 2011; Nicassio, Schoenfeld-Smith, Radojevic, & Schuman, 1995). Scores may range from 0 to 60, with higher scores signifying the presence of more symptomatology. A score of 16 or greater is recommended as the cutoff for detecting depressive disorder.
Illness beliefs
The 5-item Helplessness and 7-item Internality Subscales of the Arthritis Helplessness Index (AHI; Nicassio, Wallston, Callahan, Herbert, & Pincus, 1985; Stein, Wallston, Nicassio, & Castner, 1988) assessed patients’ beliefs about their ability to manage RA (Helplessness subscale, score range 0–30; Internality subscale, score range 0–36). While the Helplessness subscale measures the perceived inability to control RA symptoms and disease course (e.g., “No matter what I do or how hard I try, I just can’t seem to get relief from my pain”), the Internality subscale measures perceived control over RA (e.g., “Managing arthritis is my own responsibility”).
Disability
Patients’ disability was assessed by the Stanford Health Assessment Questionnaire Disability Index (HAQ-DI; Bruce & Fries, 2003). The HAQ-DI assesses the patients’ perceived difficulty in completing tasks in eight categories — dressing, arising, eating, walking, hygiene, reach, grip, and usual activities. The eight category scores were averaged into an overall HAQ-DI score and may range from 0 (no disability) to 3 (completely disabled).
Health-related quality of life (HRQOL)
The SF-36 physical and mental health functioning scores (Ware & Sherbourne, 1992) evaluated generic HRQOL. The SF-36 consists of 36 items tapping eight different components of well-being. Scores for each component may range from 0 to 100, with higher scores indicating higher levels of functioning.
Fatigue/vitality
Patients’ fatigue was assessed with the Multidimensional Assessment of Fatigue Scale (MAF; Belza, 1995) and the SF-36 Vitality Subscale (Ware & Sherbourne, 1992). Used previously in research with RA patients (Belza, 1995), the MAF consists of 16 items measuring severity and impact of fatigue. Scores range from 1 to 50, with higher scores representing more severe fatigue. The SF-36 Vitality Subscale is comprised of four items. Scores may range from 0 to 100, with higher scores indicating higher levels of vitality.
Data Analysis
Evaluation of the PSQI was initiated with examination of the scale’s coefficient alpha and the corrected component-to-scale correlations. A Cronbach’s alpha reliability statistic of .70 is considered as the minimum value for adequate internal consistency (Kline, 1993, 2000). An adjusted component-to-scale correlation exceeding .30 is considered acceptable (Nunnally & Bernstein, 1994). To assess convergent validity, Pearson’s correlation coefficients were calculated between PSQI total and factor scores and measures of RA disease activity/pain (DAS-28 and RADAR scores), depression (CES-D total score), illness beliefs (AHI Helplessness and Internality subscales), disability (HAQ-DI score), HRQOL (SF-36 physical and mental health functioning scores), and fatigue/vitality (MAF score and SF-36 vitality subscale). Additionally, a t-test was used to assess known groups validity by comparing the PSQI global scores of patients who reported sleep restlessness in the past week on the CES-D to those who did not (rarely or not at all restless versus restless).
Confirmatory factor analysis was conducted using EQS 6.1 (Bentler, 2005) with the maximum likelihood (ML) method of estimation to evaluate three alternate models. These were a single factor model (model 1) including the seven component subscales, a two-factor correlated model (model 2) comprising sleep duration and habitual sleep efficiency as one factor and perceived sleep quality, sleep latency, sleeping medications, sleep disturbances, and daytime dysfunction as factor two. In the three-factor correlated model (model 3), the sleep disturbances and daytime dysfunction subscale components are specified as loading on a separate, third factor. In view of the ordinal nature of the PSQI component subscales, Robust methodologies were used. The ML Robust approach produces accurate approximations of standard errors and fit statistics in the presence of departures from assumptions multivariate normality associated with examination of categorical variables (Bentler, 2005).
An acceptable fit between a model and the data is reflected by a non-significant Satorra-Bentler Scaled Statistic (S-Bχ2; Satorra & Bentler, 1988), an S-Bχ2 to df ratio of less than 2.0 (Tabachnick & Fidell, 2001), a robust Comparative Fit Index (CFI) of 0.95 or greater (Bentler, 2005), and a robust root mean square error of approximation (RMSEA) of less than 0.05 (Browne & Cudeck, 1993). Further, because complex models generally yield a better fit than simple models, the robust Akaike’s information criterion (AIC; Akaike, 1974), a composite measure of fit and parsimony of models, was also considered. Smaller, more negative AIC values are considered indicative of better model fit (Keith, 2006).
Results
Sample Characteristics
A total of 107 patients were included in the study. This sample size exceeds the minimum of 100 recommended for covariance structure modeling (Kline, 2005; Joreskog & Sorbom, 1989). Table 1 shows demographic characteristics of the sample. The sample consisted of 88 females and 19 males, with an average age of 56.09 years and illness duration of 10.75 years. Participants came from a range of ethnicities. Caucasians were the most prevalent group, but patients from African-American, Hispanic, and Asian ethnicities were also represented. The sample can be characterized as middle to upper middle class, possessing almost 16 years of education on average, and a median household income of greater than $50,000.
Table 1.
Sample characteristics (n = 107)
| Variable | Mean ± SD or N (%) |
|---|---|
| Age (years) | 56.09 ± 12.45 |
| Annual income ($) | 51,142 ± 18,631 |
| Education in years | 15.95 ± 2.40 |
| Gender | |
| Male | 19 (17.76) |
| Female | 88 (82.24) |
| Race/Ethnicity | |
| White | 56 (52.34) |
| Hispanic | 16 (14.95) |
| Black | 11 (10.28) |
| Asian/Pacific Islander | 8 (7.48) |
| Other race/ethnicity | 16 (14.95) |
| Marital Status | |
| Married | 46 (42.99) |
| Divorced/separated | 26 (24.30) |
| Widowed | 9 (8.41) |
| Single | 13 (12.15) |
| Other/unknown | 13 (12.15) |
| Illness duration (years) | 10.75 ± 11.23 |
Internal Consistency
Overall scale internal consistency was adequate (α = .733). Removal of the use of sleep medicine component increased the internal consistency of the scale (α = .754). Corrected component-total correlations reached an acceptable level for all components (rs ≥ .40), except for use of sleep medicine (r = .295). Therefore, in an effort to maximize scale reliability, the use of the sleep medicine component was excluded from subsequent analyses. This PSQI scale comprised of six components demonstrated acceptable reliability.
Confirmatory Factor Analysis
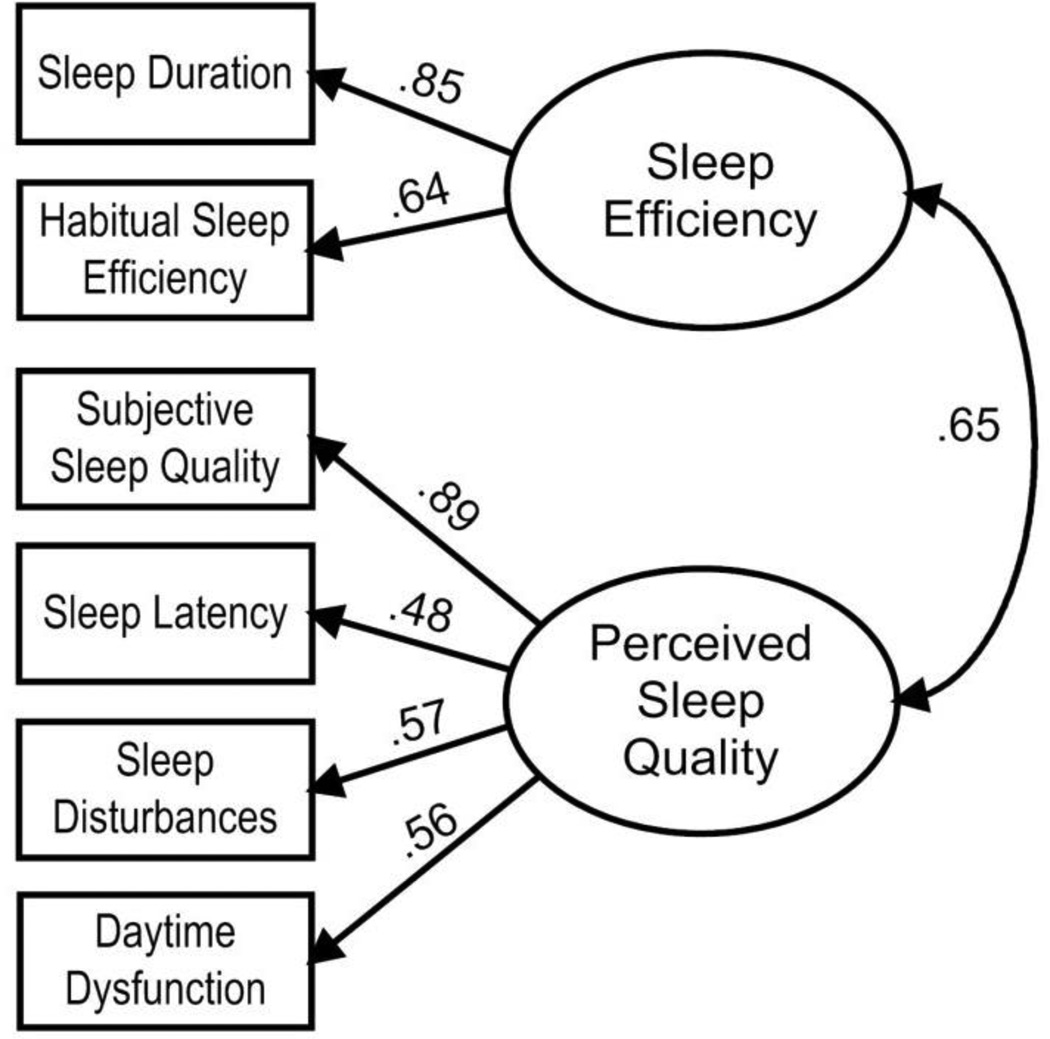
Table 2 presents the results of CFAs for the one-factor, two-factor and three-factor models. The single factor solution yielded a poor fit to the data, S-B χ2(9) = 19.88, p = .019, S-B χ2/df = 2.21, robust CFI = .894, robust RMSEA = .107, and thus was not supported in favor of the alternate models. While the three-factor solution had the best fit, S-B χ2(6) = 0.60, p = .996, S-B χ2/df = 0.10, robust CFI = 1.00, robust RMSEA < .001, it was not considered in further analyses because of unsatisfactory internal consistency for the perceived sleep quality factor (α = .58) and daily disturbances factor (α = .53). The two-factor model attained satisfactory fit statistics, S-B χ2(8) = 3.63, p = .889, S-B χ2/df = 0.45, robust CFI = 1.00, robust RMSEA < .001, and also demonstrated adequate internal consistency, with alpha values for the sleep efficiency and sleep quality factors of .70 and .71, respectively. Fit indexes, internal consistency values and model parsimony demonstrated that the two-factor solution fits the data well and warrants further attention (see Figure 1).
Table 2.
Fit Indices
| Model | S-B χ2 | df | p | Robust CFI |
Robust RMSEA |
Robust AIC |
|---|---|---|---|---|---|---|
| Model 1: Single factor model | 19.88 | 9 | .019 | .894 | .107 | 1.88 |
| Model 2: Two factor model | 3.63 | 8 | .889 | 1.00 | <.001 | −12.37 |
| Model 3: Three factor model | 0.60 | 6 | .996 | 1.00 | <.001 | −11.40 |
Note. S-B χ2 Satorra–Bentler scaled statistic; CFI = comparative fit index; RMSEA = root mean squared error of approximation; AIC = Akaike’s information criterion.
Figure 1.
Pittsburgh Sleep Quality Index 2-factor model with standardized path coefficients. All coefficients are signficant at p < .001.
Construct Validity
Correlations between the PSQI and theoretically related measures are presented in Table 3. All measures were significantly associated with the perceived sleep quality factor, with the exception of the DAS-28 and HAQ-DI. In contrast, only MAF fatigue and SF-36 vitality were correlated with the sleep efficiency factor. In addition, the mean PSQI global score among individuals who endorsed sleep restlessness on the CES-D (M = 7.08, SD = 3.29) was significantly higher than among those who reported little to no sleep restlessness (M = 4.18, SD = 3.26), t(104) = −4.51, p < .001. It is notable that the mean PSQI score was less than 5.0 in individuals who denied sleep restlessness, which is consistent with using a score of under 5.0 to indicate good sleep quality (Buysse et al., 1989).
Table 3.
Correlations between PSQI factor scores and related constructs
| Sleep Efficiency Factor |
Perceived Sleep Quality Factor |
PSQI Total | |
|---|---|---|---|
| RADAR total | .056 | .241* | .202* |
| DAS-28 total | .078 | .019 | .042 |
| CES-D total | .063 | .483*** | .337*** |
| AHI helplessness | .121 | .259** | .229** |
| AHI internality | −.146 | −.248** | −.254** |
| HAQ-DI score | .038 | .063 | .074 |
| SF-36 physical | −.068 | −.205* | −.172 |
| SF-36 mental/emotional | −.198* | −.465*** | −.424*** |
| MAF global fatigue | .227** | .577*** | .513*** |
| SF-36 vitality | −.230* | −.608*** | −.524*** |
p < .05,
p < .01,
p < .001.
Discussion
The accurate measurement of sleep quality assumes considerable clinical significance in RA as research has documented the prevalence and impact of poor sleep in this medical condition (Hirsch et al., 1994; Luyster et al., 2011). The purpose of this research was to evaluate the factor structure of the PSQI and to examine the measure’s validity through its relationship with indices of clinical functioning.
This research has provided additional evidence that the PSQI is a multi-dimensional measure with a sample of RA patients. Confirmatory factor analyses revealed that a two-factor solution consisting of sleep efficiency, comprised of sleep duration and habitual sleep efficiency, and sleep quality, comprised of subjective sleep quality, sleep latency, sleep disturbances, and daytime dysfunction, provided an optimal solution in this sample based on the criteria of goodness-of-fit and reliability of the individual factors. The two-factor solution was superior to the one-factor solution whose fit was poor, and the three-factor solution whose factors were not as reliable as those in the two-factor solution. These findings corroborate the results from studies in medical populations that have documented that two or three-factor solutions are more representative of the PSQI’s scale structure than a single factor model (Burkhalter et al., 2010; Carpenter & Andrykowski, 1998).
It is noteworthy that the two-factor model did not include the sleep medicine use component which was omitted in the CFA because its correlation with the total scale score was low, thereby reducing the internal consistency of the PSQI. The data suggest that the report of sleep medicine use may not be an accurate barometer of poor sleep, and hence, may not correlate very well with other dimensions of the PSQI that measure sleep directly. Instead, sleep medicine use assesses the way in which patients may cope with sleep problems. Patients may use sleep medicines to fall asleep to reduce their distress about sleeping or to ensure that they will sleep in order to function the next day. By itself, sleep medicine use may or may not reflect actual sleep disturbance. The sleep medicine use component may thus reduce the construct validity of the PSQI and diminish the clinical utility of the PSQI as a measure of sleep quality. Its use in assessing sleep quality in RA populations is not supported by these data.
Correlations between indices of clinical functioning and the two factors showed that self-reported disease activity, depression, helplessness, internality, fatigue, and SF-36 HRQOL measures had stronger relationships with the sleep quality factor than with sleep efficiency. Correlations with the sleep quality factor and the PSQI total were similar, indicating that the PSQI total also is sensitive to RA clinical indices. However, the sleep quality factor may serve as a valid surrogate for the total scale in future research. In contrast, sleep efficiency was correlated very modestly with fatigue, vitality, and SF-36 emotional functioning. Other studies have demonstrated that RA patients report difficulties with the quality of their sleep and fatigue the next day (Mancuso, Paget, & Charlson, 2000; Wolfe et al., 2006). Moreover, studies using polysomnography have documented the existence of sleep fragmentation, frequent awakenings from sleep, and unrestorative sleep in this population, all of which are indicators of poor sleep quality (Mahowald et al., 1989). The data converge with previous findings that sleep disturbance in RA is related to the emotional and physical functioning of patients, raising the question that a reciprocal relationship may exist between sleep disturbance and health outcomes in this medical condition (Nicassio et al., 2012). RA symptoms and emotional functioning may both affect, and be affected by, poor sleep (Irwin et al., in press). Sleep efficiency, on the other hand, as a measure of sleep duration and the degree to which time asleep matches patients’ amount of time in bed, was not shown to be as sensitive to the clinical manifestations of RA or its impact. Sleep efficiency may reflect the influence of other factors such as sleep hygiene practices or sleep behaviors that may not possess a strong association with the quality of sleep. Nevertheless, the sleep efficiency factors remains important as a separate dimension in understanding sleep disturbance in these patients. Sleep efficiency constitutes a major clinical target for behavioral interventions for sleep disturbance. However, research on behavioral interventions to address sleep efficiency in RA populations has yet to be conducted.
Despite the high prevalence of sleep complaints in pain populations, including those with arthritis (Blagestad et al., 2012; Haack et al., 2012), research on the relationship between aspects of sleep disturbance and clinical functioning has been limited. The quality of future studies will benefit from the use of sensitive and valid indices of sleep that will be appropriate for such populations. This research has furthered our understanding of the use of the PSQI in patients with RA, an autoimmune disorder chiefly characterized by chronic pain, stiffness, and fatigue. Future research on populations with other pain conditions such as osteoarthritis, fibromyalgia, and chronic low back pain will shed further light on the relevance of the factor structure of the PSQI and its sensitivity to clinical parameters in these kinds of patient groups that share similarities with RA patients. Based on the results of this research, it appears as though the PSQI sleep quality factor may be a robust indicator of pain, distress, and fatigue, and may be relevant for use with other pain conditions.
In sum, this research demonstrated that the PSQI is comprised of sleep efficiency and sleep quality factors in RA. The CFA illustrated that a two-factor solution provided an excellent fit to the data and was highly superior to a solution based on a single factor model. However, it is hoped that the findings from this study will lead to further research that would provide more definitive information on the significance of the two-factor solution. Studies on the psychometric properties and performance of the two factor model, including test-retest reliability, longitudinal analyses, and individual response theory would enhance our understanding of the value of this solution to the PSQI.
Nevertheless, the results of this study have significant clinical implications. One is that the treatment of sleep disturbance in RA should focus on enhancing the quality of sleep rather than sleep efficiency alone. While behavioral interventions for insomnia in nonmedical populations have tended to focus on sleep efficiency as the main target (Irwin, Cole, & Nicassio, 2006), it is unclear whether this would be appropriate for RA patients with sleep disturbance. Another is that the treatment of RA disease activity and symptoms may affect the quality of sleep. The use of the PSQI as a two-dimensional scale will provide important, new information regarding how the treatment of RA affects both the reported efficiency and quality of sleep in afflicted patients.
Acknowledgments
This work was supported by AR R01-049840 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health to PMN. Also supported in part by, HL R0-079955, R01-AG034588, R01-AG026364, R01-CA119159, R01 HL095799, P30-AG028748, UL RR 033176 to MI, and the Cousins Center for Psychoneuroimmunology.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. Journal of Rheumatology. 1995;22(4):639–643. [PubMed] [Google Scholar]
- Blagestad T, Pallesen S, Lunde LH, Sivertsen B, Nordhus IH, Gronli J. Sleep in older chronic pain patients: a comparative polysomnographic study. Clinical Journal of Pain. 2012;23(4):277–283. doi: 10.1097/AJP.0b013e3182313899. [DOI] [PubMed] [Google Scholar]
- Bentler PM. EQS 6 structural equations program. Encino, CA: Multivariate Software; 2005. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage; 1993. pp. 136–162. [Google Scholar]
- Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. Journal of Rheumatology. 2003;30(1):167–178. [PubMed] [Google Scholar]
- Burkhalter H, Sereika SM, Engberg S, Wirz-Justice A, Steiger J, De Geest S. Structure validity of the Pittsburgh Sleep Quality Index in renal transplant recipients: A confirmatory factor analysis. Sleep and Biological Rhythms. 2010;8(4):274–281. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Cole JC, Dubois D, Kosinski M. Use of patient-reported sleep measures in clinical trials of pain treatment: A literature review and synthesis of current sleep measures and a conceptual model of sleep disturbance in pain. Clinical Therapeutics. 2007;29(11):2580–2588. doi: 10.1016/j.clinthera.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- Drewes AM, Svendsen L, Taagholt SJ, Bjerregård K, Nielsen KD, Hansen B. Sleep in rheumatoid arthritis: A comparison with healthy subjects and studies of sleep/wake interactions. Rheumatology (Oxford) 1998;37(1):71–81. doi: 10.1093/rheumatology/37.1.71. [DOI] [PubMed] [Google Scholar]
- Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 2004;43(10):1252–1255. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Mullington JM. Pain sensitivity and modulation in primary insomnia. European Journal of Pain. 2012;16(4):522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M, Carlander B, Verge M, Tafti M, Anaya JM, Billiard M, Sany J. Objective and subjective sleep disturbances in patients with rheumatoid arthritis. Arthritis and Rheumatism. 1994;37(1):41–49. doi: 10.1002/art.1780370107. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychology. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, FitzGerald JD, Ranganath VK, Nicassio PM. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. doi: 10.5665/sleep.1742. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL 7.16: A guide to the program and application. 2nd ed. Chicago: SPSS Inc.; 1989. [Google Scholar]
- Keith TZ. Multiple regression and beyond. Boston: Allyn & Bacon; 2006. [Google Scholar]
- Kirwan J, Heiberg T, Hewlett S, Hughes R, Kvien T, Ahlmen M, Taal E. Outcomes from the Patient Perspective Workshop at OMERACT 6. Journal of Rheumatology. 2003;30(4):868–872. [PubMed] [Google Scholar]
- Kline P. The handbook of psychological testing. London: Routledge; 1993. [Google Scholar]
- Kline P. A psychometrics primer. London: Free Association Books; 2000. [Google Scholar]
- Kline RB. Principles and practices of structural equation modeling. 2nd ed. New York: Guilford Press; 2005. [Google Scholar]
- Luyster FS, Chasens ER, Wasko MC, Dunbar-Jacob J. Sleep quality and functional disability in patients with rheumatoid arthritis. Journal of Clinical Sleep Medicine. 2011;7(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- Magee Christopher A, Caputi Peter, Iverson Donald C, Huang Xu-Feng. An investigation of the dimensionality of the Pittsburgh Sleep Quality Index in Australian adults. Sleep and Biological Rhythms. 2008;6(4):222–227. [Google Scholar]
- Mahowald MW, Mahowald ML, Bundlie SR, Ytterberg SR. Sleep fragmentation in rheumatoid arthritis. Arthritis and Rheumatism. 1989;32(8):974–983. doi: 10.1002/anr.1780320806. [DOI] [PubMed] [Google Scholar]
- Mancuso CA, Paget SA, Charlson ME. Adaptations made by rheumatoid arthritis patients to continue working: A pilot study of workplace challenges and successful adaptations. Arthritis Care and Research. 2000;13(2):89–99. [PubMed] [Google Scholar]
- Mason JH, Anderson JJ, Meenan RF, Haralson KM, Lewis-Stevens D, Kaine JL. The rapid assessment of disease activity in rheumatology (RADAR) questionnaire. Arthritis and Rheumatism. 1992;35(2):156–162. doi: 10.1002/art.1780350206. [DOI] [PubMed] [Google Scholar]
- Nicassio PM, Kay MA, Custodio MK, Irwin MR, Olmstead R, Weisman MH. An evaluation of a biopsychosocial framework for health-related quality of life and disability in rheumatoid arthritis. Journal of Psychosomatic Research. 2011;71(2):79–85. doi: 10.1016/j.jpsychores.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicassio PM, Ormseth SR, Kay M, Custodio M, Irwin MR, Olmstead R, Weisman MH. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2012;153(1):107–112. doi: 10.1016/j.pain.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicassio PM, Schoenfeld-Smith K, Radojevic V, Schuman C. Pain coping mechanisms in fibromyalgia: Relationship to pain and functional outcomes. Journal of Rheumatology. 1995;22(8):1552–1558. [PubMed] [Google Scholar]
- Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. Journal of Abnormal Psychology. 1992;101(3):514–520. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- Nicassio PM, Wallston KA, Callahan LF, Herbert M, Pincus T. The measurement of helplessness in rheumatoid arthritis. The development of the arthritis helplessness index. Journal of Rheumatology. 1985;12(3):462–467. [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric theory. 3rd ed. New York: McGraw-Hill; 1994. [Google Scholar]
- Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Satorra A, Bentler PM. 1988 Proceedings of Business and Economics Sections. American Statistical Association; 1988. Scaling corrections for chi-square statistics in covariance structure analysis; pp. 308–313. [Google Scholar]
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J. Assessing sleep during pregnancy: A study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues. 2009;19(1):45–51. doi: 10.1016/j.whi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams JB, Gibbon M. Instruction manual for the Structured Clinical Interview for DSM-III-R (SCID) New York: Biometrics Research Department, New York State Psychiatric Institute; 1987. [Google Scholar]
- Stein MJ, Wallston KA, Nicassio PM, Castner NM. Correlates of a clinical classification schema for the arthritis helplessness subscale. Arthritis and Rheumatism. 1988;31(7):876–881. doi: 10.1002/art.1780310708. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4th ed. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- Verbrugge LM, Juarez L. Profile of arthritis disability: II. Arthritis and Rheumatism. 2006;55(1):102–113. doi: 10.1002/art.21694. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: evaluation by medical outcomes study and visual analog sleep scales. Journal of Rheumatology. 2006;33(10):1942–1951. [PubMed] [Google Scholar]
- Wong AL, Wong WK, Harker J, Sterz M, Bulpitt K, Park G, Paulus H. Patient self-report tender and swollen joint counts in early rheumatoid arthritis. Western Consortium of Practicing Rheumatologists. Journal of Rheumatology. 1999;26(12):2551–2561. [PubMed] [Google Scholar]