Abstract
Exercise increases resistance against stress-related disorders such as anxiety and depression. Similarly, the perception of control is a powerful predictor of neurochemical and behavioral responses to stress, but whether the experience of choosing to exercise, and exerting control over that exercise, is a critical factor in producing exercise-induced stress resistance is unknown. The current studies investigated whether the protective effects of exercise against the anxiety- and depression-like consequences of stress are dependent on exercise controllability and a brain region implicated in the protective effects of controllable experiences, the medial prefrontal cortex. Adult male Fischer 344 rats remained sedentary, were forced to run on treadmills or motorised running wheels, or had voluntary access to wheels for 6 weeks. Three weeks after exercise onset, rats received sham surgery or excitotoxic lesions of the medial prefrontal cortex. Rats were exposed to home cage or uncontrollable tail shock treatment three weeks later. Shock-elicited fear conditioning and shuttle box escape testing occurred the next day. Both forced and voluntary wheel running, but not treadmill training, prevented the exaggerated fear conditioning and interference with escape learning produced by uncontrollable stress. Lesions of the medial prefrontal cortex failed to eliminate the protective effects of forced or voluntary wheel running. These data suggest that exercise controllability and the medial prefrontal cortex are not critical factors in conferring the protective effects of exercise against the affective consequences of stressor exposure, and imply that exercise perceived as forced may still benefit affect and mental health.
Keywords: anxiety, depression, forced wheel running, learned helplessness, rat, voluntary wheel running
Introduction
Exercise can increase resistance against stress-related psychiatric disorders such as anxiety and depression (Babyak et al., 2000; Smits et al., 2008; Herring et al., 2010). One critical but relatively unexplored area of inquiry is the identification of which features of exercise are critical for producing the neuroplastic changes required for exercise-induced stress resistance (Cotman et al., 2007; Greenwood & Fleshner, 2011; Ota & Duman, in press). One potential mechanism is the psychological variable of perceived control over exercise. Studies in both humans and rodents reveal that restriction of choice or control is aversive (Clubb & Mason, 2003; Sullivan & Lewis, 2003; Crombez et al., 2008) while the opportunity to exert control is beneficial for physiology, neurochemistry and mental health (Leotti et al., 2010). Rats, for example, that are exposed to a controllable stressor during which they learn to turn a wheel to escape from shock, are protected against anxiety- and depression-like behaviors produced by later exposure to an uncontrollable stressor (Amat et al., 2006, 2010).
If choosing to exercise is a critical signal required for stress resistance, then exercise-induced stress resistance should be sensitive to exercise controllability. Whether those who may perceive exercise as being forced, such as military personnel, professional athletes or those to whom exercise is prescribed by their healthcare providers, still maximally benefit from the mental health benefits of exercise is unknown. The variable effects of forced treadmill training (TT) on animal models of anxiety and depression (Chaouloff, 1994; Dishman et al., 1996; Burghardt et al., 2004; Fulk et al., 2004; Trejo et al., 2008; Salim et al., 2010; Lalanza et al., 2012) are in contrast to the consistently protective effects of voluntary wheel running in animal models of stress-induced anxiety (Dishman, 1997; Greenwood et al., 2003, 2012; Kasimay et al., 2006; Fox et al., 2008; Van Hoomissen et al., 2011; Sciolino & Holmes, 2012; Sciolino et al., 2012; Greenwood & Fleshner, in press). Although these animal data are consistent with exercise controllability being an important factor in determining whether exercise increases stress resistance, there are many factors other than controllability that differ between typical TT paradigms and wheel running. These differences include the distance, duration and pattern of exercise, as well as administration of aversive stimuli required to force the animals to exercise. The current studies utilise a novel forced wheel-running paradigm of a similar behavioral pattern to that of voluntary wheel running, in order to determine whether controllability over exercise is an important variable in the development of exercise-induced stress resistance.
If exercise-induced stress resistance is sensitive to exercise controllability then it should also be dependent on a neural substrate sensitive to behavioral control. The ventral medial prefrontal cortex (mPFC) is a region implicated in the etiology and treatment of stress-related psychiatric disorders (Drevets et al., 1997; Goldwater et al., 2009; Sacher et al., 2012) and thought to be critical for stress resistance produced by the experience of control (Maier & Watkins, 2010). Activity and plasticity in the mPFC, for example, underlies the long-lasting protective effect of prior experience with controllable stress (Amat et al., 2006; Maier et al., 2006). Although not aversive (Belke & Wagner, 2005; Greenwood et al., 2011), voluntary exercise might be considered a type of controllable stress. Indeed, exercise stimulates sympathetic nervous system and hypothalamic– pituitary–adrenal axis activity (Fediuc et al., 2006; Campbell et al., 2009), two hallmarks of the stress response. It is possible that the experience of controllable exercise produces plasticity in the mPFC similar to that produced by controllable stress, thereby enabling stress resistance. The second goal of the present studies is to determine the involvement of the mPFC in exercise-induced stress resistance using excitotoxic lesions of the mPFC. The results of these studies will begin to reveal which features of exercise are critical for its stress-buffering and mental health benefits, and could have important implications for the design and prescription of exercise protocols to stress-vulnerable populations.
Methods and materials
Animals
Young adult (6–7 weeks upon arrival) male Fischer 344 rats (Harlan, Indianapolis, IN, USA) were housed in a temperature (22 ° C)- and humidity-controlled environment on a 12 : 12-h light : dark cycle. Although both outbred Sprague–Dawley (Greenwood et al., 2003) and inbred Fischer 344 (Greenwood et al., 2005a) rats allowed 6 weeks of voluntary access to running wheels are protected against the anxiety- and depression-like behavioral consequences of uncontrollable stress, Fischer 344 rats were used in the current studies because of the lesser variability in running behavior in this strain than in outbred rats. Rats acclimatised to the colony for 1 week prior to manipulation. Rats were weighed weekly and had ad libitum access to food (Lab Chow) and water during their active and inactive cycles. Food and water were weighed three times per week. The University of Colorado Animal Care and Use Committee approved all experimental protocols.
Exercise protocols
Rats were randomly assigned to one of five conditions: sedentary, TT, forced wheel running (FW), voluntary wheel running (VW), or voluntary wheel running in an environment matched to the FW group (VW-m). All rats were individually housed during the inactive cycle in Nalgene Plexiglas cages (45 × 25.2 × 14.7 cm). Rats in the sedentary group were randomly assigned to either remain in these cages (Fig. 1A) or, to control for effects of handling and potential enrichment provided by a wheel, were placed five nights per week into locked wheels (Lafayette Instruments, Lafayette, IN, USA), during the active cycle (Fig. 1B). Behavior of the sedentary groups (N = 6 per group) was indistinguishable within stress conditions, so these groups were pooled and sedentary groups represent rats from both treatment conditions.
Fig. 1.
Photographic depictions of the exercise conditions. (A) Sedentary, (B) Sedentary Lafayette locked wheel, (C) Treadmill training apparatus, (D) Lafayette motorised running wheel, (E) Mini Mitter voluntary running wheel, (F) Lafayette voluntary running wheel. Arrow 1 indicates the wheel locking mechanism. Arrow 2 indicates the motor.
At the start of the active cycle, rats in the TT condition were forced to run on three-lane treadmills (Columbus Instruments, Columbus, OH, USA; Fig. 1C) motivated by foot shocks (0.3 mA). TT occurred five times per week following a progressive schedule designed to facilitate acclimation to the TT procedure and minimise the number of foot shocks required (Table 1). Rats were returned to their home cages after TT, which lasted a maximum of 50 min.
Table 1.
Treadmill training protocol
| Day | Time (min) | Speed (m/min) |
|---|---|---|
| 1–4 | 10 | 10 |
| 5 | 10 | 12 |
| 6–7 | No training | |
| 8 | 5 | 10 |
| 10 | 14 | |
| 9–10 | 5 | 14 |
| 10 | 20 | |
| 11–12 | 5 | 14 |
| 15 | 20 | |
| 13–14 | No training | |
| 15 | 5 | 14 |
| 20 | 20 | |
| 16–17 | 5 | 14 |
| 30 | 20 | |
| 18 | 5 | 14 |
| 35 | 20 | |
| 19 | 5 | 14 |
| 40 | 20 | |
| 20–21 | No training | |
| 22–26 | 5 | 14 |
| 45 | 20 | |
| 27–28 | No training | |
| 29–33 | 5 | 14 |
| 45 | 20 | |
| 34–35 | No training | |
| 36–40 | 5 | 14 |
| 45 | 20 |
At the beginning of the active cycle, all rats in the FW group were removed from their home cages and placed in their assigned motorised running wheel (Lafayette Instruments; Fig. 1D). These wheels (1.1 m circumference) could not be turned voluntarily by the rat. Instead, forced wheels were driven by a motor controlled by the Activity Wheel Monitor software (Lafayette Instruments) according to a protocol pre-programed by the experimenters and designed to closely approximate rats’ natural voluntary running behavior based on analyses of prior experiments. This pattern is characterised by brief bouts of running (average of 2.04 ± 1.95 min) at various speeds (range 4–17 m/min) interspersed with frequent periods of no running (range 0.33–30 min). FW rats were confined to the forced wheels for the entire active cycle and were returned to their home cages at the start of the sleep cycle. Pilot experiments revealed that rats forced to wheel-run with no prior running experience were tumbled about in the wheel and hung onto the wheel rungs rather than running. Five days of prior experience with voluntary running minimised these non-running behaviors. For this reason, all rats in the FW condition were placed nightly into Lafayette voluntary wheels (Fig. 1F) for five consecutive nights during the first week of the study. We have previously reported that 3 weeks of VW is an insufficient duration of voluntary exercise to prevent the behavioral consequences of uncontrollable stress (Greenwood et al., 2005a). It is therefore unlikely that these 5 days of voluntary exercise would contribute to any observed effects of FW on behavior. At day 8 of the study (following 5 days of voluntary running and 2 days of no running), rats in the FW condition began the FW protocol. In order to familiarise FW rats with forced running, FW rats were forced to run only 170 m during the first day of FW.
Rats in the VW group had running wheels (1.1 m circumference; Mini Mitter, Bend, OR, USA) mounted on the inside of their home cage (Fig. 1E). Wheels in the cages of VW rats were locked on the 6th and 7th days of each week; thus VW rats had ad libitum access to their wheels 5 days/week. To control for handling and environmental differences between the Lafayette forced wheels and the Mini Mitter voluntary wheels, rats in the VW-m condition were placed during the active cycle into Lafayette voluntary wheels (Fig. 1F). All rats were exposed to their assigned exercise protocol 5 days/week for 6 weeks prior to exposure to stress or no-stress conditions. The 6-week duration of exercise was chosen because we have previously observed that several behavioral and neurochemical effects of voluntary exercise, such as resistance against behavioral effects of stress (Greenwood et al., 2005a) and neuroadaptations in the central serotonergic system (Greenwood et al., 2005a,b), take between 3 and 6 weeks to develop. Revolutions were automatically recorded by the Activity Wheel Monitor or Vital View software.
Surgical procedures
Following 3 weeks of the sedentary, FW, VW or VW-m conditions, rats were randomly assigned to either receive sham surgery or bilateral excitotoxic lesions of the ventral portion of the mPFC, including the prelimbic and infralimbic cortices. Rats were anesthetised with ketamine (75 mg/kg i.p.) and medetomidine (0.5 mg/kg i.p.) and mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Holes were drilled through the skull above the mPFC. Ibotenic acid (10 µg/µL; Sigma, St Louis, MO, USA) in 0.1 m sodium phosphate buffer was infused at a rate of 0.1 µL/min through a 25-gauge, blunt-tipped, 1.0 µL Hamilton syringe attached to a microinjector unit (David Kopf Instruments) mounted on the stereotaxic apparatus (total volume 0.2 µL per hemisphere). The target site was 2.5 mm anterior, ±0.5 mm lateral and 4.6 mm ventral from bregma, based on the rat brain atlas by Paxinos & Watson (1998). The needle remained in place for 5 min to allow for diffusion. Sham rats received the identical treatment except that no injections were made. Lesions were verified with NeuN immunohistochemistry as in our prior work (Greenwood et al., 2010). NeuN-labeled sections were inspected under a light microscope and locations of lesions were recorded. All rats were allowed to recover for 3 days in their home cages (wheels in VW cages were locked) prior to resuming their exercise protocols.
Stressor induction procedures
Rats were randomly assigned to be either left in their home cages or restrained in Plexiglas tubes and exposed to uncontrollable tail shock consisting of 100 ×5-s tail shocks (1.5 mA) delivered on a random ITI-1 min schedule as described previously (Greenwood et al., 2003; Strong et al., 2011). The entire stress procedure lasted 2 h and was started within 3 h of the start of the inactive cycle.
Behavioral testing
Behavioral testing occurred 24 h after no stress or uncontrollable stress treatment. Pre-shock freezing, shock-elicited freezing, and shuttle box escape behavior on a fixed ratio-2 (FR-2) escape contingency took place sequentially in shuttle boxes (20 inches (~51 cm) wide ×10 (~25) deep × 12 (~30) high; Coulbourn Instruments, Whitehall, PA, USA) following our previously published protocols (Greenwood et al., 2003; Strong et al., 2011). All behavioral tests were administered between 4 and 6 h following the start of the inactive cycle by an experimenter blind to treatment condition of the animals. These behaviors were assessed because exaggerated fear and shuttle box escape deficits produced by uncontrollable stress have been argued to represent animal analogs of human stress-related psychiatric disorders (Weiss et al., 1994; Maier & Watkins, 1998) and can be prevented by 6 weeks of VW (Greenwood et al., 2003, 2005a; Greenwood & Fleshner, 2008). Half of the non-stressed, non-operated rats in each group were killed 2 h following behavioral testing, and thymus, adrenals and spleens were removed and weighed.
Treadmill test to exhaustion
Half of the non-stressed, non-operated rats in each group continued their assigned running protocols for 1 week following behavioral testing. Following 2 days of treadmill acclimatisation, consisting of 10 min of forced running at 10 m/min at a 5% grade, all rats were tested in a treadmill test to exhaustion. Following a 5-min warm-up (10 m/min), speed was increased to 20 m/min (5% grade) and time to volitional exhaustion was recorded. Exhaustion was defined as a failure to keep pace with the treadmill, represented by administration of 10 consecutive foot shocks (Campisi et al., 2003).
Statistical analysis
Average daily distance, average speed while running, and average duration of running bout prior to a 10-min period of no running were calculated, and group comparisons were made using repeated-measures anova. Repeated-measures anova was used to compare body weight gain between groups. Run time to exhaustion and organ weights were compared with anova. Shock-elicited freezing scores were collapsed into 10 × 2-min blocks and analysed with two-way (exercise condition × stress) repeated-measures anova. Escape latencies were collapsed into five blocks of five trials and also analysed with two-way (exercise condition × stress) repeated-measures anova. Average shock-elicited freezing and average FR-2 escape latencies displayed by rats used in the mPFC lesion study were compared using three-way anova with exercise condition, stress and lesion condition as factors. Simple regression analysis was used to determine relationships between distance run and various dependent measures. All analyses were followed by Fisher’s protected least significant difference (PLSD) post hoc tests when appropriate. Alpha was set at 0.05 for each analysis.
Results
Running behavior
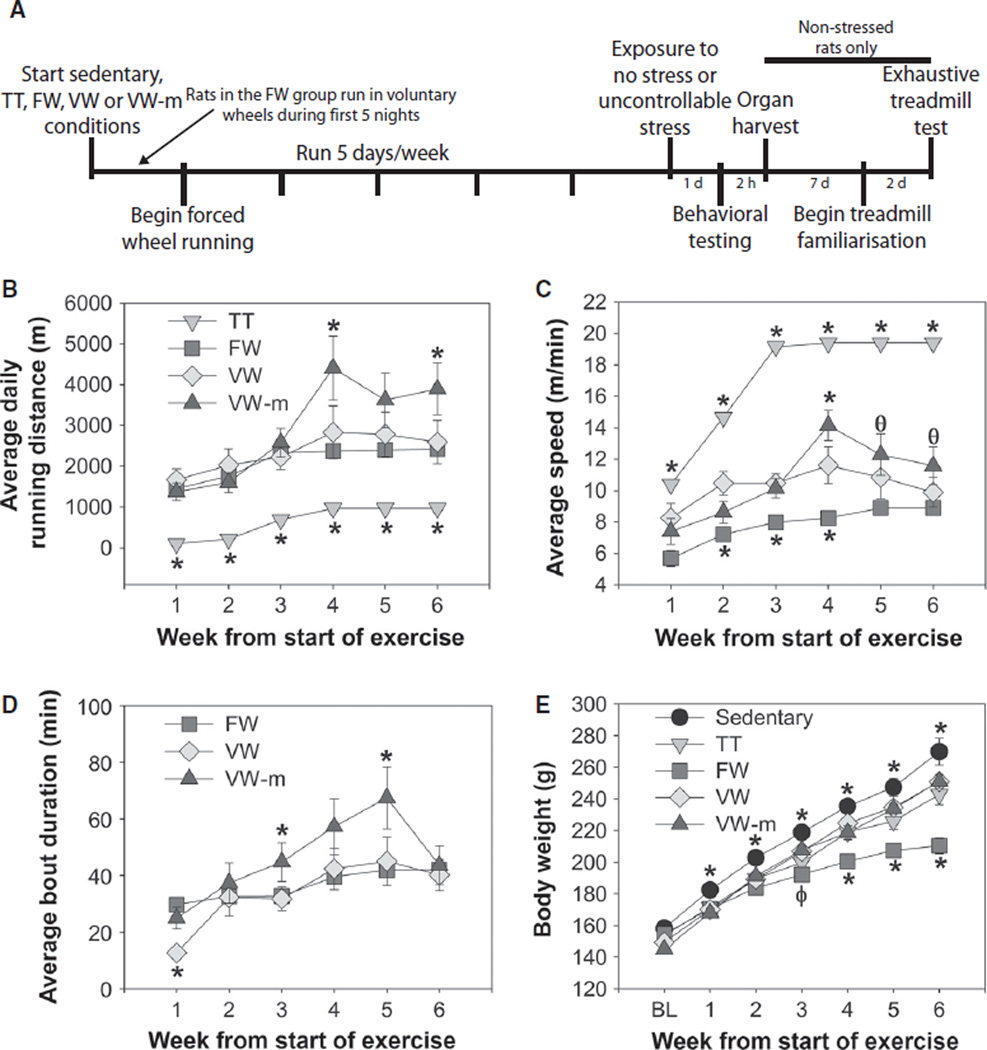
An experimental timeline appears in Fig. 2A. The distance and pattern of FW running closely resembled that of VW running. Average daily running distance (Fig. 2B; F15,300 = 5.01, P < 0.0001), average speed while running (Fig. 2C; (F15,300 = 11.26, P < 0.0001), and average length of running bout prior to a 10-min period of no running (Fig. 2D; (F10,220 = 3.38, P = 0.0004) all differed between exercise conditions. Rats in the TT group (n = 17) ran less distance, but at faster speeds, than rats in other groups. Rats in the FW group (n = 18) ran distances similar to the VW group (n = 16), but the VW-m group (n = 13) ran the greatest distance. Although FW rats ran more slowly than rats in other groups, the average duration of running bout prior to a 10-min rest was generally similar between groups, with the exception of the VW-m group which ran the longest bouts between 10-min rests. The fact that rats in the VW-m were confined to their wheels for the entire active cycle could explain why these rats ran greater distances and had longer running bouts than rats in the VW group. Indeed, during the first week of running, when rats in the FW condition were voluntarily running in an environment identical to the VW-m group, bout length of FW and VW-m groups were similar to each other and longer than those of the VW group. Body weight gain over the duration of the experiment was significantly influenced by exercise condition (F24,498 = 11.09, P < 0.0001), and rats in the FW condition gained the least amount of weight (Fig. 2E).
Fig. 2.
(A) Experimental timeline. Rats either remained sedentary or were exposed to treadmill training (TT), forced wheel running (FW), voluntary wheel running (VW), or voluntary wheel running matched to the environmental conditions of the forced wheel condition (VW-m) for 5 days/week for 6 weeks. (B) Average daily running distance ran by rats in each exercise condition. (C) Average speed during running bouts. (D) Average duration of a bout of continuous running prior to a 10-min period of no running. Rats in the TT group are not included because these rats ran only one bout during each TT session. (E) Body weight at baseline (BL) and over the course of the 6-week experiment. Data represent group means ± SEM; *P < 0.05 relative to all other groups, φP < 0.05 relative to VW and VW-m groups, θP < 0.05 relative to FW group.
Both voluntary and forced exercise prevented the behavioral consequences of uncontrollable stress
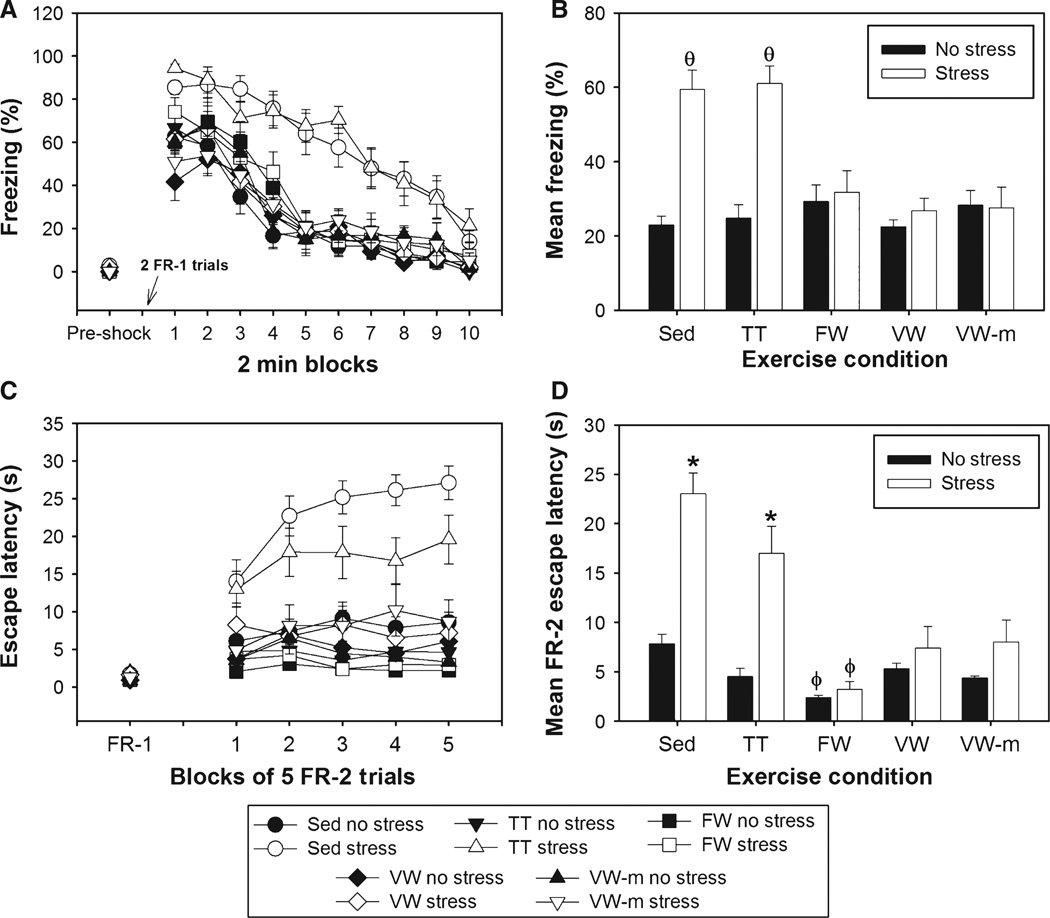
Final group sizes were as follows: Sedentary–No Stress, n = 12; Sedentary–Stress, n = 12; TT–No Stress, n = 8; TT–Stress, n = 9; FW–No Stress, n = 9; FW–Stress, n = 9; VW–No Stress, n = 8; VW–Stress, n = 8; VW-m–No Stress, n = 5; and VW-m–Stress, n = 8. Figure 3 shows the effects of exercise condition on fear and escape behaviors. Freezing behavior prior to the FR-1 trials was minimal and did not differ between groups (Fig. 3A, Pre-shock). The main effects of exercise (F4,78 = 6.79, P < 0.0001), stress (F1,78 = 30.15, P < 0.0001) and time (F9,702 = 129.42, P < 0.0001), as well as the interactions between exercise and stress (F4,78 = 9.3, P < 0.0001) and time and stress (F9,702 = 2.12, P = 0.02) on shock-elicited freezing were all significant (Fig. 3A). Starting by the third freezing block, the Sedentary–Stress group differed from all other groups except the TT–Stress group, which differed from all other groups starting at the fourth freezing block, except the Sedentary–Stress group. Mean shock-elicited freezing is shown in Fig. 3B. FR-1 escape latencies did not differ between groups (Fig. 3C). The main effects of exercise (F4,78 = 19.77, P < 0.0001), stress (F1,78 = 39.99, P < 0.0001) and time (F4,312 = 9.2, P < 0.0001), as well as the interactions between exercise and stress (F4,78 = 8.39, P < 0.01), exercise and time (F16,312 = 40.51, P < 0.0001) and exercise, stress and time (F16,312 = 2.7, P < 0.001) on FR-2 escape behavior were all significant. Sedentary and TT rats exposed to stress escaped from FR-2 trials more slowly than rats in all other groups during all five escape blocks. The FW–No Stress group escaped faster than the Sedentary–No Stress group starting in the third escape block. The Sedentary–Stress group differed from the TT–Stress group starting in the third block of FR-2 trials. The average FR-2 escape behavior is shown in Fig. 3D. No reliable correlations between running distance and behavior were revealed. Interestingly, the behavior of the TT–Stress group did not correlate with the number of shocks received by rats in this group during TT.
Fig. 3.
1998) Following 6 weeks of the sedentary or exercise conditions, rats were exposed to 100 uncontrollable tail shocks (stress) or remained in their home cages (no stress). Shock-elicited freezing and shuttle box escape behavior were measured sequentially 24 h later. (A) Percentage time spent freezing (in 2-min blocks) prior to (pre-shock) and after administration of two fixed ratio-1 (FR-1) foot-shock trials. (B) Average percentage time spent freezing during the 20-min post-shock freezing period. (C) Escape latency during FR-1 trials, during which rats were only required to run through the shuttle box door once to terminate shock, and blocks of five FR-2 trials, during which two shuttle crosses were required to terminate shock. (D) Average FR-2 escape latency across all 25 FR-2 escape trials. Although TT failed to prevent the exaggerated fear and shuttle box escape deficits following stress, FW, VW and VW-m prevented the behavioral consequences of stress. Bars represent means ± SEM; θP < 0.0001 relative to all groups except each other, *P < 0.01 relative to all other groups and φP < 0.05 relative to Sedentary–No Stress, VW–Stress and VW-m–Stress groups.
Despite the small group sizes included in these analyses due to the limited number of non-stressed rats, anova revealed that thymus weight (F4,13 = 4.23, P = 0.02), adjusted adrenal weight (F4,13 = 7.53, P = 0.002), adjusted spleen weight (F4,13 = 7.88, P = 0.002) and run time to exhaustion (F4,19 = 3.07, P = 0.04) all differed between groups (Table 2). Interestingly, all exercise groups except the FW group (P = 0.19) ran longer than sedentary rats prior to exhaustion. Daily food consumption during the 6 weeks of exercise did not differ between groups (F3,44 = 1.76, P = 0.17; data not shown).
Table 2.
Organ weights and run time to exhaustion
| Thymus |
Adrenals |
Spleen |
Time to exhaustion |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | g | g/g bw | g | g/g bw | g | g/g bw | min | n | |
| Sedentary | 6 | 0.317 ± 0.04 | 0.97 ± 0.05 | 0.053 ± 0.004 | 0.16 ± 0.01 | 0.69 ± 0.04 | 2.08 ± 0.10 | 36.17 ± 0.31 | 6 |
| TT | 3 | 0.244 ± 0.01a | 0.93 ± 0.07 | 0.06 ± 0.005 | 0.22 ± 0.01a | 0.66 ± 0.04 | 2.54 ± 0.23a | 69.40 ± 5.34a | 5 |
| FW | 3 | 0.226 ± 0.04a | 1.04 ± 0.17 | 0.055 ± 0.003 | 0.25 ± 0.02a,b | 0.68 ± 0.02 | 3.13 ± 0.19a,c | 56.50 ± 11.54 | 6 |
| VW | 4 | 0.277 ± 0.02 | 1.10 ± 0.07 | 0.05 ± 0.003 | 0.20 ± 0.01a | 0.62 ± 0.02 | 2.43 ± 0.11 | 82.0 ± 16.62a | 4 |
| VW-m | 2 | 0.259 ± 0.01 | 1.04 ± 0.09 | 0.054 ± 0.009 | 0.22 ± 0.03a | 0.70 ± 0.01 | 2.78 ± 0.12a | 90.0 ± 29.16a | 3 |
Mean values are ±SEM; bw, body weight.
P < 0.05 relative to sedentary;
P < 0.05 relative to voluntary wheel run;
P < 0.05 relative to treadmill trained and voluntary wheel run.
The mPFC was not required for exercise-induced stress resistance
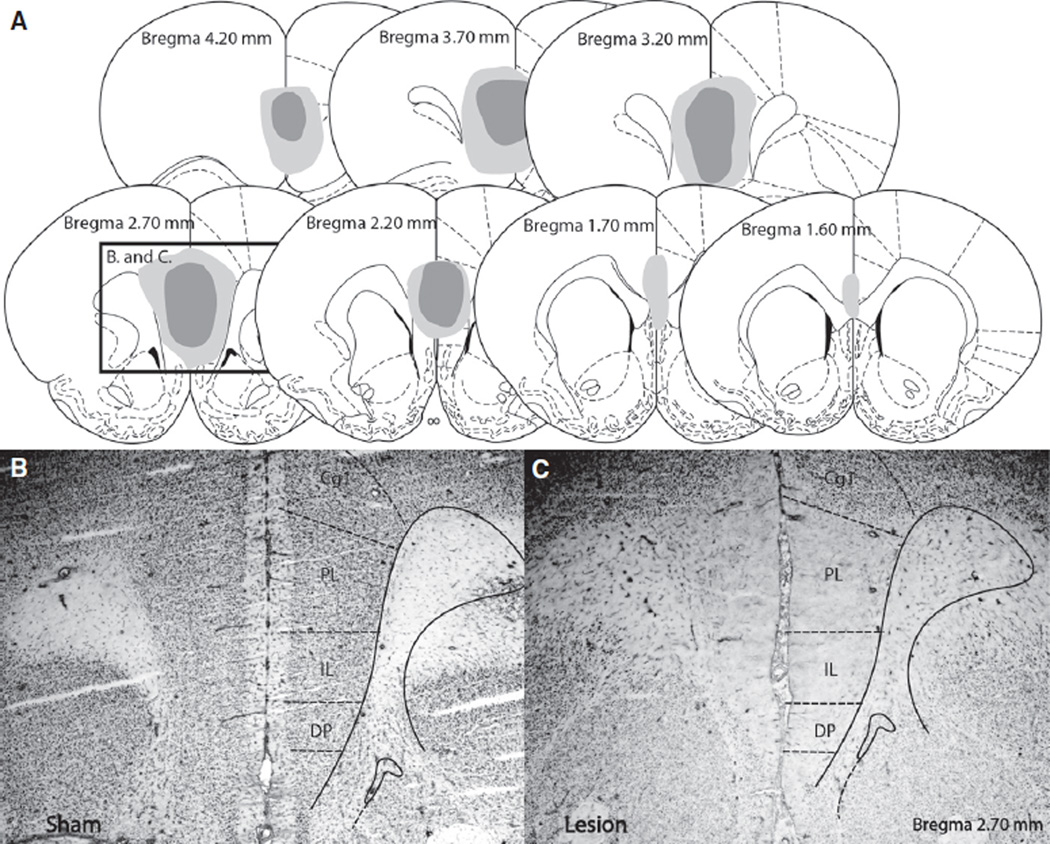
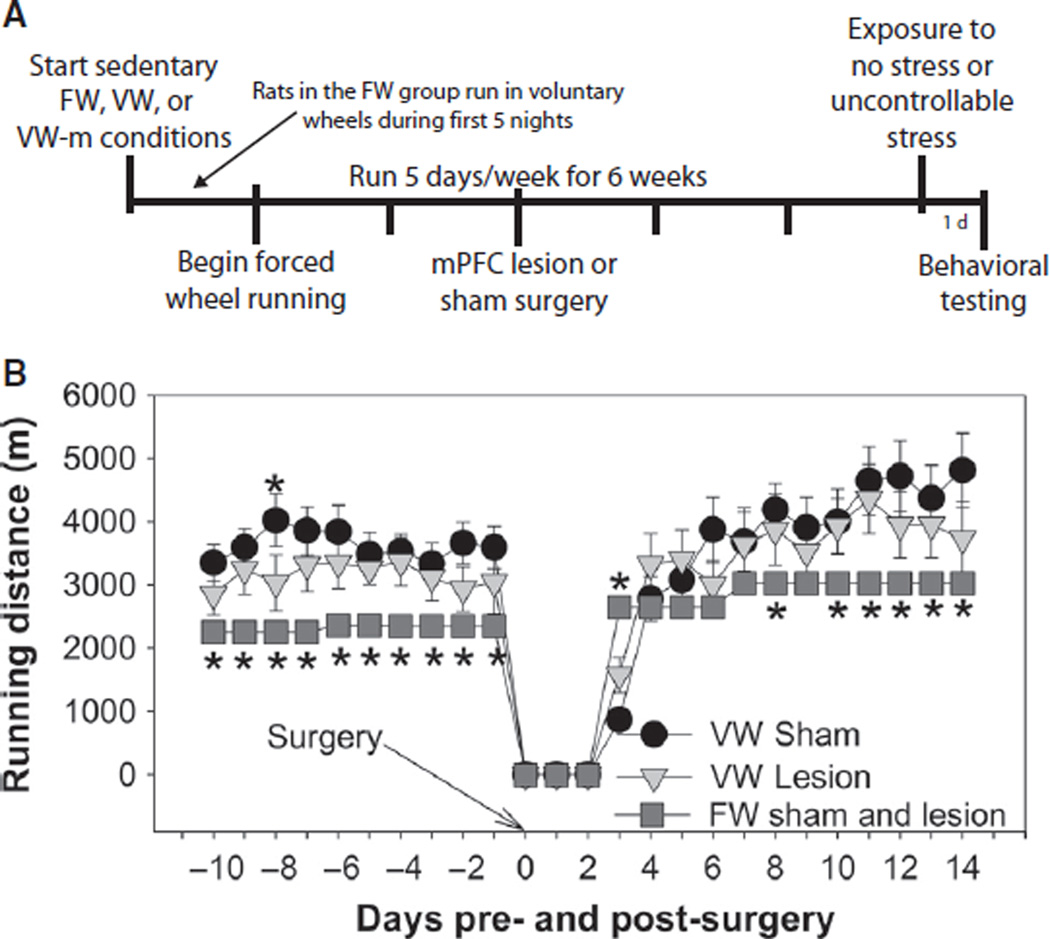
The extent of lesions produced by ibotenic acid is shown in Fig. 4. Only rats with lesions which included the majority of the prelimbic and infralimbic cortices were included. In some cases, lesions extended dorsally into the cingulate gyrus and ventrally into the dorsal peduncular cortex (Fig. 4A). Figure 4B demonstrates NeuN immunoreactivity in the mPFC of a rat exposed to sham surgery, and Fig. 4C shows the lack of NeuN immunoreactivity in mPFC of a rat injected with ibotenic acid. An experimental timeline is shown in Fig. 5A. Behavior of VW and VW-m groups (n = 4–7) was indistinguishable, so the VW groups represent animals pooled from both VW and VW-m groups. Final group sizes had n = 8–14. Rats in the VW group ran a greater distance than rats in the FW group, regardless of lesion (F2,1920 = 8.19, P = 0.0006; Fig. 5B).
Fig. 4.
(A) Graphic reconstruction of excitotoxic lesions of the medial prefrontal cortex caused by injections of ibotenic acid. Dark gray and light gray represent the smallest and largest lesions included in the analyses, respectively. Illustrations are adapted from the atlas of Paxinos & Watson (1998). (B) Representative photomicrograph (5× magnification) showing a coronal section of the mPFC of a rat exposed to sham surgery and processed for NeuN immunohistochemistry (appearing as black-stained nuclei). (C) Representative photomicrograph (5× magnification) showing a coronal section of the medial prefrontal cortex of a rat exposed to lesion surgery and processed for NeuN immunohistochemistry. Note the lack of NeuN immunoreactivity in the prelimbic (PL) and infralimbic (IL) regions in the lesioned rat compared to the sham-operated rat. Cg1, cingulate gyrus; DP, dorsal peduncular cortex.
Fig. 5.
(A) Experimental timeline. Following 3 weeks of the sedentary, FW, VW or VW-m conditions, rats received either sham surgery (sham) or bilateral lesions of the medial prefrontal cortex (Lesion). Rats were exposed to no stress or stress 3 weeks later, and tested behaviorally in shuttle boxes the next day. (B) Average daily distance run by rats in the FW (sham and lesion), VW Sham and VW Lesion groups for 10 days prior to, and 14 days following, lesion or sham surgery. Rats in the VW groups ran greater distances than rats in the FW group and lesions of the mPFC did not influence running behavior. Bars represent means ± SEM. *P < 0.05 relative to all groups.
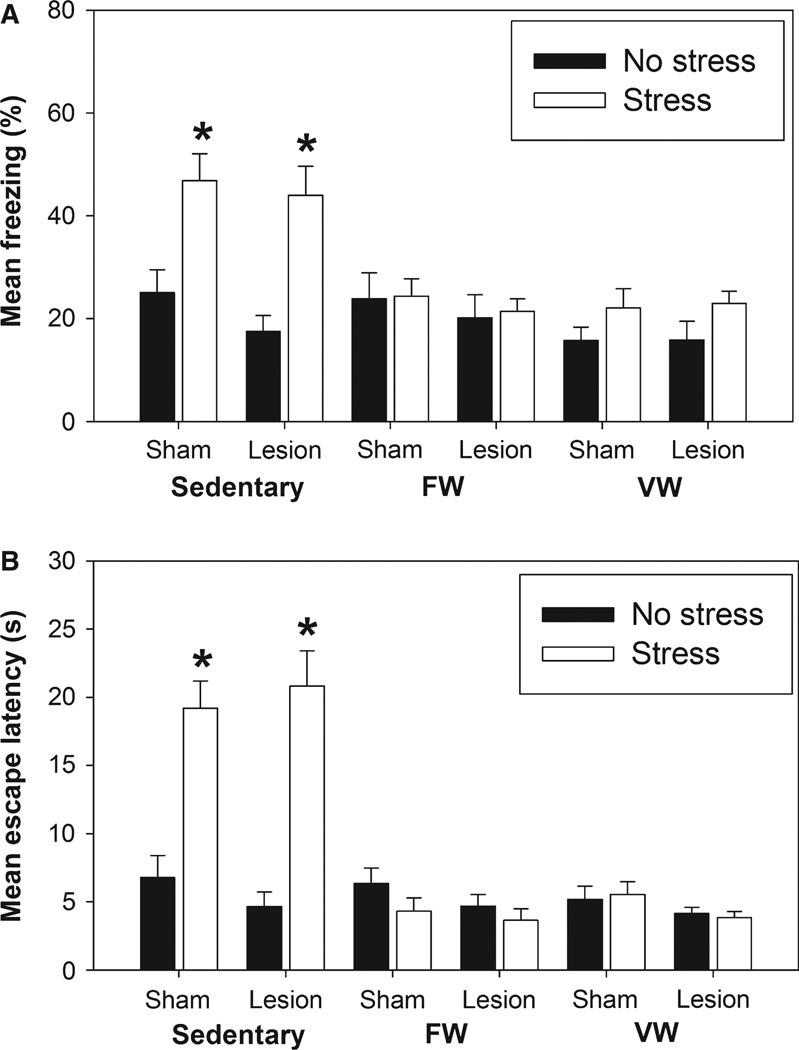
Both forced and voluntary wheel running again prevented the exaggerated fear (Fig. 6A) and escape deficit (Fig. 6B) produced by uncontrollable stress, and this protective effect of exercise was not dependent on an intact mPFC. anova revealed significant main effects of exercise (F2,128 = 13.13, P < 0.0001) and stress (F1,128 = 17.65, P < 0.0001), and a significant interaction between exercise and stress (F2,128 = 8.39, P = 0.0004) on shock-elicited freezing. Similarly, anova revealed significant main effects of exercise (F2,127 = 42.43, P < 0.0001) and stress (F1,127 = 23.4, P < 0.0001), and a significant interaction between exercise and stress (F2,127 = 36.09, P < 0.0001) on average FR-2 escape latency. Lesions of the mPFC had no impact on fear or escape behavior regardless of exercise or stress condition.
Fig. 6.
Following 3 weeks of the sedentary, FW or VW conditions, rats underwent sham surgery or received bilateral injects of ibotenic acid into the medial prefrontal cortex (Lesion). Three weeks later, rats were exposed to 100 uncontrollable tail shocks (Stress) or remained in their home cages (No Stress). Shock-elicited freezing and shuttle box escape behavior were measured sequentially 24 h later. (A) Average time spent freezing during the 20-min observation period following administration of two fixed ratio-1 foot shock trials. (B) Average FR-2 escape latency across all 25 FR-2 escape trials. Stressor exposure produced exaggerated shock-elicited fear and interfered with shuttle box escape in sedentary rats regardless of lesion. These behavioral consequences of uncontrollable stress were prevented by both FW and VW regardless of whether the medial prefrontal cortex was lesioned 3 weeks prior to stress. Bars represent means ± SEM. *P < 0.0001 relative to all groups except each other.
Discussion
The current experiments explored the role of exercise controllability and the mPFC in the protective effect of exercise against anxiety- and depression-like behavioral consequences of uncontrollable stress. Consistent with previous work (Greenwood et al., 2003, 2005a; Greenwood & Fleshner, 2008), uncontrollable stress produced exaggerated fear and interfered with shuttle box escape learning in sedentary rats, and these anxiety- and depression-like effects were prevented by 6 weeks of VW. Because restriction of choice is aversive, and forced exercise is often anxiogenic or without effect in rodent anxiety tests, the assumption has been that exercise must be voluntary in order to enable resistance against affective consequences of stress. If this is the case, then exercise-induced stress resistance should be dependent upon exercise controllability and a neural substrate sensitive to the experience of control, such as the mPFC. Little work, however, has investigated whether control over exercise is a feature critical for producing stress resistance.
Although exercise can have variable effects in animal tests of baseline anxiety, voluntary exercise consistently produces anxiolytic effects in models of stress-induced anxiety (see Sciolino & Holmes, 2012; Greenwood & Fleshner, in press, for reviews). For this reason, rats in the current study were exposed to uncontrollable stress, and anxiety- and depression-like behaviors were assessed the next day. Forced exercise on a treadmill failed to prevent the behavioral consequences of stressor exposure. Several factors, including the experience of shock or the smaller distances run during TT compared to wheel running, could have contributed to the inability of TT to prevent stress-induced behaviors. As the number of shocks received by the TT group during training was unrelated to these rats’ behavior, and if anything rats in the FW appeared more chronically ‘stressed’ than TT rats (based on the lack of body weight gain of the FW group), it is possible that the aversive nature of the foot shocks did not directly contribute to the lack of protection produced by TT. Moreover, distances run by voluntary running groups were unrelated to behavior here and in prior studies (Greenwood et al., 2003, 2005a), suggesting running distance is not a primary factor influencing exercise-induced stress resistance. Differences in the rewarding nature of running on a treadmill vs. a wheel could instead be an important difference. It is possible that a wheel is a preferred, or more rewarding, apparatus than a treadmill. Indeed, when given the choice between typical round wheels or more complex wheels requiring rats to jump over hurdles or across gaps, rats will prefer to run on the more complex wheel (Kavanau, 1967). Exercise on a more complex apparatus such as a wheel could therefore elicit greater exercise reward than exercise on a treadmill, regardless of controllability. If exercise reward is critical to stress-resistance produced by exercise, then potential differences in exercise reward between TT and forced wheel running could thus explain why TT failed to produce stress resistance. The pattern of exercise could also be a critical difference between TT and wheel running. Whereas voluntary wheel runners (and FW in the current studies) seldom run for more than a few minutes at a time prior to a break of at least a few seconds (Eikelboom & Mills, 1988), TT rats were forced to run for up to 50 min continuously. Additionally, running by the forced and voluntary wheel runners was distributed throughout the active cycle, whereas TT rats’ running was restricted to the minutes immediately following the start of the active cycle. It is possible that the repeated alternation between brief running bouts and periods of rest that are characteristic of a natural rodent running pattern are critical for enabling stress resistance.
Using a novel paradigm that forced rats to run in wheels following a distance, duration and pattern that closely resembled voluntary running, we found that forced wheel running was just as effective at preventing the affective consequences of stress as voluntary exercise. These data suggest that choosing to exercise, and exerting control over that exercise, is not required for the development of stress resistance. These results are exciting because they suggest for the first time that people who may perceive exercise as being forced could still benefit from the protective effects of exercise against stress-related psychiatric disorders. Although future work is needed to determine whether a particular pattern of forced exercise is critical for stress resistance, this knowledge could inform the development of novel exercise programs designed to encourage those in stress-vulnerable populations to exercise.
Five days of voluntary running prior to the FW protocol was necessary to allow the rats in the FW group to learn to run on wheels prior to exposure to motorised wheels. We have previously reported that 2–3 weeks of voluntary exercise is an insufficient duration to prevent (Greenwood et al., 2005a) or reverse (Greenwood et al., 2007) the behavioral consequences of uncontrollable stress, or to produce significant conditioned place preference to a chamber paired with the after-effects of nightly voluntary exercise (Greenwood et al., 2011). It is therefore highly unlikely that the initial 5 days of voluntary exercise contributed to the protective effects of subsequent forced wheel running. However, it remains possible that this initial 5 days of voluntary exercise somehow enabled the protective effects of later forced wheel running. Future studies using a forced exercise design that does not utilise prior voluntary running will be required to rule out this possibility. Nevertheless, the current paradigm of familiarisation with the motor pattern required for wheel running followed by forced exercise could more closely resemble the human condition of forced exercise than forced wheel running alone, as people who might perceive exercise as being forced are likely to at least be familiar with the motor skills required to perform the exercise.
Forced exercise, like voluntary exercise, might also be considered a form of controllable stress in that FW rats would learn to run in order to avoid being tumbled about in the motorised wheel. Similar mechanisms, therefore, might underlie the protective effect of forced exercise, voluntary exercise and controllable stress. Despite evidence that the mPFC is critical for stress resistance produced by controllable stress, lesions of the mPFC did not restore the affective consequences of stress in forced or voluntarily exercising rats. These data do not imply that the mPFC is not sensitive to exercise. In fact, exercise can improve executive function attributed to the mPFC in humans (Colcombe & Kramer, 2003). Rather, these data suggest that the mPFC is not required for exercise-induced stress resistance and that exercise recruits a mechanism for stress resistance that is distinct from stressor controllability.
Other than exercise controllability, additional factors could be important for eliciting the neuroplastic changes required for stress resistance produced by voluntary or forced exercise. These include central noradrenergic systems activated in parallel to peripheral sympathetics during exercise (Garcia et al., 2003), entrainment of central circadian or diurnal rhythms (Fleshner & Greenwood, in press), and/or repeated activation of brain reward pathways (Nestler & Carlezon, 2006; Greenwood et al., 2011). In fact, the preferred voluntary pattern of running performed by the forced and voluntary wheel running groups could uniquely entrain rhythms or activate reward circuitry. Additionally, factors released from muscle or liver during exercise, such as insulin-like growth factor, could directly or indirectly signal the brain and result in the plasticity required for stress resistance (Trejo et al., 2001; Fabel et al., 2003; Ding et al., 2006; Duman et al., 2009; Kobilo et al., 2011). The current data indicate that fitness per se may not be a critical factor, however, as rats in the FW group did not run longer than sedentary rats during an exhaustive treadmill test. Future work is required to determine whether forced and voluntary exercise confer stress resistance via similar or independent signaling mechanisms.
Acknowledgments
Studies were funded by National Institutes of Mental Health (R01-MH068283 and R03-MH086665) and Defense Advanced Research Projects Agency Award# W911NF-10-1-0050.
Abbreviations
- FR-2
fixed ratio-2
- FW
forced wheel running
- mPFC
medial prefrontal cortex
- TT
treadmill training
- VW
voluntary wheel running
- VW-m
voluntary wheel running in an environment matched to the FW group
References
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom. Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav. Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC. Voluntary wheel running initially increases adrenal sensitivity to adreno-corticotrophic hormone, which is attenuated with long-term training. J. Appl. Physiol. 2009;106:66–72. doi: 10.1152/japplphysiol.91128.2008. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R520–R530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Influence of physical exercise on 5-HT1A receptor- and anxiety-related behaviours. Neurosci. Lett. 1994;176:226–230. doi: 10.1016/0304-3940(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Clubb R, Mason G. Animal welfare: captivity effects on wide-ranging carnivores. Nature. 2003;425:473–474. doi: 10.1038/425473a. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, De Vlieger P, Van Damme S, De Clercq A. Is it better to have controlled and lost than never to have controlled at all? An experimental investigation of control over pain. Pain. 2008;137:631–639. doi: 10.1016/j.pain.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med. Sci. Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol. Behav. 1996;60:699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces anti-depressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 2009;198:366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague–Dawley rats. J. Appl. Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Greenwood BN. Impact of physical activity on diurnal rhythms: a potential mechanism for stress resistance or stress resilience. In: Hamer M, editor. The Handbook of Physical Activity and Mental Health. New York: Routledge; (In Press), in press. [Google Scholar]
- Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behav. Neurosci. 2008;122:943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int. J. Sports Med. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119:721–732. doi: 10.1016/s0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc. Sport Sci. Rev. 2011;39:140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Mechanisms Underlying the Relationship Between Physical Activity and Anxiety: Animal Data. New York, NY: Routledge; (In Press) [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J. Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005a;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol. Psychiatry. 2005b;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behav. Neurosci. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Fleshner M. Lesions of the basolateral amygdala reverse the long-lasting interference with shuttle box escape produced by uncontrollable stress. Behav. Brain Res. 2010;211:71–76. doi: 10.1016/j.bbr.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav. Brain Res. 2012;233:314–321. doi: 10.1016/j.bbr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring MP, O’ Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch. Intern. Med. 2010;170:321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Kasimay O, Guzel E, Gemici A, Abdyli A, Sulovari A, Ercan F, Yegen BC. Colitis-induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp. Physiol. 2006;91:897–906. doi: 10.1113/expphysiol.2006.034439. [DOI] [PubMed] [Google Scholar]
- Kavanau JL. Behavior of captive white-footed mice. Science. 1967;155:1623–1639. doi: 10.1126/science.155.3770.1623. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanza JF, Sanchez-Roige S, Gagliano H, Fuentes S, Bayod S, Camins A, Pallas M, Armario A, Escorihuela RM. Physiological and behavioural consequences of long-term moderate treadmill exercise. Psychoneuroendocrino. 2012;37:1745–1754. doi: 10.1016/j.psyneuen.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Leotti LA, Iyengar SS, Ochsner KN. Born to choose: the origins and value of the need for control. Trends Cogn. Sci. 2010;14:457–463. doi: 10.1016/j.tics.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability, anxiety, and serotonin. Cogn. Ther. Res. 1998;22:595–613. [Google Scholar]
- Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Ota KT, Duman RS. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiol. Dis. doi: 10.1016/j.nbd.2012.05.022. (In Press) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Sacher J, Neumann J, Funfstuck T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav. Brain Res. 2010;208:545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 2012;36:1965–1984. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Dishman RK, Holmes PV. Voluntary exercise offers anxiolytic potential and amplifies galanin gene expression in the locus coeruleus of the rat. Behav. Brain Res. 2012;233:191–200. doi: 10.1016/j.bbr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. Reducing anxiety sensitivity with exercise. Depress Anxiety. 2008;25:689–699. doi: 10.1002/da.20411. [DOI] [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN. 5-Hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–144. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MW, Lewis M. Contextual determinants of anger and other negative expressions in young infants. Dev. Psychol. 2003;39:693–705. doi: 10.1037/0012-1649.39.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol. Cell. Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen J, Kunrath J, Dentlinger R, Lafrenz A, Krause M, Azar A. Cognitive and locomotor/exploratory behavior after chronic exercise in the olfactory bulbectomy animal model of depression. Behav. Brain Res. 2011;222:106–116. doi: 10.1016/j.bbr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin- releasing factor. Brain Res. Bull. 1994;35:561–572. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]