Abstract
Adolescence is associated with heightened mortality rates due in large measure to negative consequences from risky behaviors. Theories of adolescent risk taking posit that immature cognitive control coupled with heightened reward reactivity drive adolescent risk-taking, yet surprisingly few empirical studies have examined these neurobiological systems together. In this paper, we describe a related series of studies from our laboratory aimed at further delineating the maturation of cognitive control through adolescence, as well as how rewards influence a key aspect of cognitive control, response inhibition. Our findings indicate that adolescents can exert adult-like control over their behavior, but that they have limitations regarding the consistency with which they can generate optimal responses compared to adults. Moreover, we demonstrate that the brain circuitry supporting mature cognitive (inhibitory) control is still undergoing development. Our work using the rewarded antisaccade task, a paradigm that enables concurrent assessment of rewards and inhibitory control, indicates that adolescents show delayed but heightened responses in key reward regions along with concurrent activation in brain systems that support behaviors leading to reward acquisition. Considered together, our results highlight adolescent-specific differences in the integration of basic brain processes that may underlie decision-making and more complex risk taking in adolescence.
Keywords: prefrontal, antisaccade, inhibition, dopamine, oculomotor
INTRODUCTION
Adolescence is associated with normative increases in novelty and sensation seeking (Dahl, 2004; Spear, 2000). These drives may have adaptive benefit as they may motivate youth to explore novel environments and engage in new social relationships, leading to the acquisition of skills and experiences critical in adulthood (Steinberg, 2004). However, these drives may also result in risky behavior (e.g. experimenting with drugs; unprotected sex), which can lead to aversive outcomes (e.g., substance abuse; unwanted pregnancy). Indeed, increased morbidity and mortality rates during the adolescent period are due largely to consequences of preventable risk taking (Eaton et al., 2012). Consequently, understanding why adolescents are prone to risk taking represents not only a major scientific but also a pressing public health objective.
Risk-taking may be viewed as arising from decision-making, particularly choices made in the context of heightened emotional arousal and/or salient incentives. Decision-making, in turn, consists of more basic elements, of which incentive (reward/loss) processing and inhibitory control are fundamental. From this perspective, examining the interaction of incentive processing and inhibitory control may provide insight on the basic mechanisms contributing to the complex behavioral phenomena of risky behavior. Increasingly, developmental neuroscientists suggest that adolescents may be more vulnerable to risk-taking because of limitations in the ability of prefrontal regulatory systems (prefrontal circuits) to effectively regulate heightened reward and loss signals (striatal circuits) (Casey, Jones, & Hare, 2008; Ernst, Pine, & Hardin, 2006; Geier & Luna, 2009). That is, while adolescents may engage similar circuitry as adults to correctly perform a cognitive task, the addition of rewarding or aversive (e.g., stressful) stimuli may alter brain activity and, by extension, the resulting behavior (Steinberg, 2008).
Below, we review work from our laboratory investigating the neural bases of cognitive control and reward processing in adolescence. Our studies utilize the antisaccade (an eye-movement) task, a paradigm widely used in human and non-human primate studies to understand brain-behavior relationships, specifically inhibitory control (Munoz & Everling, 2004). We operationalize cognitive control as the ability to suppress attention to compelling yet goal-irrelevant stimuli in order to generate a planned response, though cognitive control may be more generally thought of as top-down control over task-relevant processes, or the ability to coordinate thoughts and actions toward realizing goals (Koechlin et al., 2003; MacDonald et al., 2000). During the antisaccade (AS) task, participants are told that a light will appear at an unexpected location on a computer screen and instructed to suppress the automatic urge to move their eyes towards the light, but instead generate an eye-movement to its mirror location. To investigate the relation between reward and inhibitory control, we modified the AS task by providing incentives based on task performance. As such, this simple task engages the relevant brain systems (importantly, prefrontal cortex and striatum) required for reward processing and cognitive control of behavior, which may inform our understanding of the development of circuitry underlying more complex behaviors like risk-taking.
DEVELOPMENT OF COGNITIVE CONTROL
The ability to control behavior and make goal-directed responses is evident early in development when even infants show instances of inhibitory control (Johnson, 1995). The ability to exert cognitive control in a consistent manner, however, continues to improve through adolescence (Bjorklund & Harnishfeger, 1995; Klein, 2001; Luna, Garver, Urban, Lazar, & Sweeney, 2004). Our studies have found continued improvements in the rate of correct responses through childhood (Luna et al., 2004) and through adolescence (Velanova, Wheeler, & Luna, 2009) similar to those of other laboratories (Fischer et al., 1997; Klein et al., 2005). As such, the processes supporting a single controlled response are available early on, but processes supporting the ability to effectively engage them continue to strengthen into adulthood.
Furthermore, our studies suggest that brain function during the antisaccade task continues to develop over adolescence. In a study of 8- to 27-year-olds performing the antisaccade task, we demonstrated that a similar brain network (including prefrontal cortex and motor control areas) were engaged across ages during successful trials (Velanova, Wheeler, & Luna, 2008). Children younger than 13 years of age, however, showed increased activation of these regions, implying greater effort to perform at mature levels. However, when looking at activity sustained across blocks of trials, which reflect circuits that support a cognitive response state, children and adolescents demonstrated decreased activation in prefrontal and posterior regions, suggesting age-related inefficiency in brain function to engage systems that support the ability to consistently inhibit responses (Velanova et al., 2008). Differences in the magnitude of activation across age groups (greater or lesser) are interpreted as limitations in neural processing, based on the assumption that the adult mature model is the ideal model of comparison (Luna et al., 2010). In this manner, greater magnitude of activation in a young group compared to an adult group is interpreted as limitations in the neural components supporting activation in a region that may not have reached efficient mature levels (e.g., synaptic pruning may still be occurring) resulting in greater activity. Further, greater magnitude of activation in a region by younger groups can be understood as reflecting greater effort, similar to how adults show increased activation in relevant regions with increased task difficulty (Braver et al., 1997). When younger groups show decreased activation compared to the ideal mature adult model, this can be interpreted as reflecting qualitative limitations in younger groups in accessing an optimal region that may underlie ease of cognitive responses in adults.
In addition, there are age-related differences in processing an antisaccade error for subsequent adjustment of behavior (e.g. when participants accidently looked at the light and then immediately looked away) (Velanova et al., 2009). When adults made antisaccade errors, the dorsal anterior cingulate cortex in the prefrontal cortex was utilized. This area of the brain has been previously associated with error monitoring potentially regulating the ability to improve behavior on subsequent trials (MacDonald et al., 2000). Adolescents and children did not engage this region to the same degree as adults, and overall made more antisaccade errors. Taken together, these results indicate that the ability to make an inhibitory response is available early in development while the ability to monitor performance and identify when errors are committed so as to adjust subsequent performance in order to effectively engage inhibitory control in a sustained fashion, continues to mature into adulthood.
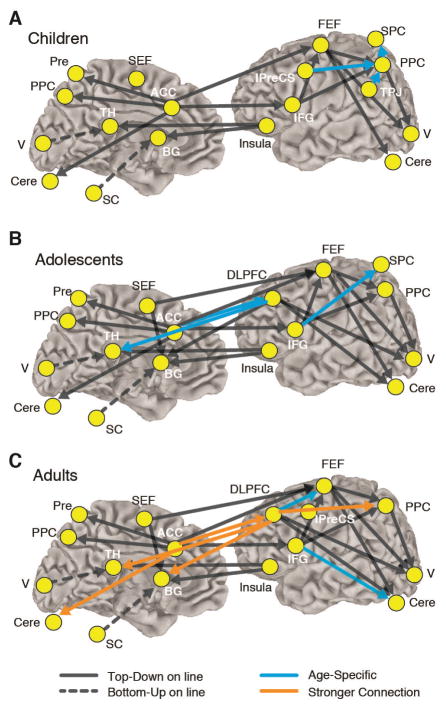
Cognitive control of behavior is supported by a wide network of brain regions including prefrontal and parietal cortices that interact with subcortical areas including the striatum, thalamus, cerebellum, and brain stem, forming a circuitry that supports top-down control of behavior (Sweeney et al., 1996). In a study assessing the direction in which distinct brain regions communicate with one another (using a method called Granger Causality) to support inhibitory control, we found that from childhood to adolescence connections that were directed by prefrontal cortex became established and were subsequently strengthened into adulthood (Hwang, Velanova, & Luna, 2010) (Figure 1). These results are in agreement with other effective connectivity studies indicating that strengthening of causal connection is associated with age related improvements in cognitive processes (Bitan et al., 2009; Bitan et al., 2006; Stevens et al., 2007).
Figure 1.
The results of connectivity analysis during the antisaccade task for 78 participants including 26 children (aged 8–12 years, 11 males and 15 females), 25 adolescents (13–17 years, 10 males and 15 females), and 27 adults (18–27 years, 11 males and 16 females). Left- and right-hemisphere connections are placed on the right hemisphere to aid with visualization. Arrows connecting two ROIs represent significant effective (causal) connectivity from one ROI to another. Only significant connections are displayed. Children showed reliance on parietal regions (light blue), while by adolescence prefrontal connections become predominant (dark blue). By adulthood there is a strengthening and increase in number of prefrontally guided connections (red/orange).
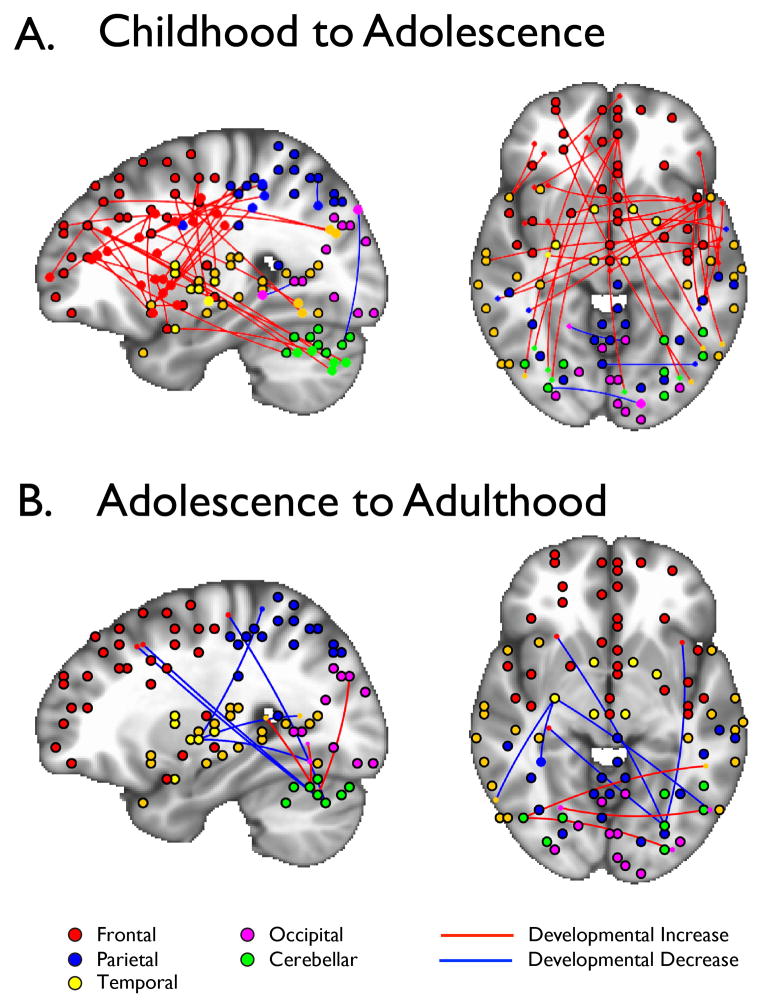
In a subsequent study, we identified connections between brain regions when participants (aged 10–20) were not engaged in a task (referred to as “rest”) in the scanner in order to capture the core architecture of the brain (Hwang, Hallquist, & Luna, 2012). We specifically identified “hubs” in the brain, which are regions in the brain that have the greatest number of connections with other regions. Similar to airports that serve as “hubs” for certain airlines, these functional brain hubs are believed to function as way stations for information (traffic) flow directing high volumes of information across the brain (Buckner et al., 2009). Throughout development, the basic architecture of the brain remained stable (e.g. areas that served as hubs and the regions they connected to did not change) and were organized in an optimal small world arrangement as has been found previously (Fair et al., 2009). However, similar to our functional connectivity studies during inhibitory control, we found that connectivity of frontal hubs to the rest of the brain became established from childhood to adolescence (Fig. 2A) in agreement with previous findings of reorganization of functional networks with development (Fair et al., 2009). However, we did not find evidence for a qualitative shift from short to long connections in the hub architecture that has been evidenced when characterizing predefined controlled networks (Fair et al., 2007). By adolescence, frontal hub connections were stable (Fig. 2B). These findings highlight the stability of the basic architecture of the brain throughout development. This stability supports subsequent refinements in neural connectivity during the bio-behavioral transition into adulthood. Importantly, prefrontal executive networks are established by adolescence, which further support the notion that adolescents can often behave like adults and that differences in behavior might be context dependent due to immaturities in connection strengths between brain regions.
Figure 2.
Connectivity of frontal, cognitive control hubs are primarily established between childhood and adolescence (A), which by adolescence are relatively stable into adulthood (B)
Overall, our cognitive development studies indicate that the foundation for cognitive control is available in childhood, becomes guided by executive prefrontal systems by adolescence, but continues to mature through adolescence in specific areas including performance monitoring and sustaining a cognitive manner of responding that support mature adult level of controlled behavior. These results are in agreement with behavioral studies of the development of executive function indicating significant improvements in processes including working memory and inhibitory control from childhood to adolescence (Diamond, 2002; Zelazo, Craik, & Booth, 2004).
Next we review our work on reward processing in adolescence and its influence on cognitive control.
REWARD PROCESSING IN ADOLESCENCE
Human behavior is strongly motivated by rewards and punishments. Indeed, a vast majority of the behaviors that we engage in are done directly or indirectly to obtain rewards or to avoid loss or punishment. For example, many of us wake before dawn and head to work everyday in order to earn money to buy the material goods that we want or need. Given its central role in behavior, the study of rewards has unsurprisingly been the focus of extensive scientific inquiry.
A large literature in animals and human adults has identified distinct brain circuitry underlying incentive (reward, loss) processing. Key components of this circuitry include the dorsal and ventral striatum (Delgado et al., 2000), ventral medial prefrontal cortex (Knutson et al., 2005), amygdala (De Martino et al., 2010), and orbitofrontal cortex (O’Doherty et al., 2001), among others ( see Schultz, 2000, for review). Previous work has also demonstrated that central to the function of this circuitry is the neurotransmitter dopamine. Cells that produce dopamine respond to different properties of incentives, including salience and valence, and modulate reciprocal interactions between the prefrontal cortex and the ventral striatum (which includes the nucleus accumbens), an area highly implicated in the processing of rewards (Cools, 2008). Developmentally, converging evidence from rodent, non-human primate, and human studies indicate a peak in the availability and function of dopamine suggesting that adolescence is a period of heightened reward sensitivity. Importantly, this sensitivity seems to parallel peaks in reward-seeking and sensation-seeking behaviors (Rosenberg & Lewis, 1994; Spear, 2000; Wahlstrom, White, & Luciana, 2010).
Ventral striatal function has been of particular interest in developmental neuroimaging studies. For the most part, studies have shown increased activation in the ventral striatum during adolescence relative to adulthood during the processing of rewards (Ernst et al., 2005; Galvan et al., 2006; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Padmanabhan, 2011). Increased activation in ventral striatum has been demonstrated during passive reward tasks (van Leijenhorst et al., 2009) as well as active tasks with relatively simple response demands (Ernst et al., 2005; Galvan et al., 2006) and cognitive control requirements (Geier et al., 2010; Padmanabhan, 2011). However, when an abstract cue associated with a reward is used in the context of a simple speeded response adolescents show decreased involvement of ventral striatum compared to adults (Bjork et al., 2004; Bjork, Smith, Danube, & Hommer, 2007). Considered together, these studies suggest that the adolescent reward system may be highly influenced by contextual demands and the specific component or stage of reward processing under investigation (Galvan, 2010; Geier & Luna, 2009; Bjork, Smith, Chen, & Hommer, 2010), and that adolescents show differential reward sensitivity relative to younger and older comparison groups.
It is important to recognize that characterization of adolescents’ reward responses in isolation provides an incomplete picture of motivated behavior. Reward processes function together with cognitive control processes to enable goal-directed behavior. Our work has focused on characterizing the interaction of rewards and inhibitory (cognitive) control. In order to characterize the effect of reward on inhibitory control unique to the adolescent period, we tested 8–21 year old subjects using an incentive modulated antisaccade task, modified for use in an event related fMRI design (Padmanabhan et al., 2011). Three rectangles with “$” signs to resemble a ‘slots’ machine was used to depict the possibility of a reward dependent on correct performance while neutral trials were depicted using “#” signs. Participants could win up to US $25 depending on performance. Children and adolescents committed more inhibitory errors compared to adults during neutral trials, consistent with our prior work. However, children and adolescents showed a decrease in the number of errors on rewarded trials, while adult performances remained at high levels. All participants showed increased speed of responses in rewarded trials, indicating that rewards were more compelling than neutral trials. Although activation in reward and cognitive circuitries were evident across age groups, only adolescents showed increased activation in ventral striatum in rewarded vs. neutral trials, concurrent with increased activation in intraparietal sulcus and putamen, regions important for antisaccade performance. These results suggest increased adolescent sensitivity to rewards that may enhance performance by elevating activity in regions that support behaviors that lead to a reward, although we did not find correlations between activation in these regions and effects of rewards on behavior. Children showed greater activation in frontal eye movement control regions across different trials, perhaps reflecting overall increased effort in generating inhibitory responses, a finding evident across antisaccade studies (Velanova et al., 2009). Together, these findings are in line with literature finding enhanced reward sensitivity during adolescence (Galvan, 2010) and advance the model by showing that rewards may contribute to increased recruitment of brain circuitry that enhances the ability to generate responses that result in rewards.
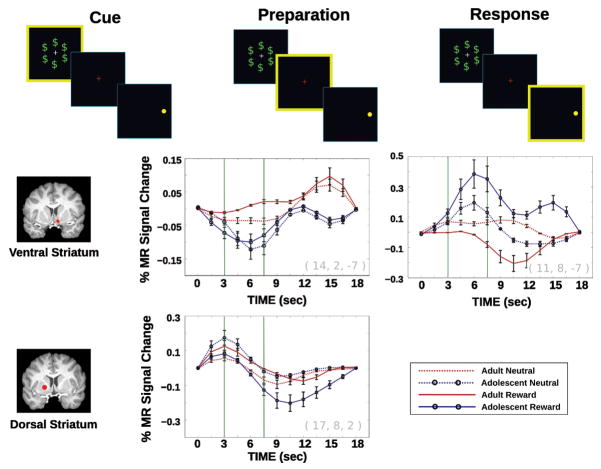
Reward processing consists of multiple stages that may be temporally dissociable, e.g. initial reward assessment, anticipation, feedback processing. Identifying which stages of reward processing show developmental differences is important, not only because each stage implicates different ensembles of neural regions, but also because they can influence the interpretation of changes in rewarded behavior. For example, differences in reward assessment could implicate changes in motivation, while differences in feedback processing could implicate changes in error monitoring, updating, or learning. In order to better identify which stages of reward processing may show differences during adolescence, we used a rewarded antisaccade task designed to independently assess brain responses during distinct stages of reward processing, including reward assessment (processing of the rewarded/neutral cue), response preparation/reward anticipation (presentation of fixation/instruction cue), and motor response (correct inhibitory response) (Geier et al., 2010). We were able to dissociate and separately characterize blood-oxygen-level-dependent (BOLD) activation associated with each reward processing stage that had been previously identified in the literature to be distinct (Schultz, 2000) by using a fast, event-related fMRI design with partial “catch” trials (Ollinger et al., 2001). In Geier et al., 2010, we focused on the adolescent period and tested subjects aged 13–17 years compared to 18- to 30-year-olds. Behaviorally, all subjects responded faster when making correct rewarded vs. neutral trial responses, but only adolescents showed a significant improvement in rate of correct responses, which reached adult levels. Imaging results indicated increased activation in the ventral striatum by adults compared to adolescents during the initial cue assessment period, suggesting that the ventral striatum may play a key role in the assessment of reward value and that adults may have earlier access to this signal relative to adolescents. Adolescents compared to adults showed a subsequent dramatic increase in ventral striatal activation later during the response preparation/reward anticipation period (see Figure 3). The recruitment of ventral striatum in adolescents later in reward processing, and immediately preceding the response, may expose vulnerability to impulsive decision making in the sense that adolescents may have comparably less time than adults to assess and appropriately integrate rewards with goal-oriented behavior. Similar to the Padmanabhan et al. (2011) study described above, Geier et al. (2010) demonstrated that critical regions of the oculomotor control network were recruited to higher levels in adolescents in conjunction with heightened ventral striatal activation, suggesting a possible mechanism for how behavior improves with reward incentives in adolescence. Together, these results provide evidence suggesting that adolescents have delayed reward reactivity that is enhanced during the period when response preparation and reward anticipation occurs enhancing activity in regions that support the behavior that leads to reward receipt. The proximity of enhanced reward reactivity in adolescence immediately preceding a reward motivated response may underlie the impulsive nature of adolescent sensation seeking.
Figure 3.
Estimated impulse response functions from deconvolution analysis for the cue, preparation, and response stages of the reward antisaccade task (highlighted in yellow) for striatal regions showing age and/or incentive interactions across time (for methodological details see Geier et al., 2010). Error bars represent ±1 standard error of the mean. Upon cues, adults show greater activation in ventral striatum compared to adolescents. By the preparation period adolescents show greater reactivity in ventral striatum compared to adults. During the response stage there are no age group differences in the dorsal striatum.
CONCLUSIONS
Adolescents’ propensities for risk-taking may be strongly linked to a normative neurobiological sensitivity to rewards coupled with a still-emerging cognitive control over behavior. Brain systems that support executive control of behavior are close to adult levels in adolescence, supporting abstract thought and the ability to generate voluntary executive responses, but these systems are still limited in the ability to be flexibly and effectively engaged. Adolescence is also a period during which heightened reactivity to reward incentives may bias the ability to exert executive control of behavior. Our studies investigating the interaction of rewards and inhibitory control suggest that adolescents show a heightened engagement of key reward regions along with parallel enhancements in inhibitory control related circuitry. Notably, the timing and pattern of neural recruitment we identify appears uniquely adolescent. Importantly, reward reactivity in adolescence occurs immediately preceding the response, which may make them vulnerable to impulsivity. In naturalistic settings, heightened reactivity to rewards and activation of response systems directed towards reward acquisition may bias reward-driven decisions towards potentially risky behavior.
References
- Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam MM, et al. Weaker top-down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Booth JR. Developmental increase in top-down and bottom-up processing in a phonological task: an effective connectivity, fMRI study. Journal of Cognitive Neuroscience. 2009;21:1135–1145. doi: 10.1162/jocn.2009.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. Journal of Neuroscience. 2007;27(18):4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. Interference & Inhibition in Cognition. San Diego, CA: Academic Press, Inc; 1995. The evolution of inhibition mechanisms and their role in human cognition and behavior; pp. 141–173. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepilcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008;14(4):381–395. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proceedings of the National Academy of Sciences. 2010;107(8):3788. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Nystrom L, Fissell C, Noll D, Fiez J. Tracking the Hemodynamic Responses to Reward and Punishment in the Striatum. Journal of Neurophysiology. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, Harris WA, et al. Youth risk behavior surveillance - United States, 2011. MMWR Surveill Summ. 2012;61(4):1–162. [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4(6):1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–2692. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and its Influence on Inhibitory Control in Adolescence. Cerebral Cortex. 2010;20(7):1613–29. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B. The Development of Hub Architecture in the Human Functional Brain Network. Cerebral Cortex. 2012:1047–3211. doi: 10.1093/cercor/bhs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of Top-Down Frontal Cognitive Control Networks Underlying the Development of Inhibitory Control: An fMRI Effective Connectivity Study. Journal of Neuroscience. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. The inhibition of automatic saccades in early infancy. Developmental Psychobiology. 1995;28(5):281–291. doi: 10.1002/dev.420280504. [DOI] [PubMed] [Google Scholar]
- Klein C. Developmental functions for saccadic eye movement parameters derived from pro- and antisaccade tasks. Experimental Brain Research. 2001;139(1):1–17. doi: 10.1007/s002210100711. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F, Hartnegg K, Fischer B. Lifespan development of pro- and anti-saccades: multiple regression models for point estimates. Developmental Brain Research. 2005;160:113–123. doi: 10.1016/j.devbrainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. The Journal of Neuroscience. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach M, Rolls E. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Developmental Cognitive Neuroscience. 2011:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Sweeney JA, Gillen JS, Kim J, Varanelli MJ, O’Hearn KM, Erb PA, et al. Magnetic resonance imaging of children without sedation: preparation with simulation. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):853–859. doi: 10.1097/00004583-199706000-00024. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021(1):51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behavioural Brain Reserach. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75(1):454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2009;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience & Biobehavioral Reviews. 2010;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Craik FI, Booth L. Executive function across the life span. Acta Psychologica (Amst) 2004;115:167–183. doi: 10.1016/j.actpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]
RECOMMENDED READINGS
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. -Detailed discussion on interpreting findings from developmental neuroimaging studies on cognitive control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. -Comparative review on risk-taking behavior and the processing of rewarding, aversive, and emotional, stimuli in adolescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. -Review on the development of the dopamine system and how it may relate to adolescent brain development and behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]