Abstract
This study aimed to characterize social cognition, executive functions (EFs), and everyday social functioning in adolescent girls with fragile X syndrome, and identify relationships among these variables. Participants were 20 girls with FXS and 20 typically developing peers. Results showed significant between-groups differences in social cognition, accounted for by differences in IQ and language. Within the FXS group, IQ and language were related to social cognition; parent-reported social functioning was related to language and EFs; and self-reported social functioning was generally good and not related to cognitive or social cognition variables. Results suggest that intervention might focus on managing language and cognitive contributions to social functioning, rather than social cognition, and underscore the importance of considering parent and adolescent perspectives. (120 words)
Keywords: social cognition, fragile X syndrome, executive function, social outcome, adolescent
Fragile X syndrome (FXS) is the leading known cause of intellectual disability in females, with the full mutation occurring in an estimated 1 in 6000–9000 females (Crawford, Acuna, & Sherman, 2001) and the premutation form occurring in an estimated 1/161females in a U.S. sample (Seltzer et al., 2012). The associated cognitive deficits range from mild to severe. Intellectual disability is less common in females with FXS than in males, although as many as 25% of affected females have IQs of 70 or below (Hagerman, et al., 1992).
FXS in females is associated with a variety of behavioral impairments. Chief among these are social impairments, which range from frankly autistic behavior to atypical social behaviors, social “oddness”, and social isolation (Hagerman, 1999; Hagerman, et al., 1992; Keysor & Mazzocco, 2002). Females with FXS, like males with FXS, have been reported to show gaze avoidance, social anxiety, and shyness (Hagerman, 1999), and may receive a childhood diagnosis of avoidant disorder (Freund, Reiss, & Abrams, 1993). Social deficits have been linked to abnormalities of the hypothalamic-pituitary axis and in autonomic habituation to stimuli (Hessl, et al., 2002; Hessl, Rivera, & Reiss, 2004; Keysor, Mazzocco, McLeod, & Hoehn-Saric, 2002), although the relation of these physiological findings to performance on specific tasks is only beginning to be studied.
Studies that have addressed social cognition as a contributor to social dysfunction in individuals with FXS have generally focused on impairments in Theory of Mind (ToM), defined as the ability to understand that others have thoughts and that these thoughts influence their behavior (Baron-Cohen, Leslie, & Frith, 1985). The results of these studies, however, have been mixed. Cornish and colleagues (2005) administered two commonly used tests of ToM, a false belief task and an appearance-reality task, to boys with FXS (n = 28) or Down Syndrome (DS) (n = 26). The false belief task was the classic Sally Anne, which involves distinguishing between the participant’s accurate beliefs about an object’s location and a story character’s false belief (Wimmer & Perner, 1983), and the altered reality task, which requires the participant to state the color of an object that was covered by a cloth of a different color (e.g., state that an orange was really orange although it was covered by a white cloth). Although Cornish et al. found that the groups were similar in average accuracy on ToM items (50%), most of the errors of the FXS group were realist errors (i.e., failing to see an object in its altered state), whereas boys in the DS group made mostly phenomenalist errors (i.e., seeing the altered state as the true state, regardless of reality). Cornish et al. interpreted this finding to suggest that males with FXS might have difficulty differentiating appearance from reality, supporting a deficit in ToM. By contrast, Mazzocco and colleagues (1994) found no differences between a group of adult females with FXS (n = 19) and a comparison group of intellectually typical adult females (n = 56) on a task requiring the participant to tell a story in way that distinguished her perspective from that of another person, once IQ was controlled. The task used by Mazzocco et al. was developed for children, however, and might have been insufficiently sensitive to deficits in social-cognitive constructs that might be problematic for adolescents or adults. In summary, the extent to which social cognition is impaired relative to age expectations, and thus a source of impairments in social functioning, in females with FXS is unclear. The present study was designed to address this issue by focusing on a young group – adolescent girls with FXS – and comparing them to age-matched typically developing girls using a more developmentally appropriate measure of social cognition.
It also is possible that deficits in social functioning observed in girls with FXS are due to their well-documented impairments in executive function (EF; Keysor & Mazzocco, 2002; Kirk, Mazzocco, & Kover, 2005; Sobesky, et al., 1996; Wilding, Cornish, & Munir, 2002) rather than to impairments in social cognition per se. EFs are the cognitive functions that allow us to achieve goal-directed behaviors (Mesulam, 2002) and include the ability to update working memory (WM), shift from one mental set to another, and inhibit thoughts and actions according to the context or task at hand. Impairments in the WM and inhibitory control facets of EF are particularly common in females with FXS (Hagerman, 1999; Keysor & Mazzocco, 2002). It has been suggested that EF impairments influence social functioning indirectly through their relationship with ToM, as ToM requires EF skills such as the ability to inhibit one’s own perspective and shift perspectives according to the context. Indeed, the notion that ToM impairments are related to deficits in EF has been discussed in the context of a variety of clinical groups that share some social features with FXS, including individuals with autism (Perner, Stummer, & Lang, 1999), Down Syndrome (Zelazo, Burack, Benedetto, & Frye, 1996), schizophrenia (Bowie & Harvey, 2005), frontotemporal dementia (Lough, et al., 2006), and focal frontal lobe lesions (Channon & Watts, 2003; Stuss & Alexander, 2000; Turkstra, Dixon, & Baker, 2004; Turkstra, McDonald, & DePompei, 2001; Turkstra, McDonald, & Kaufmann, 1996). Note that EF impairments are not fully accounted for by lower IQ in FXS (Bennetto, Pennington, Porter, Taylor, & Hagerman, 2001; Keysor & Mazzocco, 2002; Mazzocco, Pennington, & Hagerman, 1993).
The contribution of EFs to social cognition in FXS was suggested by Mazzocco and colleagues (1993, 1994). In a follow-up analysis in the study of social cognition described above, the authors compared social cognition test scores to scores on 11 neuropsychological tests reported in an earlier study (Mazzocco, et al., 1993). Tests included the Wisconsin Card Sort Test (WCST; Heaton, 1981) and the Contingency Naming Test (CNT; Anderson, Anderson, Northam, & Taylor, 2000), each of which measures inhibitory control, an aspect of EF. Details were not provided, but Mazzocco et al. stated that there was “no main effect [of perspective-taking scores] on any of the dependent variables” (p. 482) and “no consistent relations” (p 482) between any of the neuropsychological test scores and emotion recognition. Thus, it appeared that EF test scores were not related to social cognition in that cohort of women with FXS. There is a need, however, to examine this issue further given the well-documented relationship between EF and social cognition in typically developing individuals and in various disorders, as noted in the previous paragraph, as well as the problems already noted with the measure of social cognition used by Mazzocco and colleagues.
In summary, deficits in social cognition and EFs have been reported in children and adults with FXS, but the relationship between these two types of functions is not clear. There is a gap in knowledge about girls with FXS, a group that includes individuals with social problems despite IQs in the normal range. The hypothesis that impairments in EFs and social cognition are related in girls with FXS has implications for intervention, as interventions aimed at improving social cognition per se (e.g., training in emotion recognition, use of scripted social scenarios) are likely to be ineffective if the core problem is in the flexible implementation of goal-oriented social behavior in real-life contexts.
In addition to studying the relation of EFs to social cognition, the present study aimed to situate cognitive deficits in the larger context of social functioning in everyday life. Impairments in both EFs and social cognition are likely to affect the social functioning of girls with impairments in these domains by affecting the ability to adapt thinking and behavior to the social context (Crone & Dahl, 2012), but the extent to which social acceptance is a problem for adolescent girls with FXS is unknown. The literature on individuals with autism and other developmental disorders suggests that social cognitive skills are a prerequisite for peer-appropriate social performance (Adolphs, 1999; Siegal & Varley, 2002) and may permit the acquisition of culture-specific social knowledge (Marschark, Green, Hindmarsh, & Walker, 2000; Russell, et al., 1998). Thus, individuals with social-cognitive impairments may experience progressive separation from peers due to a combination of poor ability to “read” a social situation and a lack of knowledge of appropriate responses. This may lead to isolation and reduced opportunity for social activity, which , in turn, may undermine growth of social competence (Russell, et al., 1998). Adolescence is a time of increasing socialization with – and enjoyment of – peers, and separation from family (Csikszentmihalyi, Larson, & Csikszenthi, 1984; Raffaelli & Duckett, 1989). If an adolescent with FXS is unable to meet typical age expectations for social behavior, isolation may result, which may produce a variety of negative effects on subjective well-being and health (Baumeister & Leary, 1995). Thus, the study of adolescent social acceptance is important not only for theoretical and clinical reasons but also as a public health issue.
Restated within the World Health Organization International Classification of Functioning, Disability, and Health (2001), the present study explored relations among impairments at the level of body structures or functions (i.e., social cognition and EFs) and between these impairments and limitations in activities and participation in social life. To assess the latter two factors, participants completed a social self-perception measure, the Self-Perception Profile for Adolescents (Harter, 1988). Self-perception was included because of evidence that higher levels of self-perceived competency (that is, seeing oneself as better off than others do) are associated with enhanced coping (Armor & Taylor, 1998; Hoffman, Cole, Martin, Tram, & Seroczynski, 2000; Taylor & Brown, 1988). Participants’ parents completed the Vineland Adaptive Behavior Scales (Sparrow, Cicchetti, & Balla, 2005) to assess others’ perspectives on participants’ competence.
In summary, there is a need for more data on the social functioning of adolescent females with FXS, including data on how their social functioning is viewed by others and themselves, whether they have impaired social cognition, and the relationships among social functioning, social cognition, and EF impairments. There also is a need for understanding the social functioning of these girls relative to their typically developing age peers so that we can determine the extent of impairments and whether the determinants are similar across the two groups of girls. Thus, the present study was designed to address the following questions:
Do adolescent girls with FXS have impairments in social cognition, and do they and others judged them to have impaired social functioning? It was hypothesized that adolescent girls with FXS would have significantly lower scores than typically developing age-matched peers on tests of social cognition and measures of parent- and self-reported social functioning in everyday life.
Is the social-cognitive functioning of adolescent girls with FXS predicted by their level of EF? It was hypothesized that scores on tests of social cognition would have a significant positive relationship to EF test scores over and above the contribution of language and general intelligence. Are the patterns of relationships different relative to typically developing age-matched peers?
Is the social behavior of adolescent girls predicted by their levels of social-cognitive functioning? It was hypothesized that social cognition test scores would have a significant positive relationship to parent and self-ratings of social functioning in everyday life. Are the patterns of relationships different relative to typically developing age-matched peers?
Although hypotheses 2 and 3 related primarily to girls with FXS, in general the literature on social cognition in adolescent girls is limited. Thus, these two hypotheses were tested both in girls with FXS alone and also in typically developing peers.
Method
Participants
Participants were 20 adolescent females with a confirmed diagnosis of FXS (mean age = 14.91 years) and 20 typically developing (TD) females individually matched for age + 1 year (mean age = 15.43 years). Because the goals of the study included interests in determining whether adolescent girls with FXS had social-cognitive impairments and in examining perceptions of their skill in navigating the adolescent social world, the groups were matched on chronological age. As age matching leads to a mismatch on important dimensions of competence and behavior, we used ANCOVA and multiple regression to examine the contributions of IQ and other variables to between- and within-group differences in social cognition and social functioning. All participants were Caucasian. Participants were recruited through local and national organizations, and through a school-based participant registry for typical adolescents.
Inclusion criteria for all participants were as follows:
-
1
A pure tone, air-conduction threshold of 30 dB HL or better in each ear (averaged across 500, 1000, and 2000 Hz); scores at or above the age-equivalent score for 8 years 6 months (approximately Grade 3) on the Synonyms (raw score = 18) and Grammaticality Judgment (raw score = 38) tests of the Comprehensive Assessment of Spoken Language (CASL; Carrow-Woolfolk, 1999). The preceding two criteria ensured participants could hear and understand experimental stimuli. Based on transcription of a random sample of stimuli used in the social cognition experimental tasks, the language level was Grade 1 or below (Flesch, 1994); thus, requiring comprehension at a Grade 3 level controlled for the possibility that language comprehension affected task performance.
-
3
English as a first language and primary language spoken at home, by caregiver report.
Parents of adolescents with FXS were asked to supply a copy of results of molecular genetic testing (i.e., PCR and Southern blot analysis of a peripheral blood sample) confirming that each adolescent had the full mutation of the FMR1 gene (i.e., > 200 CGG repeats). Participants with FXS were recruited if they used speech as their primary means of communication, regularly used three-word or longer utterances, had no uncorrected physical or sensory impairments that would limit participation, and did not meet criteria for a primary diagnosis of Autism Spectrum Disorder (i.e., participants may have had social problems but were not diagnosed with autism).
Girls in the TD group were required to have no history of medical or neurological disease affecting the brain, language or learning disability, receipt of special education services, or gifted status, by parent report, for minors, or self-report, for participants ages 18–21 years.
Cognitive Tests
Intelligence
IQ was determined by using the subtests comprising the Brief IQ composite of the Leiter International Performance Scales Revised (Roid & Miller, 1997). The Leiter is a completely nonverbal test for individuals ages 2–20 years. The test was normed on 1719 persons matched to the 1993 U.S. census and has a high correlation with IQ as measured by the Wechsler Intelligence Scales for Children – Third Edition (Wechsler, 1991); and reliability and validity coefficients range from .67 to .90. The Leiter was designed to be “uninfluenced by educational, social, and family experience” (Roid & Miller, 1997), and was chosen to minimize potential effects of expected differences in verbal skills between groups. The subtests comprising the Brief IQ are Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. It should be noted that visuospatial deficits have been reported in some girls with FXS (Cornish, Munir, & Cross, 1998), but these were primarily on tests of visual construction rather than visual perception. Scaled scores were entered into data analysis.
Language
Participants were administered the Comprehensive Assessment of Spoken Language (CASL; Carrow-Woolfolk, 1999). The CASL provides comprehensive assessment of oral language in children and adolescents ages 3–21 years. The battery was standardized on 1700 individuals from across the United States, and meets nationally accepted criteria for reliability and validity (American Educational Research Association, 1999). At the time of the study, the CASL was the only standardized language test that included measures of social communication skills specific to adolescents, had norms for adolescents ages 13–21 years, and met standard criteria for validity and reliability. The CASL is a collection of tests, including several that are specifically aimed at language functions developing during adolescence, such as comprehension of ambiguous and nonliteral language. The CASL also includes a Pragmatic Judgment (PJ) Test, in which examinees are asked to generate appropriate responses in hypothetical social contexts. Thus, it was possible to generate scores for both age-appropriate general language skills as well as social language skills. Scaled scores for the CASL core composite were used in data analysis. PJ Test scores were reported for descriptive purposes.
EFs
The Tasks of Executive Control (TEC; Isquith, Roth, & Gioia, 2010) is a computerized battery of tasks designed to overcome the limitations of previous tests of EFs, particularly the difficulty disentangling the contributions of WM and inhibitory control to task performance. The TEC was chosen for the present study because WM and inhibitory control were two aspects of EFs that were found to be impaired in FXS in previous research (Bennetto, et al., 2001; Cornish, Munir, & Cross, 2001; Hagerman, 1999; Keysor & Mazzocco, 2002; Sobesky, et al., 1996; Wilding, et al., 2002). The TEC shares features with the CNT (Anderson, et al., 2000), which also manipulates demands for WM and inhibitory control, but has the added advantage of computerized presentation and specific tests for each parametric manipulation of target constructs. The design of the TEC involves serial presentation of visual stimuli in six different tasks, with stepwise increases in WM load and response-inhibition demands across tasks. Stimuli are simple two-dimensional drawings of common objects familiar to children, and are presented in pseudorandom order within each task. There are two parametric manipulations of WM and inhibitory demand across tasks: 1) tasks progress from simple target detection (i.e., press the X key when you see the target stimulus and the Y key for all other stimuli) to one-back and two-back target detection (e.g., press the X key when the stimulus is the same as the one presented two stimuli previously and otherwise press the Y key); and 2) every other trial is an inhibit trial in which there is an exception to the rule (e.g., follow the preceding rule except when the stimulus has a box around it, then press the Y key). Participants were administered a research version of the TEC (Gioia, Isquith, & Roth, 2006), provided by test authors. On the recommendation of the test authors (P. Isquith, personal communication, January 3, 2011), total number correct across trials was converted to a percent and entered into data analysis.
Social Cognition Tests
Faux Pas Test (Gregory, et al., 2002; Stone, Baron-Cohen, & Knight, 1998). Baron-Cohen and colleagues (1999) originally developed the Faux Pas Test as an advanced test of ToM that would be appropriate for older children, and it was subsequently adapted for use with adults (Gregory, et al., 2002; Stone, et al., 1998). The test is comprised of a series of short, spoken vignettes, half of which include a faux pas (e.g., a girl insults cafeteria staff when a boy, out of her hearing, just said his mother worked in the cafeteria). The examinee is asked whether someone said something they shouldn’t have said (i.e., if a faux pas had occurred; if so, what it was; and the mental state of the person who made the faux pas (e.g., did the girl know the boy’s mother worked in the cafeteria). The Faux Pas Test was chosen for the study for several reasons. First, comprehension of faux pas is thought to be the most developmentally advanced use of ToM (Baron-Cohen, et al., 1999), maturing by around age 11 years; thus, the Faux Pas Test was expected to be more sensitive to between-groups differences in adolescents than a simple first-order false belief test. Second, the Faux Pas Test has revealed between-groups differences in studies of other clinical populations with social disabilities, such as autism and Asperger Syndrome (Baron-Cohen, et al., 1999), acquired brain injury (Martin-Rodriguez & Leon-Carrion, 2010; Muller, et al., 2009), and frontotemporal dementia (Gregory, et al., 2002). Third, there is evidence that faux pas comprehension is impaired in children with social anxiety (Banerjee & Henderson, 2001), which is common among girls with FXS. Last, in a longitudinal study of typically developing children (Banerjee, Watling, & Caputi, 2011), faux pas comprehension was related to measures of social acceptance; thus, Faux Pas test scores were expected to correlate with measures of everyday social functioning.
Participants listened to a series of 10 stories, which were recorded on audiotape to eliminate visual cues to comprehension. Each story was followed by a series of questions requiring detection of the faux pas and description of what that person did or did not know in the scenario and his or her intent. There were two follow-up questions requiring recall of main facts from each story. An additional 10 stories served as controls. The adult version was used, as an ongoing study by the first author revealed a ceiling effect on the child version when administered to older adolescents. The test yielded a maximum score of 60 for faux pas items and 20 for control items. The total score for faux pas items was converted to a percent for data analysis.
Reading the Mind in the Eyes Test-Child Version (Baron-Cohen, Wheelwright, Scahill, Lawson, & Spong, 2001). The Reading the Mind in the Eyes Test (“Eyes Test”) was developed as a measure of “mentalizing”, the ability to read an individual’s thoughts by looking only at his or her eyes. The Eyes test was used for the present study because, like Faux Pas, the Eyes Test is considered an advanced test of social cognition (Muller, et al., 2009) and thus, is appropriate for adolescents. The Eyes Test has been widely used to study social cognition in children, adolescents, and adults with a variety of developmental and acquired disorders, including autism spectrum disorders (Baron-Cohen, Wheelwright, Scahill, et al., 2001), acquired brain injury (Turkstra, 2008), and psychiatric disorders such as schizophrenia (Bora, Eryavuz, Kayahan, Sungu, & Veznedaroglu, 2006) and depression (Wang, Wang, Chen, Zhu, & Wang, 2008). Scores on the Eyes Test differentiate premutation carriers from typical peers, even after controlling for IQ and age (Cornish, Kogan, et al., 2005), and a test of emotion recognition (an element of the Eyes Test) has revealed social cognition impairments specifically in women and girls with FXS (Mazzocco, et al., 1994). Of interest given estimates of social anxiety ranging from 23% to 50% in girls with FXS (Keysor & Mazzocco, 2002), Eyes Test scores in typically developing young women with high social anxiety were higher than in women with low social anxiety (Sutterby, Bedwell, Passler, Deptula, & Mesa, 2012)
The Eyes Test has a child version (Baron-Cohen, Wheelwright, Scahill, et al., 2001) and an adult version (Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). The child version was used in the present study as it is not subject to a ceiling effect in adolescents, and the vocabulary of the adult version includes low-frequency words (e.g., incredulous, despondent, pensive) that might have been beyond the vocabulary level of younger TD adolescent participants and those in the FXS group. The test consists of 28 black and white photographs of the eye region of the face of individual men and women. Each photograph has two words printed above it and two below. Participants are asked to choose one of the four words that best describes what the pictured person is thinking or feeling. The test yields a maximum score of 28, which was converted to a percent correct for data analysis.
Measures of Everyday Social Functioning
Self-Perception
Social self-perception was measured using the Harter Self-Perception Profile for Adolescents (SPPA; Harter, 1988), a 45-item questionnaire. The SPPA and its pediatric equivalent, the Self-Perception Profile for Children (Harter, 1985), are the most widely used and studied measures of their kind (John, 2001). The SPPA has been studied extensively in typical adolescents (e.g., Shapka & Keating, 2005; Todd & Kent, 2003; Wichstrom, 1995), and has shown discriminant validity and reliability in a wide variety of clinical groups, including adolescents with physical, social, and intellectual disabilities. The SPPA has nine subscales: academic, physical, physical appearance, job competence, romantic appeal, behavioral conduct, social, close friendships, and general self-worth. Raw scores from the last five subscales (in italics) were averaged for use in data analysis.
Todd and Kent (2003) used a version of the SPPA developed previously by Wichstrøm (1995), who modified the format be more easily comprehensible to respondents. In its original version, the SPPA had two contrasting descriptions of adolescents on opposite sides of the page (e.g., “some teenagers have a lot of friends” on the left; “other teenagers do not have very many friends” on the right) and thus, the participant was asked to choose which of the two statements best described him or her, and how closely (is it “really” vs. “sort of” true). Wichstrøm (1995) modified the item presentation so that only a single description appeared, the original item on the left, which was rephrased in the first person (e.g., “I have a lot of friends”), and the respondent then chose one of four responses: (1) describes me very well, (2) describes me fairly well, (3) describes me a little, and (4) does not describe me at all. Wichstrøm (1995) reported that the modified version had higher internal consistency than the original (mean alpha of .77 vs. 67), and had acceptable convergent validity and reliability (data were not provided). The use of the modified test ensured that even participants with lower IQs were able to understand the questionnaire format.
Parent perception
Parent perception of social life was measured using the Socialization domain of the Parent/Caregiver Report Form of the VABS-II (Sparrow, et al., 2005). The VABS-II was designed to measure personal and social skills for the purpose of diagnosis, determining eligibility for services, intervention planning, and research (Sparrow, et al., 2005). The VABS-II was normed on 3695 persons from early childhood to age 90 years, with demographic characteristics matching the 2001 U.S. census. Reliability and validity coefficients range from .58–.91, and VABS-II scores have a high correlation with scores on the Adaptive Behavior Assessment System – Second Edition (Harrison & Oakland, 2003). The VABS-II is widely used for the evaluation of individuals with clinical disorders such as intellectual disability and autism spectrum disorders. Unlike other commonly used parent measures of social behavior (e.g., the Child Behavior Checklist; Achenbach & Edelbrock, 1980), the Parent/Caregiver Report Form of the VABS-II includes norms for individuals up to age 21 years 11 months and thus, was well-suited for the present study. Scaled scores for the Socialization domain were used in data analysis.
Procedures
Once informed assent and consent was obtained from participants and their caregiver each participant completed standardized language and cognitive assessments and experimental tasks. Caregivers completed the VABS. Tasks and tests were administered in random order. Participants were tested at the XXX. One participant was unable to complete two of the CASL tests on site due to travel constraints, and the missing tests were administered by a speech-language pathologist in the participant’s home community. Hearing screenings were completed by a trained research assistant or certified audiologist, and the results of school- or hospital-based hearing testing within the previous two years were used when available.
Participants were seen in one session of approximately 3 hours, with breaks as needed, and were paid $50 for their participation. Travel costs were reimbursed for participants driving from outside of the local area.
Data Analysis
Hypothesis 1 was tested using one-way analyses of variance (ANOVAs), with Bonferroni correction for multiple comparisons (criterion alpha =.017). Follow-up analyses of covariance (ANCOVAs) were conducted to explore the relative contribution of IQ, language, and EF test scores to any group differences on social measures. Hypothesis 2 was tested by conducting a multivariate regression analyses with IQ, language, and EFs as predictors of the scores on the two social cognition tests. Hypothesis 3 was tested using separate regression analyses for each of the two measures of everyday social functioning, with the two social cognition test scores as predictors and age as a covariate for the SPPA analysis. The criterion alpha level for Hypotheses 2 and 3 was 05/2 = .025.
Standard scores created from age-stratified normative samples were used where available (e.g., for the CASL, Leiter, VABS-II, and IQ tests). Previous studies by the authors (e.g., Turkstra, Dixon, & Baker, 2004) and others have not shown age effects on social cognition tests from ages 13–21 years; thus, social cognition test scores were not corrected for age. Age was significantly correlated with SPPA scores, r = .39, p = .01, and the correlation of age and TEC scores approached significance, r = .32, p = .06. Thus, age was entered as a covariate in regression and correlation analyses involving SPPA and TEC scores.
Results
Hypothesis 1: Between-Groups Differences
Average scores for the FXS and TD groups on all measures are listed in Table 1. TD group scores were significantly higher than FXS group scores on the CASL, t(37) = 6.77, p < .001; and Leiter, t(38) = 7.32, p < .001. The CASL and Leiter scaled scores of all TD participants were above 85 (i.e., 1 SD below the mean of the standardization sample). CASL core composite scores were more than 1 SD below 85 for 11 of 19 girls in the FXS group (data from one participant were missing), and CASL Pragmatic Judgment Test scores were below average for 11 of 20 girls in the FXS group. Leiter scores were more than 1 SD below average for 14 girls in the FXS group.
Table 1.
Test scores for TD and FXS groups. Standard deviations are in parentheses. All between-groups differences are significant (p’s < .01). CASL = Comprehensive Assessment of Spoken Language; TEC = Tasks of Executive Control; SPPA = Self-Perception Profile for Adolescents; VABS-II = Vineland Adaptive Behavior Scales, Second Edition.
| TD (n = 20) | FXS (n = 20) | |||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Range | Mean (SD) | Range | |
| Leiter IQ | 111.30 (12.11) | 87–137 | 73.15 (19.91) | 42–111 |
| CASL Core Composite | 114.75 (7.61) | 98–123 | 85.63 (17.59) | 40–119 |
| TEC Total Correct | 515.35 (18.52) | 473–540 | 402.67 (84.98) | 248–491 |
| Faux Pas Percent Correct | 91.75 (7.17) | 75–100 | 73.69 (13.00) | 47.5–95 |
| Eyes Test Percent Correct | 75.60 (7.56) | 60.71–90.48 | 61.97 (15.64) | 39.29–89.29 |
| SPPA Score | 3.40 (.42) | 2.6–4 | 2.94 (.62) | 1.6–4 |
| VABS-II Scaled Score for Socialization | 108.63 (15.45) | 77–135 | 77.44 (13.53) | 59–100 |
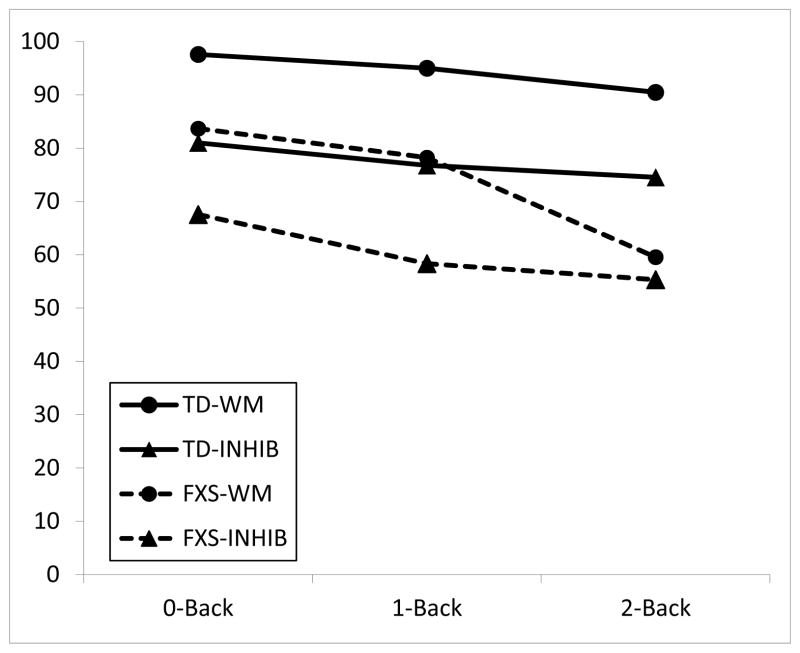
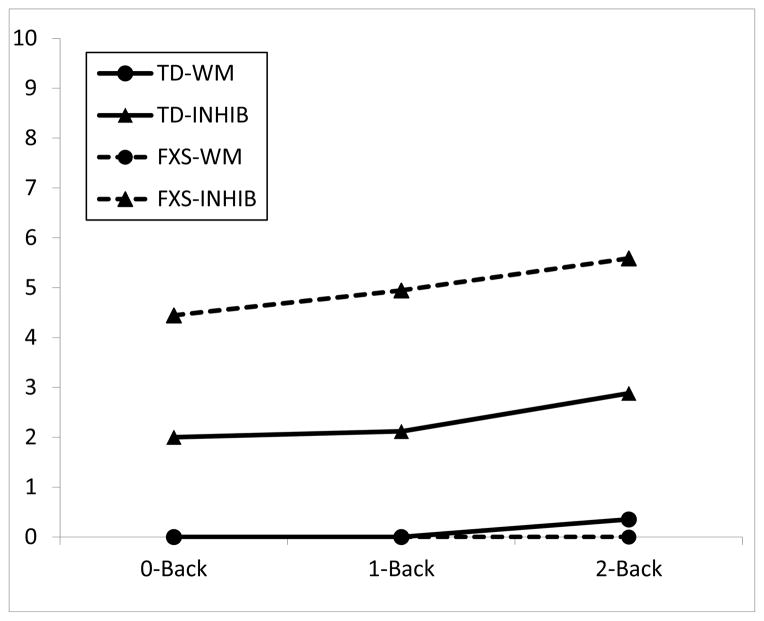
TEC data are shown in Figure 1 (percent accuracy) and Figure 2 (commission errors). For percent accuracy, there also was a significant effect of group, F(1,99) = 35.24, p < .001; and condition, F(2, 99) = 16.98, p < .001; and no significant interaction of group by condition, F(2, 99) = .07, p = .93. For inhibition, there was a significant effect of group, F(1,99) = 63.65, p < .001; and condition, F(2, 99) = 4.34, p < .05; and no significant interaction of group by condition, F(2, 99) = 2.38, p = .10. Figure 2 shows that participants in both groups made commission errors primarily on inhibition trials; that is, errors were not false positive responses on the basic n-back trials, but rather were errors inhibiting responses on the target-in-box trials. The between-groups difference was no longer significant if Leiter scores were entered as a covariate, F (1, 31) = 1.87, p = .18.
Figure 1.
Total correct responses on the TEC. TD = typical peers, FXS = fragile X group. WM = working memory trials (n-back only), INHIB = inhibition trials (n-back + inhibit response to target picture if picture is in a box).
Figure 2.
Commission errors on the TEC. TD = typical peers, FXS = fragile X group. WM = working memory trials (n-back only), INHIB = inhibition trials (n-back + inhibit response to target picture if picture is in a box).
There were significant between-groups differences on the SPPA, t(37)= 2.03, p > .05; and VABS-II, t(35) = 6.52, p < .001. VABS-II questionnaires were returned by parents of 18 participants in the FXS group (all mothers) and 19 participants in the TD group (14 mothers, 4 fathers, and 1 for which the identity of the parent could not be determined). Scores for 13 of 18 participants in the FXS group were in the clinical range, vs. 2 of 19 in the TD group.
An ANOVA revealed a significant between-groups difference on the Eyes Test, F(1,38) = 12.30, p = .001. This difference was no longer significant when Leiter, CASL, and TEC scores were added as covariates, F(1,27) = .33, p = .57, with only IQ contributing significantly, F(1,27) = 5.06, p < .05. Similarly, an ANOVA revealed a significant between-groups difference on the Faux Pas Test, F(1,38) = 29.61, p < .001, but this difference was no longer significant when Leiter, CASL, and TEC scores were added as covariates, F(1,27) = .57, p = .46, with a significant contribution of CASL scores only, F(1,27) = 9.59, p < .005.
Hypothesis 2: Language, Executive Functions, and IQ vs. Social Cognition
Regression results are shown in Table 2. For the FXS group, the combination of Leiter, CASL, and TEC scores and age accounted for 69% of variance in Faux Pas scores, p < .005, with significant contributions of TEC scores, t = -3.14, p < .01, and CASL scores, t = 5.13, p < .001; and no significant contribution of Leiter scores, t = .35, p = .73; or age, t = 1.47, p = .17. A univariate regression revealed no significant correlation between TEC scores and Faux Pas test scores, adjusted R-squared = 1.04, p = .59; but when language test scores were taken into account lower TEC scores are associated with higher scores on the Faux Pas test. The combination of Leiter, CASL, and TEC scores and age accounted for 54% of variance in Eyes Test scores, p < .05, with only Leiter scores contributing significantly, t = 2.52, p < .05; and no significant contribution of TEC scores, t = .17, p = .87; CASL scores, t = .57, p = .58; or age, t = 1.48, p = .16. Thus, language and executive functions predicted scores on the verbal test of social cognition, and nonverbal IQ predicted scores on the visuospatial test of social cognition.
Table 2.
Regression of IQ, language, executive function test scores, and age on social cognition test scores. FXS = fragile X, TD = typically developing; CASL = Comprehensive Assessment of Spoken Language; TEC = Tasks of Executive Control.
| FXS Group | TD Group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Measure | Predictor | B | SE B | β | B | SE B | β |
| Faux Pas Test | Leiter | .06 | .12 | .04 | .44 | .15 | .28 |
| CASL | 1.12** | .16 | .82 | .12 | .23 | .26 | |
| TEC | −.78** | .04 | −.12 | −.28 | .12 | −.11 | |
| Age | .27 | .10 | .14 | .09 | .09 | .03 | |
| Eyes Test | Leiter | .55* | .18 | .44 | .37 | .16 | .24 |
| CASL | .15 | .23 | .13 | .26 | .25 | .26 | |
| TEC | .05 | .06 | .01 | .21 | .13 | .09 | |
| Age | .33 | .14 | .20 | .13 | .10 | .05 | |
p < .05
p < .01
For the TD group, the combination of Leiter, CASL, and TEC scores and age accounted for 24% of variance in Faux Pas scores, which was not significant, p = .47. Likewise, the combination of Leiter, CASL, and TEC scores and age accounted for 31% of variance in Eyes Test scores, p = .31, with no significant contribution of any test variable.
Hypothesis 3: Social Cognition vs. Everyday Social Functioning
Regression results are shown in Table 3. For the FXS group, the regression of Eyes and Faux Pas scores on SPPA scores approached significance, adjusted R-squared = .32, p = .026, with only Eyes Test scores contributing significantly, t(3, 16) = 2.20, p = .04. The regression of Eyes and Faux Pas scores on VABS scores was not significant, adjusted R-squared = .17, p = .14.
Table 3.
Regression of social cognition tests on self- and parent-reported social functioning. FXS = fragile X, TD = typically developing; SPPA = Self-Perception Profile for Adolescents; VABS-II = Vineland Adaptive Behavior Scales, Second Edition.
| FXS Group | TD Group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Measure | Predictor | B | SE B | β | B | SE B | β |
| SPPA | Eyes Test | .43* | .004 | .01 | .17 | .01 | ..01 |
| Faux Pas Test | .13 | .006 | .003 | −.10 | .01 | −.004 | |
| Age | .36 | .003 | .005 | .23 | .003 | .003 | |
| VABS-II | Eyes Test | .26 | .29 | .21 | −.32 | .51 | −.64 |
| Faux Pas Test | .37 | .27 | .45 | .24 | .53 | .51 | |
| Age | .21 | .12 | .11 | .04 | .15 | .03 | |
p < .05
p < .01
For the TD group, the regression of Eyes and Faux Pas scores on SPPA scores was not significant, adjusted R-squared = −.10, p = .73. Likewise, the regression of Eyes and Faux Pas scores on VABS scores was not significant, adjusted R-squared = −.06, p = .59.
Discussion
Adolescent girls with FXS are at high risk for social problems, but the mechanisms underlying these problems are unknown. The aims of the present study were to describe social cognition in this at-risk group and test hypotheses about factors contributing to performance, specifically language, EFs, and IQ. A main motivation for the study was that intervention for a core impairment in social cognition would be quite different from intervention for social performance problems related to underlying deficits in domain-general cognitive functions. A second motivation was to link social cognition to everyday social functioning, not only as rated by parents but also as rated by girls with FXS themselves.
Results of the study provided partial support for the study hypotheses, and also revealed unexpected findings that could have important clinical implications. In the following sections, we discuss each study hypothesis, then consider ways in which the results can inform clinical assessment and intervention for adolescent girls with FXS.
Hypothesis 1: Between-groups differences in social cognition and everyday social functioning
Differences in social cognition
There were statistically significant differences between the FXS and typical groups on two measures of social cognition: “reading” thoughts and feelings from a photograph of the eye region of a face, and understanding faux pas in spoken stories. These findings supported the first study hypothesis. These differences, however, were accounted for by between-groups differences in IQ and language, and thus did not suggest a core deficit in social cognition in girls with FXS. Findings were similar to those of previous research in women with FXS, which showed no difference in social cognition between women with FXS and typical peers once IQ was controlled (Mazzocco, et al., 1994).
Differences in everyday social functioning
There were statistically significant differences between the FXS and typical groups in self- and parent-reported social functioning in everyday life. Although there was a between-groups difference in self-reported acceptance, adolescents in both groups rated their social acceptance as generally good, and overall mean scores for both groups were similar to those for typical Norwegian adolescents who completed the modified version of the SPPA (Wichstrom, 1995) that was used in the present study (Norwegian N = 11,315, M = 3.09, SD = .49; vs. FXS M = 2.82, SD = .31; and TD M = 3.06, SD = .30). By contrast, 13 of 18 parent ratings of social functioning in the FXS group (76%) were below the average range for the standardization sample, compared to 2 of 19 ( 11%) in the typical group. The effect size for group differences in parent-reported social functioning (ES = 1.46) also was substantially larger than for self-reports (ES = .63); that is, parents perceived a greater difference in social functioning than their daughters did themselves, and the majority of parents in the FXS groups reported clinically significant social problems in their daughters.
The finding of higher self- than parent ratings of social functioning in adolescents with FXS is consistent with results of other studies of adolescents with disabilities (e.g., Burgess & Turkstra, 2010; Hughes, Turkstra, & Wulfeck, 2007), in which adolescents rated their own social lives as being better than their parents perceived. The underlying cause of the discrepancy in self- vs. parent-rated outcomes is unknown. It might be due to failure of girls with FXS to accurately report problems, or possibly a lack of metacognitive skills in girls with FXS, resulting in failure to appreciate their social problems or understand their social standing in relation to peers. It also might be due to a need to depict one’s social life in a positive light, which is not uncommon in typical adolescents (Ames & Kammrath, 2004; Pakaslahti & Keltikangas-Jarvinen, 2000). Discussing a similar pattern in self- vs. parent-reported social anxiety in girls with FXS, Keysor and Mazzocco (2002) stated:
“This discrepancy may reflect that parents either attribute or perceive more anxiety in their daughter than she actually experiences, a failure of girls with FraX to report their symptoms accurately, or a combination of these. An alternative explanation…is that girls with FraX may acknowledge their symptoms without recognizing the magnitude of their impairment.” (p. 183)
One possible explanation for the discrepancy between parent and self-reports does not invoke lack of insight or disclosure on the part of adolescent participants: parents might be over-reporting problems. Adolescents in general have hyper-acute perception of social life and their place within it (Sumter, Bokhorst, Steinberg, & Westenberg, 2009), suggesting they are quite able to identify their own social strengths and limitations. In a qualitative study by Jones (2012), adolescents with intellectual disabilities shared comments such as, “I hate being MR because people make fun of me” (p. 35), showing awareness of peer social judgments even in adolescents with impaired cognition. It should be noted that reports from girls in the FXS group, albeit within the average range, were statistically lower than those of typical peers (i.e., indicating a perception of less social acceptance). Thus, as observed in other adolescent clinic groups (Daley & Weisner, 2003; Hughes, Turkstra, & Wulfeck, 2009), the present cohort might have shown the typical developmental tendency to overestimate their social competence, but nevertheless seemed to be aware of social differences between themselves and their peers.
Burgess and Turkstra (2010) found similar discrepancies in self- vs. mothers’ reports of social functioning in adolescent boys with high-functioning autism/Asperger syndrome, and suggested that the two parties might have different but equally accurate perceptions. The authors suggested that mothers’ ratings were based on an “adult perspective” of what adolescent experience was like for their child, possibly in comparison to recollections of parents’ own childhoods or their hopes for their children, and noted the importance of recognizing that perceptions can be different but still accurate. Thus, everyday social experiences of girls in the present study could be positive at the same time as their parents saw challenges. It is worth noting that typical adolescents’ behaviors with parents often differ substantially from their behaviors with peers, in part because time with peers typically is more desirable than time with parents (Larson, 1983). An addition, adults’ recollection of adolescent social life tends to have a negative bias that is inconsistent with actual experience (Hendry & Reid, 2000); as a result, parents’ ratings might be confounded by their own recollection biases. Parent ratings also might be influenced by concerns for the future. Thus, a discrepancy in scores does not necessarily imply that girls with FXS lacked awareness of their problems.
Regardless of the cause of self- vs. parent-report discrepancies, on a self-perception measure of social acceptance the person completing the ratings is the “gold standard.” If participants have insufficient metacognitive skills to evaluate their own social lives by adult criteria, they also might have insufficient metacognitive skills to fully appreciate their social problems, and thus might feel satisfied with their current status. This raises questions about the wisdom of intervention that aims to “improve” social skills and social acceptance, if the adolescent herself does not perceive a problem.
Hypothesis 2: Correlates of social cognition
Cognitive test scores accounted for a statistically significant proportion of scores on social cognition measures in girls with FXS. Contrary to predictions, however, EF scores were not among the positive predictors. Instead, the contribution of language test scores to scores on the most language-demanding social cognition test was statistically significant, and nonverbal IQ contributed at a statistically significant level to scores on the most visuospatially demanding social cognition test. An unexpected result was that in the FXS group, there was a statistically significant negative correlation between EF scores and scores on the Faux Pas test (i.e., higher EF scores were associated with lower Faux Pas scores). The lack of a positive correlation between Faux Pas comprehension and EFs was surprising. Faux pas comprehension is a prototypical test of WM and inhibitory control. Stimuli are spoken stories that are 53 to 96 words in length and each includes several units of meaning, including proper names of two or more characters. All of this information must be kept in mind while answering a series of follow-up questions. In addition, faux pas comprehension requires the examinee to maintain two competing interpretations in WM (literal vs. nonliteral) and inhibit the literal meaning in favor of the nonliteral meaning. In theory, the EF demands of the Faux Pas test were well matched by the TEC, which required participants to hold information in mind and inhibit a prepotent tendency to respond in favor of an alternative choice.
Before concluding that EFs truly have a negative relationship with faux pas comprehension in girls with FXS, it is important to consider alternative explanations for the results. First, examination of the raw data suggested that the negative correlation could be due to the influence of two participants in the FXS group who had very low nonverbal IQ but language test scores in the average range. In a sample of this size, two participants with extreme scores could have exerted a disproportionate effect. Second, non-construct factors might have influenced performance. The most likely confound was language ability, suggested by the strong positive correlation between CASL and Faux Pas test scores. It is possible that in a clinical group with known language impairments, such as the girls with FXS tested here, the effect of language could outweigh the influence of EFs. Using the Flesch-Kincaid formula (Flesch, 1994), the Faux Pas stimulus paragraphs vary from Grade 1 to Grade 5, which should have been within the comprehension ability of all participants. The Flesch-Kincaid formula, however, has been shown to underestimate reading level by as much as two grades (Mailloux, Johnson, Fisher, & Pettibone, 1995), however, so the reading level of some stimuli might have been challenging for participants in the FXS group. Flesch-Kincaid also measures only the number of syllables, words, and sentences, which does not account for syntax such as embedded sentence complement structures, which are inherent in ToM-type questions (e.g., what does [Y think about X]). Thus, the effects of language might have outweighed any contribution of EFs, so that EF scores acted as a suppressor variable in the regression. Future research could use written or pictured stimuli to decrease language demands, but this could limit ecological validity, as pictures and writing are not available in participants’ everyday social lives.
A final possibility is that another EF measure might have revealed different results. Although the TEC was chosen specifically because it measures the types of EFs thought to be involved in faux pas comprehension, several studies of EFs in women with FXS have used the WCST and CNT (Bennetto, et al., 2001; Kirk, et al., 2005; Simon, Keenan, Pennington, Taylor, & Hagerman, 2001; Sobesky, et al., 1996), and these might have had a stronger relationship with social cognition. It should be noted, however, that the WCST and CNT appear to test cognitive processes similar to those tested on the TEC.
EF test scores likewise did not make a statistically significant contribution to scores on the Eyes Test. This null finding might have resulted from the fact that the Eyes Test had a minimal WM load, as the stimuli were individual pictures, shown for an unlimited time, and word choices were visible throughout. The demands on inhibitory control likewise were low, as participants had no constraints on responding other than to choose only one of four words to describe the feelings or thoughts of the person pictured. In a previous study of typical adults (Ahmed & Stephen Miller, 2011), Eyes Test scores did not correlate with scores on tests of EFs, although EF tests in that study focused on cognitive flexibility rather than inhibitory control and WM. A lack of correlation between EF tests and Eyes Test scores has been reported in other populations as well, however, including adults with traumatic brain injury (Muller, et al., 2009) and Huntington Disease (Eddy, Sira Mahalingappa, & Rickards, 2012), suggesting that the Eyes Test indeed has low EF demands. For this reason, the Eyes Test is unlikely to capture social cognition challenges of everyday life, in which stimuli must be processed rapidly in complex environments. Perhaps a more dynamic test of emotion recognition, such as the Emotions subtest of the video-based Awareness of Social Inference Test (TASIT; McDonald, Flanagan, & Rollins, 2002) would be more linked to EFs, although TASIT includes only basic emotions presented at relatively long durations, and thus might miss subtle and fleeting social emotions that are typical in adolescent life (e.g., disdain, impatience, desire).
The trend toward a positive correlation between Eyes Test scores and IQ was consistent with results of previous studies in typical young adults (Ahmed & Miller, 2011; Peterson & Miller, 2012), and suggests that the Eyes Test performance is influenced by domain-general cognitive functions. The most likely non-social contributor to Eyes Test performance is vocabulary. Consistent with this, Peterson and Miller (2012) found a correlation of .49 between Eyes Test scores and scores on the Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), in a study of 42 college students (23 females). As in the present study, participants were provided with the vocabulary definitions included in the Eyes Test, and were encouraged to consult the definitions if they did not know the meaning of a word. Results suggest, however, that this did not counter effects of variable vocabulary knowledge. It is noteworthy that researchers have used a variety of measures in attempts to understand the relationship between social cognition and EFs in clinical and nonclinical samples, and results have been mixed (see review in Ahmed & Miller, 2011). Standardized assessment of both social cognition and EFs is relatively new; thus, it may be that as tests improve the relationship between these two constructs will be clarified.
In typically developing girls, there were no statistically significant relationships among cognition and social cognition test scores. This was not surprising for several reasons. First, scores on both types of measures were higher and less variable in the TD group than the FXS group, so there was not much variance for which cognitive test scores could account. High scores on the Faux Pas test were expected, as the construct of faux pas typically is mastered by about age 11 years (Baron-Cohen, et al., 1999). Eyes Test scores also were high, although well below ceiling levels, and like Faux Pas scores had a relatively limited range. A second possible reason for the lack of a statistically significant correlation is that language, EFs, and IQ play a more important role in development of ToM vs. using ToM on everyday tasks. Language in particular has been hypothesized to play a critical role in the development of ToM (de Villiers & Pyers, 1997; Miller, 2004), as the main way for young children to learn about others’ thoughts is by hearing others talk about them. For example, mothers’ use of mental-state terms was correlated with performance of 4- and 5-year-old children on a false belief task (Adrian, Clemente, Villanueva, & Rieffe, 2005). By contrast, children with developmental language impairments perform like their typical peers on ToM tasks, once language demands are controlled (Miller, 2004), suggesting that language impairments per se might not be critical to successful ToM performance once core ToM constructs have developed. Perhaps EFs, language, and IQ are most influential either early in typical development or when one or more of these cognitive functions is impaired. Other potential reasons for the lack of a statistically significant correlation between cognitive and social cognitive tests were that the social cognition measures used were insensitive to the aspects of ToM that develop during adolescence, or that the sample size was too small and homogeneous to detect any effects. Potential limitations related the social cognition tasks and sample characteristics are discussed further below.
Hypothesis 3: Relation of social cognition to everyday social functioning
Social cognition test scores correlated at statistically significant levels with parent and self-ratings of social functioning in the FXS group, with moderate-sized effects. The one correlation that was not statistically significant – between the Faux Pas test and VABS – was in the expected direction, so the lack of statistically significant findings may have been due to inadequate statistical power.
The findings suggest that problems in social cognition can play a role in social outcome, regardless of the cognitive mechanisms underlying performance (i.e., if language impairments contribute to errors on tasks requiring social cognition). Girls and women with FXS are known to be at risk for social problems (Hagerman, 1999; Hagerman, et al., 1992; Keysor & Mazzocco, 2002), which would be expected given the high prevalence of autism spectrum disorders among the broader FXS population (Moss & Howlin, 2009). Less is known, however, about females with FXS who do not have an autism diagnosis but still experience social challenges. It appears that even in this group, social cognition is an important consideration in overall social outcome.
It was interesting that different social cognition tests contributed to different social outcomes in girls with FXS, with Eyes Test scores relating to self-reports and Faux Pas test scores relating to parent reports. This was consistent with the finding of overall differences between adolescents’ self-perceptions and perceptions of their parents (Burgess & Turkstra, 2010; Daley & Weisner, 2003; Hughes, et al., 2009), and supports the inclusion of both types of data when considering social outcomes in this age group.
For the TD group, social cognition variables did not predict self- or parent-reported social functioning in typical adolescent girls. While the lack of a correlation between social cognition and social functioning might have been due to limited variance in the former, as was suggested in regard to cognitive tests, similar findings have been reported previously in the literature on typical adolescent development (Cavell, 1990). Also as noted in regard to cognitive predictors, it might be the case that social cognition only plays a role in social functioning if it is impaired. This type of nonlinear relationship has been observed in other domains of adolescent functioning, such as parenting style vs. adolescent psychosocial outcomes, which are related only if parental control is high (Kurdek & Fine, 1994). If social cognition is adequate for everyday interactions, social functioning might be more strongly influenced by non-cognitive factors known to play an important role in adolescence, such as appearance, income, race, sex, and personal factors such as motivation (Cavell, 1990).
Limitations
The present study was limited by the small sample size. Although effect sizes were medium or large, further interrelationships among cognitive, social cognition, and social functioning variables might have emerged in a larger sample. Despite the small sample size, participants with FXS were representative of the general FXS psychological phenotype in females (Bennetto, et al., 2001; Keysor & Mazzocco, 2002), including IQs lower than those of typical peers, with about two thirds in the average or borderline range; impairments in EFs, language, and social cognition; social withdrawal, shyness, and social anxiety; and parent-reported everyday social problems that, although statistically significant, did not meet criteria for a primary diagnosis of autism. Thus, overall the study results might be applicable to the broader population of adolescent girls with FXS. Nevertheless, it is important to recognize the variability in psychological presentation among girls with FXS (Keysor & Mazzocco, 2002), which must be kept in mind when interpreting group data such as those reported here.
Our interest in determining whether FXS is associated with social-cognitive impairments and altered perception of skill in navigating the social world of the adolescent led us to compare age-matched groups. It would be helpful, however, to also compare females with FXS to a comparison group matched for age and IQ. Although IQ was considered in the analyses, as Bennetto and colleagues (Bennetto, et al., 2001) noted that there might be “something more general about having a lower IQ that leads to an uneven [cognitive] profile” (p. 295). Likewise, it might have been helpful to compare the FXS group to females matched for language ability. At the age studied here, girls who were even a few years younger would be likely to have had very different social experiences and perceptions, which would confound interpretation of self- and parent-report data. Moreover, we were able to evaluate the contributions of IQ and language ability statistically. And finally, Bennetto and colleagues (2001) found no difference in results between covarying on IQ and equating for IQ through matching, so at least for IQ it is not clear that a matched comparison would have altered the results.
The study was limited by the tools used to measure social cognition and EFs. Both of these constructs are performance-based and highly context-dependent, which is a challenge for standardized assessment. EF tests have been criticized for their lack of ecological and predictive validity (Burgess, Alderman, Evans, Emslie, & Wilson, 1998; Turkstra, et al., 2005), and a large body of literature attests to the continued debate about how EFs should be parsed and measured. Similarly, as discussed earlier in this section, there continues to be debate about the constructs included in social cognition and the best approach for evaluation. The Eyes Test has the advantage of widespread use in studies of clinical populations with social disorders, which permits comparison of results across studies and populations, but it is an experimental task rather than a standardized test and its construct validity has not been established. The Eyes test has been referred to as a test of automatic detecting or decoding mental states (Sabbagh, 2004), but item response choices mix emotion recognition and detection of more complex mental states, not only between items but also within items (e.g., choices for item 8 include “remembering” and “happy”). It is possible that these two aspects of social cognition might be differentially impaired or differentially related to other cognitive functions, but it is not possible to disambiguate these two with the test as constructed.
A fourth potential limitation was that mothers completing the VABS might have had FXS or been premutation carriers, and thus might have either underestimated their daughters’ problems because of their own social cognition impairments, or overestimated problems because of knowledge about social consequences of living with FXS. Future studies might include tests of parents’ social cognition or perhaps ratings by family members known to be without the FXS mutation.
Considerations for Intervention
We undertook the present study to better understand social functioning in adolescent girls with FXS, many of whom have IQs in the typical range and interact in mainstream classrooms with typically developing peers. An understanding of social functioning is important at this stage, as social interactions are the cornerstone of adolescent life and social developments are the foundation for successful work, school, and community outcomes in adulthood. Adolescence also is a critical stage for development of EFs and high-level language skills (Ciccia, Meulenbroek, & Turkstra, 2009), and presents a window of opportunity for intervention. Thus, results can inform identification of appropriate assessment and treatment strategies.
Although preliminary, results of the present study suggest that the social cognition performance gap in girls with FXS is not due to impaired social cognition per se, but rather can be attributed to general cognitive functions such as language and IQ. This raises questions about the type of intervention that would be most appropriate for this group. Any intervention must consider the views of adolescents with FXS themselves, a principle that was underlined by the finding that girls with FXS reported generally positive views of their everyday social lives. As Keysor and Mazzocco (2002) noted, competing hypotheses about the discrepancy between parent and self-reports of social functioning have important implications for assessment and treatment. If self-reports of adolescent girls are valid, what does it mean to attempt intervention for problems they do not perceive? Perhaps intervention could focus on making the transition to independent social life in the future, rather than “remediating” current problems perceived by parents.
Conclusion
Mazzocco and colleagues (1994) first described social cognition deficits in women with FXS almost two decades ago. Since that time, there have been important advances in our understanding of the FXS phenotype, but relatively few studies have provided information about females as a unique group. When compared to males, females with FXS have higher levels of independence and employment and participate in more leisure activities, but also have higher levels of anxiety and social phobia (Hartley, et al., 2011). Thus, they are more likely to interact socially with typically developing peers but might experience challenges in those situations. Knowledge about social functioning in this group can help identify supports and intervention strategies to maximize social participation.
The present study revealed deficits in social cognition among adolescent girls with FXS, which could be accounted for by language problems and nonverbal IQ. Despite their impairments in social cognition and their parents’ concerns about everyday social functioning, however, girls with FXS reported levels of social acceptance that were generally high. Discrepancies among social skills, social beliefs, and parent perceptions of social functioning have important implications not only for our understanding of the underlying mechanisms of the FXS psychological phenotype, but also for assessment and intervention.
Acknowledgments
This study was supported in part by a core grant to the Waisman Center (P30 HD03352) and grants to the first author (R03 HD054586) and second author (R01 HD024356), from the National Institutes on Child Health and Human Development. The authors wish to thank Dan Bolt for his assistance with statistical analysis, Lindsey Byom for her assistance with participant testing, and Susen Schroeder for her assistance with recruitment. We are indebted to the participants and their families. Leonard Abbeduto has received financial support to develop outcome measures for fragile X syndrome clinical trials from F. Hoffman- LaRoche, Ltd. and Roche TCRC, Inc.
References
- Achenbach TM, Edelbrock C. Child Behavior Checklist. Burlington, VT: Achenbach, T M; 1980. [Google Scholar]
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adrian JE, Clemente RA, Villanueva L, Rieffe C. Parent-child picture-book reading, mothers' mental state language and children's theory of mind. Journal of Child Language. 2005;32(3):673–686. doi: 10.1017/s0305000905006963. [DOI] [PubMed] [Google Scholar]
- Ahmed FS, Stephen Miller L. Executive function mechanisms of theory of mind. Journal of Autism and Developmental Disorders. 2011;41(5):667–678. doi: 10.1007/s10803-010-1087-7. [DOI] [PubMed] [Google Scholar]
- American Educational Research Association. Standards for educational and psychological testing. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Ames DR, Kammrath LK. Mind-reading and metacognition: Narcissism, not actual competence, predicts self-estimated ability. Journal of Nonverbal Behavhior. 2004;28:187–209. [Google Scholar]
- Anderson P, Anderson V, Northam E, Taylor HG. Standardization of the Contingency Naming Test (CNT) for school-aged children: A measure of reactive flexibility. Clinical Neuropsychological Assessment. 2000;1(4):247–273. [Google Scholar]
- Armor DA, Taylor SE. Situated optimism: Specific outcome expectancies and self-regulation. Advances in Experimental Social Psychology. 1998;30:309–379. [Google Scholar]
- Banerjee R, Henderson L. Social–cognitive factors in childhood social anxiety: A preliminary investigation. Social Development. 2001;10(4):558–572. [Google Scholar]
- Banerjee R, Watling D, Caputi M. Peer relations and the understanding of faux pas: longitudinal evidence for bidirectional associations. Child Development. 2011;82(6):1887–1905. doi: 10.1111/j.1467-8624.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, O'Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. Journal of Autism and Developmental Disorders. 1999;29(5):407–418. doi: 10.1023/a:1023035012436. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Scahill V, Lawson J, Spong A. Are intuitive physics and intuitive psychology independent? A test with children with Asperger Syndrome. Journal of Developmental and Learning Disorders. 2001;5:47–78. [Google Scholar]
- Baumeister R, Leary M. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Porter D, Taylor AK, Hagerman RJ. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 2001;15(2):290–299. [PubMed] [Google Scholar]
- Bora E, Eryavuz A, Kayahan B, Sungu G, Veznedaroglu B. Social functioning, theory of mind and neurocognition in outpatients with schizophrenia; mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Research. 2006;145(2–3):95–103. doi: 10.1016/j.psychres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatric Clinics of North America. 2005;28(3):613–633. 626. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Evans J, Emslie H, Wilson BA. The ecological validity of tests of executive function. Journal of the International Neuropsychological Society. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- Burgess S, Turkstra LS. Quality of Communication Life in Adolescents with High Functioning Autism and Asperger Syndrome: A Feasibility Study. Language, Speech, a nd Hearing Services in Public Schools. 2010 doi: 10.1044/0161-1461(2010/09-0007). [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Comprehensive Assessment of Spoken Language. Circle Pines, MN: American Guidance Service, Inc; 1999. [Google Scholar]
- Cavell TA. Social adjustment, social performance, and social skills: a tri-component model of social competence. Journal of Clinical Child Psychology. 1990;19(2):111–122. [Google Scholar]
- Channon S, Watts M. Pragmatic language interpretation after closed head injury: Relationship to executive functioning. Cognitive Neuropsychiatry. 2003;8(4):243–260. doi: 10.1080/135468000344000002. [DOI] [PubMed] [Google Scholar]
- Ciccia AH, Meulenbroek P, Turkstra LS. Adolescent brain and cognitive developments: Implications for clinical intervention. Topics in Language Disorders. 2009;29(3):249–265. doi: 10.1097/TLD.0b013e3181b53211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Burack JA, Rahman A, Munir F, Russo N, Grant C. Theory of mind deficits in children with fragile X syndrome. Journal of Intellectual Disability Research. 2005;49(Pt 5):372–378. doi: 10.1111/j.1365-2788.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Cornish K, Kogan C, Turk J, Manly T, James N, Mills A, et al. The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain and Cognition. 2005;57(1):53–60. doi: 10.1016/j.bandc.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. The nature of the spatial deficit in young females with Fragile-X syndrome: a neuropsychological and molecular perspective. Neuropsychologia. 1998;36(11):1239–1246. doi: 10.1016/s0028-3932(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Differential impact of the FMR-1 full mutation on memory and attention functioning : a neuropsychological perspective. Journal of Cognitive Neuroscience. 2001;13(1):144–150. doi: 10.1162/089892901564126. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genetic Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Csikszenthi M. Being adolescent: Conflict and growth in the teenage years. New York: Basic Books; 1984. [Google Scholar]
- Daley TC, Weisner TS. “I speak a different dialect”: teen explanatory models of difference and disability. Medical Anthropology Quarterly. 2003;17(1):25–48. doi: 10.1525/maq.2003.17.1.25. [DOI] [PubMed] [Google Scholar]
- de Villiers J, Pyers J. Complementing cognition: the relationship between language and theory of mind. In: Hughes E, Hughes M, Greenhill A, editors. Proceedings of the Twenty-first Annual Boston University Conference on Language Development. Vol. 1. Somerville; Cascadilla: 1997. pp. 136–147. [Google Scholar]
- Eddy CM, Sira Mahalingappa S, Rickards HE. Is Huntington's disease associated with deficits in theory of mind? Acta Neurologica Scandinavicia. 2012 doi: 10.1111/j.1600-0404.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- Flesch R. How to write, speak, and think more effectively (Reissued) New York: New American Library; 1994. [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. Psychiatric disorders associated with fragile X in the young female. Pediatrics. 1993;91(2):321–329. [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Roth RM. Tests of Executive Control Software Portfolio Standardization Version. Lutz, FL: Psychological Assessment Resources; 2006. Beta Version. [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: Theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Neurodevelopmental Disorders: Diagnosis and Treatment. New York: Oxford University Press; 1999. [Google Scholar]
- Hagerman RJ, Jackson C, Amiri K, Silverman AC, O'Connor R, Sobesky W. Girls with fragile X syndrome: physical and neurocognitive status and outcome. Pediatrics. 1992;89(3):395–400. [PubMed] [Google Scholar]
- Harrison P, Oakland T. Adaptive Behavior Assessment System. 2. San Antonio, TX: Pearson Assessment; 2003. [Google Scholar]
- Harter S. Manual for the Self-Perception Profile for Children. Denver, CO: University of Denver; 1985. [Google Scholar]
- Harter S. Manual for the Self-Perception Profile for Adolescents. Denver, CO: University of Denver; 1988. [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, Bailey DB. Exploring the adult life of men and women with fragile X syndrome: results from a national survey. American Journal of Intellectual Developmental Disabilities. 2011;116(1):16–35. doi: 10.1352/1944-7558-116.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test (WCST) Odessa, Florida: Psychological Assessment Resources; 1981. [Google Scholar]
- Hendry LB, Reid M. Social relationships and health: The meaning of social “connectedness” and how it relates to health concerns for rural Scottish adolescents. Journal of Adolescence. 2000;23:705–719. doi: 10.1006/jado.2000.0354. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, et al. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27(7):855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Cole D, Martin M, Tram J, Seroczynski AD. Are the discrepancies between self and others' appraisals of competence predictive or reflective of depressive symptoms in children and adolescents: A longitudinal study, part II. Journal of Abnormal Psychology. 2000;109(4):651–662. doi: 10.1037//0021-843x.109.4.651. [DOI] [PubMed] [Google Scholar]
- Hughes D, Turkstra LS, Wulfeck B. Parent and Self-Ratings of Executive Function in Adolescents with Specific Language Impairment. Paper presented at the Student-Run Conference on Language Disorders; 2007. [DOI] [PubMed] [Google Scholar]
- Hughes DM, Turkstra LS, Wulfeck BB. Parent and self-ratings of executive function in adolescents with specific language impairment. International Journal of Language and Communication Disorders. 2009;44(6):901–916. doi: 10.1080/13682820802425693. [DOI] [PubMed] [Google Scholar]
- Isquith PK, Roth RM, Gioia GA. Tasks of Executive Control. Lutz, FL: Psychological Assessment Resources, Inc; 2010. [Google Scholar]
- John K. Measuring Children's Social Functioning. Child Psychology and Psychiatry Review. 2001;6(4):181–188. [Google Scholar]
- Jones JL. Factors associated with self-concept: adolescents with intellectual and development disabilities share their perspectives. Intellectual Developmental Disabilities. 2012;50(1):31–40. doi: 10.1352/1934-9556-50.1.31. [DOI] [PubMed] [Google Scholar]
- Keysor CS, Mazzocco MM. A developmental approach to understanding Fragile X syndrome in females. Microscience Research and Technology. 2002;57(3):179–186. doi: 10.1002/jemt.10070. [DOI] [PubMed] [Google Scholar]
- Keysor CS, Mazzocco MM, McLeod DR, Hoehn-Saric R. Physiological arousal in females with fragile X or Turner syndrome. Developmenal Psychobiology. 2002;41(2):133–146. doi: 10.1002/dev.10060. [DOI] [PubMed] [Google Scholar]
- Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the Contingency Naming Test (CNT) Developmental Neuropsychology. 2005;28(3):755–777. doi: 10.1207/s15326942dn2803_2. [DOI] [PubMed] [Google Scholar]
- Kurdek LA, Fine MA. Family acceptance and family control as predictors of adjustment in young adolescents: linear, curvilinear, or interactive effects? Child Development. 1994;65(4):1137–1146. doi: 10.1111/j.1467-8624.1994.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Larson RW. Adolescents' daily experience with family and friends: Contrasting opportunity systems. Journal of Marriage and Family. 1983;54(4):739–751. [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44(6):950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mailloux SL, Johnson ME, Fisher DG, Pettibone TJ. How reliable is computerized assessment of readability? Computers in Nursing. 1995;13(5):221–225. [PubMed] [Google Scholar]
- Marschark M, Green V, Hindmarsh G, Walker S. Understanding theory of mind in children who are deaf. Journal of Child Psychology and Psychiatry. 2000;41:1067–1073. [PubMed] [Google Scholar]
- Martin-Rodriguez JF, Leon-Carrion J. Theory of mind deficits in patients with acquired brain injury: a quantitative review. Neuropsychologia. 2010;48(5):1181–1191. doi: 10.1016/j.neuropsychologia.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. Journal of Developmental Behavioral Pediatrics. 1993;14(5):328–335. [PubMed] [Google Scholar]
- Mazzocco MM, Pennington BF, Hagerman RJ. Social cognition skills among females with Fragile X. Journal of Autism and Developmental Disorders. 1994;24(4):473–485. doi: 10.1007/BF02172129. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J. The Awareness of Social Inference Test (TASIT) Austin, TX: Harcourt Assessment; 2002. [Google Scholar]
- Mesulam MM. The human frontal lobes: Transcending the default mode through contingent encoding. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 8–30. [Google Scholar]
- Miller C. False belief and sentence complement performance in children with specific language impairment. International Journal of Language and Communication Disorders. 2004;39(2):191–213. doi: 10.1080/13682820310001616994. [DOI] [PubMed] [Google Scholar]
- Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. Journal of Intellectual Disabilies Research. 2009;53(10):852–873. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- Muller F, Simion A, Reviriego E, Galera C, Mazaux JM, Barat M, et al. Exploring theory of mind after severe traumatic brain injury. Cortex. 2009 doi: 10.1016/j.cortex.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Pakaslahti L, Keltikangas-Jarvinen L. Comparison of peer, teacher and self-assessments on adolescent direct and indirect aggression. Educational Psychology. 2000;20(2):177–190. [Google Scholar]
- Perner J, Stummer S, Lang B. Executive functions and theory of mind: Cognitive complexity or functional dependence? In: Zalazo PD, Astington JW, Olson DR, editors. Developing theories of intention: Social understanding and self-control. Mahwah, N J: Lawrence Erlbaum Associates; 1999. pp. 133–153. [Google Scholar]
- Peterson E, Miller SF. The Eyes Test as a Measure of Individual Differences: How much of the Variance Reflects Verbal IQ? Frontiers in Psychology. 2012;3:220. doi: 10.3389/fpsyg.2012.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli M, Duckett E. “We were just talking...”: conversations in early adolescence. Journal of Youth and Adolescence. 1989;18(6):567–582. doi: 10.1007/BF02139074. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. The Leiter International Performance Scale. Lutz, Florida: Psychological Assessment Resources; 1997. Revised. [Google Scholar]
- Russell PA, Hosie JA, Gray CD, Scott C, Hunter N, Banks JS, et al. The development of theory of mind in deaf children. Journal of Child Psychology and Psychiatry. 1998;39:903–910. [PubMed] [Google Scholar]
- Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain and Cognition. 2004;55(1):209–219. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Shapka JD, Keating DP. Structure and change in self-concept during adolescence. Canadian Journal of Behavioural Science. 2005;37(2):83–96. [Google Scholar]
- Siegal M, Varley R. Neural systems involved in Theory of Mind. Nature Reviews Neuroscience. 2002;3:463–471. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Simon JA, Keenan JM, Pennington BF, Taylor AK, Hagerman RJ. Discourse processing in women with fragile X syndrome: Evidence for a deficit establishing coherence. Cognitive Neuropsychology. 2001;18:1–8. doi: 10.1080/02643290126042. [DOI] [PubMed] [Google Scholar]
- Sobesky WE, Taylor AK, Pennington BF, Bennetto L, Porter D, Riddle J, et al. Molecular/clinical correlations in females with fragile X. American Journal of Medical Genetics. 1996;64(2):340–345. doi: 10.1002/(SICI)1096-8628(19960809)64:2<340::AID-AJMG21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to Theory of Mind. Journal of Cognitive Neuroscience. 1998;10(5):640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Steinberg L, Westenberg PM. The developmental pattern of resistance to peer influence in adolescence: will the teenager ever be able to resist? Journal of Adolescence. 2009;32(4):1009–1021. doi: 10.1016/j.adolescence.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Sutterby SR, Bedwell JS, Passler JS, Deptula AE, Mesa F. Social anxiety and social cognition: The influence of sex. Psychiatry Research. 2012;197(3):242–245. doi: 10.1016/j.psychres.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Illusion and well-being: A social psychological perspective on mental health. Psychological Bulletin. 1988;103:193–210. [PubMed] [Google Scholar]
- Todd SY, Kent A. Student athletes' perceptions of self. Adolescence. 2003;38(152):659–667. [PubMed] [Google Scholar]
- Turkstra LS. Conversation-based assessment of social cognition in adults with traumatic brain injury. Brain Injury. 2008;22(5):397–409. doi: 10.1080/02699050802027059. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, Coelho C, Ylvisaker M, Kennedy M, Sohlberg MM, Avery J, et al. Practice Guidelines for Standardized Assessment for Persons with Traumatic Brain Injury. Journal of Medical Speech Language Pathology. 2005;13(2):ix–xxviii. [Google Scholar]
- Turkstra LS, Dixon TM, Baker KK. Theory of Mind and social beliefs in adolescents with traumatic brain injury. NeuroRehabilitation. 2004;19(3):245–256. [PubMed] [Google Scholar]
- Turkstra LS, McDonald S, DePompei R. Social information processing in adolescents: data from normally developing adolescents and preliminary data from their peers with traumatic brain injury. Journal of Head Trauma Rehabilitation. 2001;16(5):469–483. doi: 10.1097/00001199-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, McDonald S, Kaufmann PM. Assessment of pragmatic communication skills in adolescents after traumatic brain injury. Brain Injury. 1996;10(5):329–345. doi: 10.1080/026990596124359. [DOI] [PubMed] [Google Scholar]
- Wang YG, Wang YQ, Chen SL, Zhu CY, Wang K. Theory of mind disability in major depression with or without psychotic symptoms: a componential view. Psychiatry Research. 2008;161(2):153–161. doi: 10.1016/j.psychres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence 1999 [Google Scholar]
- Wichstrom L. Harter's Self-Perception Profile for Adolescents: reliability, validity, and evaluation of the question format. Journal of Personality Assessment. 1995;65(1):100–116. doi: 10.1207/s15327752jpa6501_8. [DOI] [PubMed] [Google Scholar]
- Wilding J, Cornish K, Munir F. Further delineation of the executive deficit in males with fragile-X syndrome. Neuropsychologia. 2002;40(8):1343–1349. doi: 10.1016/s0028-3932(01)00212-3. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International classification of functioning, disability and health (Report) Geneva: Switzerland: 2001. [Google Scholar]
- Zelazo PD, Burack JA, Benedetto E, Frye D. Theory of mind and rule use in individuals with Down's syndrome: a test of the uniqueness and specificity claims. Journal of Child Psychology and Psychiatry. 1996;37(4):479–484. doi: 10.1111/j.1469-7610.1996.tb01429.x. [DOI] [PubMed] [Google Scholar]