Abstract
Using guidelines of the Meta-analysis of Observational Studies in Epidemiology Group, we systematically reviewed the literature on neonatal jaundice (unconjugated hyperbilirubinemia) and Autism Spectrum Disorder (ASD) in term and preterm infants. Thirteen studies were included in a meta-analysis. Most used retrospective matched case–control designs. There was significant heterogeneity (Q = 31, p = 0.002) and no evidence of publication bias (p = 0.12). Overall, jaundice, assessed by total serum bilirubin (TSB), was associated with ASD (OR, 1.43, 95% CI 1.22–1.67, random effect model). This association was not found in preterms (OR 0.7, 95% CI 0.38–1.02) but deserves further investigation since other measures of bilirubin such as unbound unconjugated bilirubin may be better predictors of neurotoxicity than TSB in preterms.
Keywords: Unconjugated hyperbilirubinemia, Free bilirubin, Premature infants, Meta-analysis
Introduction
Autism Spectrum Disorders (ASD, including autism, Asperger’s Disorder, and Pervasive Developmental Disorder Not Otherwise Specified) are a group of pervasive developmental disorders characterized by impaired communication, impaired social interaction, and restricted and repetitive interests and behaviors. In recent years, several studies have reported a higher prevalence rate of approximately 1 in 112 in general population when milder variants of the disorder were included (Bertrand et al. 2001; Chakrabarti and Fombonne 2001; Kuehn 2007; Yeargin-Allsopp et al. 2003). In addition, emerging evidence suggests that premature infants are at even higher risk for ASD than the general population (Buchmayer et al. 2009; Johnson et al. 2010; Moster et al. 2008).
Although the precise cause for ASD is unknown, the current high prevalence estimates for ASD suggest that some common perinatal and/or postnatal factor during the neonatal period may be responsible for this increased risk. Further insight into biologically plausible perinatal and/or postnatal risk factors for ASD may be derived from the existing knowledge regarding neuropathology of ASD. ASD is a neurodevelopmental disorder and the most consistent neuropathological finding in infants with ASD is cerebellar hypoplasia most likely resulting from cerebellar injury secondary to exposure to etiologic agents (Bauman and Kemper 2005; Courchesne 1995; Kemper and Bauman 1993; Palmen et al. 2004). The neuropathological findings from autopsies suggest that autistic children have cerebellar hypoplasia involving both vermis and cerebellar hemispheres with significantly decreased number of purkinje and granule cells of the cerebellum (Bauman and Kemper 2005; Kemper and Bauman 1993; Palmen et al. 2004; Ritvo et al. 1986). Cerebellar hypoplasia was also demonstrated by Courchesne et al. (1988) and Courchesne (1991, 1995) in autistic children using in vivo neuroimaging studies. Recently, Limperopoulos (2009) using neuroimaging studies demonstrated that cerebellar injury and impaired cerebellar growth in premature infants were independently associated with higher (abnormal) Modified Checklist for Autism in Toddlers (M-CHAT) scores. Furthermore, recent evidence from neuroimaging and electrophysiological studies suggest that cerebellum is not only important for motor function but is also actively involved in sensory function and cognitive task (Bower 1997; Kern 2002, 2003; Pierce and Courchesne 2001). Based on emerging evidence, Limperopoulos et al. (2007) and Kern (2003) have postulated that because of anatomic and functional interactions between cerebellum and cerebrum including sensory cortices of the brain, cerebellar injury may play a central role in the abnormal fetal brain development characteristic of ASD (Yip et al. 2007). Cerebellar injury has been recently shown to result in disruption of multisensory feedback loop, potentially explaining the clinical characteristics of ASD (Kern 2002).
For these reasons, perinatal and/or postnatal risk factors that can cause cerebellar injury are biological plausible factors that may be associated with subsequent development of ASD. Several investigators have postulated that exposure to some biological factor during the critical period of rapid brain growth that occurs during the second and third trimester of gestation may lead to abnormal fetal brain development in ASD. However, this biological factor is yet to be identified. Based on the emerging evidence, it appears that the biological factor should meet at least two criteria: (a) occurring frequently enough to explain the high prevalence of ASD, specifically in the premature population, and (b) having the potential to cause cerebellar injury.
One possible biological factor that meets both these criteria is unconjugated hyperbilirubinemia.
Neonatal unconjugated hyperbilirubinemia (jaundice) as routinely evaluated by measuring total serum bilirubin concentration (TSB) occurs in varying degrees in most term and premature infants during the first 2 weeks after birth (Watchko and Maisels 2010). In both term and preterm infants, the concentration of conjugated bilirubin is very negligible or undetectable during the neonatal period; therefore, TSB mainly reflects unconjugated bilirubin concentration (Fig. 1).
Fig. 1.
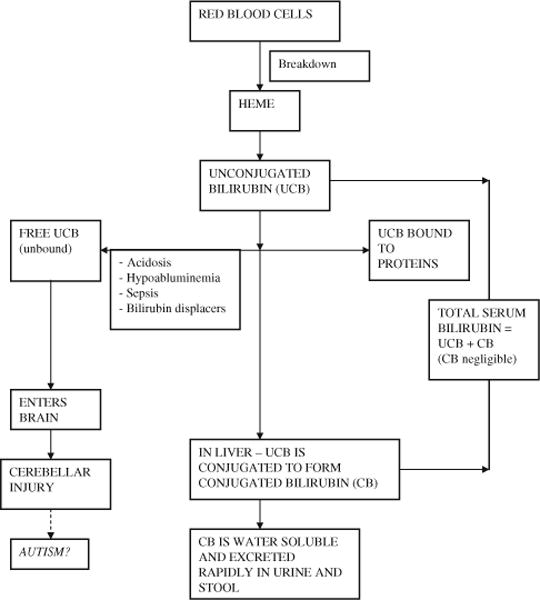
An overview of bilirubin metabolism, bilirubin biochemical markers, and putative pathogenesis of jaundice mediated autism
Unconjugated bilirubin is produced from the breakdown of red blood cells in the vascular system. The majority of unconjugated bilirubin (99.9%) is immediately bound to protein (mainly albumin) and circulates as a bound form until taken up by hepatic cells where it is conjugated to form conjugated bilirubin. The protein binding prevents partially lipid soluble unconjugated bilirubin from crossing the blood brain barrier and subsequently causing brain injury. However, a fraction of unconjugated bilirubin may remain free (unbound to proteins). This free or unbound unconjugated bilirubin has the potential to cross the blood brain barrier and cause neurotoxicity. Compared to unconjugated bilirubin, conjugated bilirubin is water soluble that not only renders it safe but also allows rapid excretion in the urine and stool.
The neuropathological finding of bilirubin induced neurotoxicity is characterized by cerebellar injury with decreased number of purkinje cells (Ahdab-Barmada and Moossy 1984).
In addition, several other clinical features lend indirect support for the causative role of jaundice in ASD. Premature infants are at higher risk of bilirubin-induced neurotoxicity compared to term infants consistent with the findings of increased prevalence of ASD among premature infants compared to term infants (Amin 2004; Burd et al. 1999; Eaton et al. 2001; Larsson et al. 2005; Maimburg and Vaeth 2006). Both autism and bilirubin-induced neurotoxicity may also manifest clinically with abnormal auditory evoked responses and impaired communication further suggesting biological plausibility (Amin 2004; Amin et al. 2009; Funato et al. 1996; Perlstein 1960; Russo et al. 2008; Rosenhall et al. 2003; Whitehouse and Bishop 2008). Several investigators have demonstrated prolongation of absolute and interpeak latencies of auditory brainstem evoked response in autistic children (Rosenhall et al. 2003; Thivierge et al. 1990; Wong and Wong 1991). Similarly, unconjugated hyperbilirubinemia has also been associated with changes in auditory brainstem evoked responses including prolongation of absolute and interpeak wave latencies (Amin et al. 2001; Funato et al. 1994, 1996). We therefore hypothesize that neonatal unconjugated hyperbilirubinemia during the critical period of brain development may be associated with ASD. Our objective was therefore to perform systematic review of the existing literature and evaluate possible association between neonatal jaundice and ASD in neonates.
Methods
We performed a systematic review using published guidelines of the Meta-analysis of Observational Studies in Epidemiology Group (MOOSE) and the Assessment of Multiple Systematic Reviews (AMSTAR) (Stroup et al. 2000; Shea et al. 2007).
Eligibility Criteria
Inclusion criteria: (a) publication in English, and (b) studies with evaluation of neonatal jaundice and ASD. Exclusion criteria: case reports, case series studies, multiple publications of the same material from the same author, and sample size <30 infants. The latter criterion was used because the asymptotic method for meta-analysis tends to overestimate the precision of small studies which would carry too much weight in a meta-analysis (Petitti 2000).
Identification of Studies
Our search strategy was to identify all published completed studies until December 2009 in MEDLINE (1966–2009), PubMed (1966–2009), and references of published manuscripts. Index terms included premature infants, neonates, perinatal factors, neonatal factors, jaundice, hyperbilirubinemia, autism, and autism spectrum disorder.
Data Extraction
Data were collected from each study independently by two investigators using pre-designed form including first author, publication year, study design, study matching criteria, gestational age (GA) of subjects, number of subjects, and raw data using 2 × 2 tables for neonatal jaundice and ASD.
Statistical Analyses
Meta-analysis was performed for the association between neonatal jaundice and ASD using Stata 10 (Stata Corporation, College Station, TX). Briefly, the analysis software produces forest plots as a schematic description of the meta-analysis results. Summary random effect estimates are reported using pooled odds ratios (OR), and 95% confidence intervals (CI) were calculated around each summary effect estimate. The random effect model assumes that analyzed studies are a random sample of a hypothetical population of studies. Heterogeneity testing using Q statistics was performed to evaluate variance between the studies. If the between-study variance was large (i.e. when there is heterogeneity) enough to make the test of heterogeneity significant (p < 0.05), random effects models are considered most appropriate. Each of these studies was examined for sources of clinical heterogeneity including design of the study, year the study was performed, characteristic of study population, procedures for ascertaining autism and jaundice, and analysis of confounders (Table 1). Finally, a Begs funnel plot was produced and an Eggers test was performed to examine publication bias. A p-value <0.05 was considered significant for publication bias.
Table 1.
Observational studies of the association between neonatal jaundice and autism
| Author (year) | Study design and population | Number of subjects | Autism diagnosis | Odds ratio (95% CI) | Heterogeneity |
|---|---|---|---|---|---|
| Finegan and Quarrington (1979) | Matched case control. 23 preterm and term autistic children (<30 months old) matched with a sibling closest in age | 46 | Clinical | 8 (0.39–164) | Medical charts; Jaundice defined as TSB > 16 mg/dl; not controlled for confounders |
| Deykin and MacMahon (1980) | Matched case control. 118 preterm and term autistic children matched with 246 siblings | 364 | Clinical | 0.99 (0.45–2.18) | Degree of jaundice not defined; Medical charts and maternal reports; controlled for confounders |
| Gillberg and Gillberg (1983) | Matched case control involving preterm and term infants. 25 autistic children compared with gender and maternity-clinic matched controls | 50 | Clinical | 3.12 (0.12–80) | Medical charts, Jaundice defined as TSB > 20 mg/dl for birth weight > 2.5 kg; not controlled for confounders |
| Mason-Brothers et al. (1990) | Matched case–control involving preterm and term infants. 223 autistic children versus 57 non-autistic siblings | 280 | DSM-III | 1.18 (0.65–2.13) | Medical charts; degree of jaundice not defined; not controlled for confounders |
| Lord et al. (1991) | Matched case–control study involving low and normal birth weight with cases matched with normal siblings | 100 | DSM-III R | 0.89 (0.30–2.63) | 8–25 years old children, Maternal reports and medical records, degree of jaundice not defined; adjusted for confounders |
| Piven et al. (1993) | Matched case control involving 39 term and preterm infants with ASD matched with normal sibling (5–25 years old) | 78 | ADI and ADOS | 6.9 (0.79–60) | Maternal interviews and medical records; Jaundice defined as TSB > 20 mg/dl for birth weight > 2.5 kg; adjusted for confounders. |
| Matsuishi et al. (1999) | Prospective study involving term and preterm infants. 18 children with autism compared with normal subjects | 232 | DSM-III R | 2.05 (0.77–5.4) | Degree of jaundice not defined; NICU survivors; not controlled for confounders |
| Juul-Dam et al. (2001) | 74 term and preterm infants (2.4–4 years old) with autism or PDD-NOS compared with general population | 128 | ADI-R ADOS | 2.73 (1.09–6.8) | Parental interview and review of medical charts; Jaundice defined as TSB > 10 mg/dl; not controlled for confounders |
| Sugie et al.(2005) | Case control. 209 late preterm and term infants with ASD versus 1,580 controls (3 years or older) | 1,789 | DSM-IV | 2.3 (1.5–3.6) | Maternal interview and medical records; not controlled for confounders; Jaundice defined based on the need of phototherapy |
| Croen et al. (2005) | Matched case control. Each of 338 term infants aged 4–7 years with ASD compared with 5 controls matched with gender, age, and birth hospital | 2,028 | ICD diagnosis | 0.8 (0.55–1.18) | Outpatient and inpatient clinical database; only 28% of subjects had bilirubin level measured |
| Maimburg et al. (2008) | Matched case control. 473 term and preterm autistic children <10 years old matched with randomly selected control for gender, age, and birth country | 946 | ICD diagnosis of infantile autism | 5.9 (2.2–15.4) | Danish Civil Registration System and medical records; controlled for confounders; Jaundice defined based on birth weight or infant’s weight |
| Jangaard et al. (2008) | Retrospective cohort study. Infants ≥35 weeks GA with TSB ≥13.5 mg/dl compared to infants with TSB <13.5 mg/dl | 55,671 | Not defined | 1.7 (1.07–2.7) | Perinatal database and discharge abstract database. Controlled for confounding variables; Jaundice defined |
| Buchmayer et al. (2009) | Matched case control involving term and preterm infants. Each case (1,095) matched with 5 randomly selected controls for gender, birth year, and hospital | 6,776 | ICD diagnosis | is 1.29 (0.99–1.7) | Hospital Discharge Register; controlled for confounders; jaundice defined as that requiring treatment |
ADI Autism Diagnostic Interview, ADI-R Autism Diagnostic Interview-Revised, ICD International Classification of Diseases, ADOS Autism Diagnostic Observation Schedule, PDD-NOS Pervasive Developmental Disorder Not Otherwise Specified, GA gestational age, TSB total serum bilirubin, ASD Autism Spectrum Disorder, DSM Diagnostic and Statistical Manual of Mental Disorders, DSM-R Diagnostic and Statistical Manual of Mental Disorders Revised
Results
Thirteen observational studies met the inclusion criteria (Table 1) (Buchmayer et al. 2009; Croen et al. 2005; Deykin and MacMahon 1980; Finegan and Quarrington 1979; Gillberg and Gillberg 1983; Jangaard et al. 2008; Juul-Dam et al. 2001; Lord et al. 1991; Maimburg et al. 2008; Mason-Brothers et al. 1990; Matsuishi et al. 1999; Piven et al. 1993; Sugie et al. 2005).
Three studies that did not report basic information on number of subjects with jaundice and/or autism but included jaundice in the overall optimal risk scores were excluded (Bryson et al. 1988; Deb et al. 1997; Levy et al. 1988). Data from one research project were published twice with one published in a non-English literature and therefore the non-English manuscript was excluded (Sugie and Sugie 2009). There was an excellent inter-rater reliability with no disagreement among two independent raters regarding identification of studies and data collection. Among 13 included studies, there was a statistically significant association between neonatal jaundice and ASD (OR 1.43, 95% CI 1.22–1.67, p = 0.000, Fig. 2).
Fig. 2.
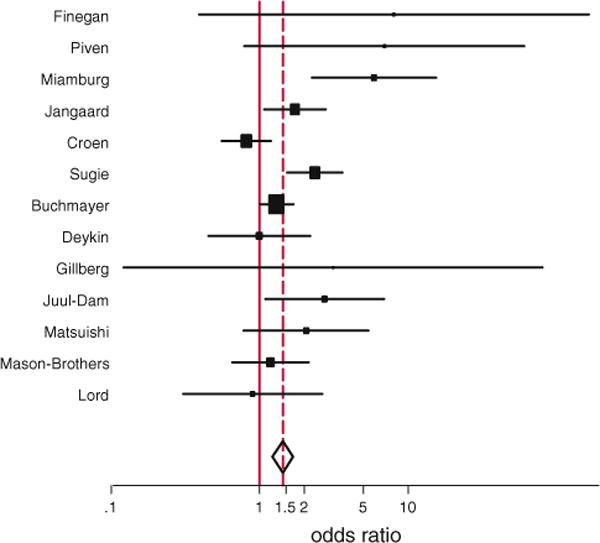
Forrest plot showing individual and combined effect size estimates and 95% confidence interval (CIs) in the studies that evaluated the effect of neonatal jaundice on autism spectrum disorder. The weighting (filled square) given to the study in the overall pooled estimate, taking into account the number of subjects and the amount of between-study variation (heterogeneity). Error bars (−) indicate 95% CIs. The rhombus indicates combined effect size of 1.43
There was no evidence of publication bias (Fig. 3, p = 0.12).
Fig. 3.
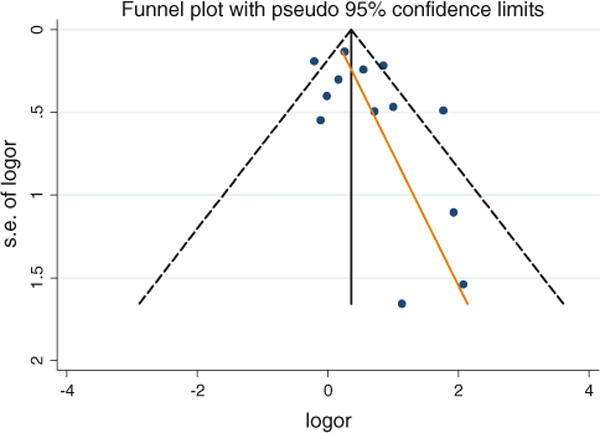
Begg’s funnel plot (with pseudo 95% CI) of the log odds ratio (logor) versus the standard errors of log odds ratio (SE of logor) in studies that evaluated the effect of neonatal jaundice on autism spectrum disorder. There was no publication bias (no asymmetry)
There was significant heterogeneity (Q = 31, p = 0.002). Most studies were designed as retrospective matched case–control studies with the exception of studies performed by Matsuishi (1999) and Jangaard (2008) (Table 1). Most earlier studies were smaller and used siblings as matched controls (Table 1). Studies also differed in procedures used for ascertaining autism and most studies did not use standardized diagnostic assessments for autism such as Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview-Revised (ADIR) (Table 1). Regarding other sources of clinical heterogeneity, all five studies that demonstrated significant positive association (Buchmayer et al. 2009; Jangaard et al. 2008; Juul-Dam et al. 2001; Maimburg et al. 2008; Sugie et al. 2005) defined jaundice based on magnitude of total serum bilirubin concentration and evaluated association between ASD and moderate to severe jaundice (TSB > 10 mg/dl). In comparison, the majority of studies that demonstrated no association (OR<1) between jaundice and ASD included infants with any degree of jaundice (Deykin and MacMahon 1980; Lord et al. 1991). Secondly, most studies that demonstrated significant positive association between jaundice and ASD (Buchmayer et al. 2009; Jangaard et al. 2008; Maimburg et al. 2008; Sugie et al. 2005) were larger and more recent compared to studies that failed to demonstrate significant association between jaundice and ASD (Deykin and MacMahon 1980; Lord et al. 1991) (Table 1).
Although most studies included both premature (<37 weeks gestational age) and term infants with jaundice, there were only two studies that evaluated and reported separately the findings of the association between neonatal jaundice and ASD in premature and term infants (Buchmayer et al. 2009; Maimburg et al. 2008). Both these studies reported significant positive association between neonatal jaundice and ASD in only term infants. Buchmayer et al. (2009) reported that there was no significant association between neonatal jaundice and ASD (OR 0.7, 95% CI 0.45–1.09) in premature infants. Similarly, Maimburg et al. (2008) also reported that there was no significant association between neonatal jaundice and ASD in premature infants <37 weeks GA (OR 1.0, 95% 0.06–15.99). On meta-analysis including only these two studies involving premature infants, there was no significant association between neonatal jaundice and ASD in premature infants (OR 0.7, 95% CI 0.38–1.02).
Discussion
There is ample evidence in the literature to suggest that cerebellar injury is the most consistent neuropathology findings among children with ASD (Bauman and Kemper 2005; Courchesne 1995; Kemper and Bauman 1993; Palmen et al. 2004). Although a well designed prospective study has not yet been conducted, the findings of our meta-analysis of observational studies suggest an association between unconjugated hyperbilirubinemia and ASD (Fig. 2). However, contrary to our hypothesis and biological plausibility, our findings suggest that the association appears to be significant in term infants but not in premature infants.
This contrary finding can be explained by bilirubin metabolism and the pathogenesis of bilirubin-induced neurotoxicity. As noted in Introduction section, the TSB concentration in the absence of phototherapy is comprised of unconjugated and conjugated bilirubin, free or bound to proteins. However, only free unconjugated bilirubin is neurotoxic. The free unconjugated bilirubin concentration is determined by TSB concentration, protein (or albumin) concentration, and the binding affinity between protein and bilirubin (Amin 2004; Ahlfors 2001). According to the current theory of the pathogenesis of bilirubin-induced neurotoxicity, unbound or free unconjugated bilirubin crosses intact blood brain barrier and causes neurotoxicity. Compared to term infants, in very premature infants, not only the protein concentration may be lower but the binding affinity may also be altered because of the presence of several endogenous and exogenous competitors of bilirubin for albumin binding. In such conditions, free unconjugated bilirubin concentration can be significantly high despite low TSB concentration. Thus, it may be important to measure free unconjugated bilirubin in premature infants rather than relying on TSB. In contrast, for term neonates, TSB concentration correlate with free unconjugated bilirubin concentration and has been shown to be a sensitive marker for bilirubin-induced neurotoxicity (Amin 2004; Wennberg et al. 2006). Therefore, in term infants, the magnitude of jaundice (measured by TSB) may be important, and, there may be dose–response association between jaundice and ASD.
We also found that studies that defined jaundice based on high cut-off levels of TSB were the only studies that demonstrated significant positive association between jaundice and ASD suggesting that degree of jaundice may be an important factor for developing ASD (Buchmayer et al. 2009; Jangaard et al. 2008; Juul-Dam et al. 2001; Maimburg et al. 2008; Sugie et al. 2005). Additionally, dose–response association between jaundice and ASD were demonstrated by Maimburg et al. (2008) and Sugie et al. (2005). The later demonstrated that the association was slightly stronger if jaundice was defined based on the need of phototherapy to treat jaundice compared to degree of hyperbilirubinemia (OR 2.5 vs. OR 2.3) (Sugie et al. 2005). However, in premature infants, TSB has failed to predict bilirubin-induced neurotoxicity and neurotoxicity has been reported at lower TSB concentration (Amin 2004; Gartner et al. 1970). In support of the free bilirubin theory, several studies have reported that free bilirubin is a better predictor than TSB of bilirubin-induced neurotoxicity in premature infants (Amin et al. 2001, 2005; Amin 2004, Ahlfors and Parker 2008, Ahlfors et al. 2009; Cashore and Oh 1982; Funato et al. 1994; Nakamura et al. 1985, 1992; Ritter et al. 1982). Therefore, the reported absence of association between ASD and unconjugated hyperbilirubinemia as assessed using TSB concentration in premature infants is not surprising. Unless evidence from prospective studies involving free bilirubin measurement is available, the association between unconjugated hyperbilirubinemia and ASD in premature infants cannot be ruled out.
A limitation of meta-analysis of observational studies is that it is difficult to control for the confounding factors and selection bias often associated with observational studies. Therefore, a causal relationship between jaundice and ASD cannot be established from this literature. However, individual studies that demonstrated significant association between jaundice and ASD had reported adjusted odds ratio after controlling for confounding factors (Buchmayer et al. 2009; Jangaard et al. 2008; Maimburg et al. 2008). Secondly, the majority of these observational studies were exploratory and not hypothesis driven, specifically regarding neonatal jaundice and autism. Thirdly, although the majority of studies involved preterm and term infants, only two reported separate findings for premature infants. Fourthly, the majority of studies that used siblings for matching controls involved siblings of either gender, heterogeneous ages, and unrelated to birth order in the family. Because of gender, environmental, and age related developmental influences on diagnosis of autism, use of age (next in birth order) and gender matched sibling may be more appropriate controls when using family members or siblings as controls in autism research (Shaked and Yirmiya 2004). Fifthly, different diagnostic criteria were used for diagnosis of ASD and very few studies used standardized diagnostic instruments for diagnosis of ASD. Despite these limitations, the findings of these observational studies strongly suggest that neonatal jaundice may be associated with ASD.
The significance of our systematic review is that it may provide much needed impetus and direction for a timely and focused prospective research to evaluate the hypothesis that unconjugated hyperbilirubinemia is a significant cause of ASD in premature and term infants. The hypothesis that unconjugated hyperbilirubinemia is a significant cause of ASD is easily testable, implies prevention, and is of public health importance. The hypothesis can be easily tested in a prospective observational study which will involve prospective collection of information on important bilirubin biochemical markers including free bilirubin concentration during the first two postnatal weeks when neonatal jaundice peaks and then performing prospective assessment for ASD using diagnostic methods such as Autism Diagnostic Observation Schedule. Subsequently, randomized doubleblind clinical trials can be conducted to confirm the association between jaundice and ASD and evaluate the effect of therapeutic intervention on the incidence of autism spectrum disorder. Clinical trials may involve therapeutic interventions such as heme-oxygenase inhibitors which have been used to decrease bilirubin production in premature and term infants (Valaes et al. 1994). The reported increased prevalence of ASD in recent years is a cause for concern. With the recent trend in improved survival of very premature infants, the population of children with ASD may rise, adding to the personal and economic cost of ASD to the society (Leslie and Martin 2007; Stephens and Vohr 2009). The estimated annual cost of ASD to the society is as high as $35 billion dollars per year and an individual lifetime cost is ~$3.2 million (Ganz 2007). If neonatal jaundice predisposes infants to ASD, neonatal interventions aimed at preventing and correcting jaundice could be a promising strategy for reducing jaundice associated ASD and ASD related expenditures in the future.
In summary, unconjugated hyperbilirubinemia may be linked to ASD and may have affected the prevalence of ASD globally. The severity of unconjugated hyperbilirubinemia defined based on free or unbound unconjugated bilirubin concentration may determine the risk of ASD in neonates. Future research should attempt to elucidate the relationship between the level of unbound bilirubin in premature infants and the development of autism spectrum disorders.
Acknowledgments
The paper was supported by NIH K-23 DC 006229-05 and RO3 HD061084-01.
Contributor Information
Sanjiv B. Amin, Email: Sanjiv_amin@urmc.rochester.edu, Division of Neonatology, Department of Pediatrics, The University of Rochester School of Medicine and Dentistry, P.O. Box 651, 601 Elmwood Avenue, Rochester, NY 14642, USA
Tristram Smith, Division of Developmental Pediatrics, Department of Pediatrics, The University of Rochester School of Medicine and Dentistry, Rochester, NY, USA.
Hongyue Wang, Department of Biostatistics, The University of Rochester School of Medicine and Dentistry, Rochester, NY, USA.
References
- Ahdab-Barmada M, Moossy J. The neuropathology of kernicterus in the premature neonate: Diagnostic problems. Journal of Neuropathology and Experimental Neurology. 1984;43(1):45–56. doi: 10.1097/00005072-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Ahlfors CE. Bilirubin–albumin binding and free bilirubin. Journal of Perinatology. 2001;21(Suppl. 1):S40–42. doi: 10.1038/sj.jp.7210631. discussion S59–62. [DOI] [PubMed] [Google Scholar]
- Ahlfors CE, Amin SB, Parker AE. Unbound bilirubin predicts abnormal automated auditory brainstem response in a diverse newborn population. Journal of Perinatology. 2009;29(4):305–309. doi: 10.1038/jp.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlfors CE, Parker AE. Unbound bilirubin concentration is associated with abnormal automated auditory brainstem response for jaundiced newborns. Pediatrics. 2008;121(5):976–978. doi: 10.1542/peds.2007-2297. [DOI] [PubMed] [Google Scholar]
- Amin SB. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Seminars in Perinatology. 2004;28(5):340–347. doi: 10.1053/j.semperi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Amin SB, Ahlfors C, Orlando MS, Dalzell LE, Merle KS, Guillet R. Bilirubin and serial auditory brainstem responses in premature infants. Pediatrics. 2001;107(4):664–670. doi: 10.1542/peds.107.4.664. [DOI] [PubMed] [Google Scholar]
- Amin SB, Charafeddine L, Guillet R. Transient bilirubin encephalopathy and apnea of prematurity in 28 to 32 weeks gestational age infants. Journal of Perinatology. 2005;25(6):386–390. doi: 10.1038/sj.jp.7211295. [DOI] [PubMed] [Google Scholar]
- Amin SB, Prinzing D, Myers G. Hyperbilirubinemia and language delay in premature infants. Pediatrics. 2009;123(1):327–331. doi: 10.1542/peds.2007-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. International Journal of Developmental Neuroscience. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P. Prevalence of autism in a United States population: The Brick Township, New Jersey, investigation. Pediatrics. 2001;108(5):1155–1161. doi: 10.1542/peds.108.5.1155. [DOI] [PubMed] [Google Scholar]
- Bower JM. Is the cerebellum sensory for motor’s sake, or motor for sensory’s sake: The view from the whiskers of a rat? Progress in Brain Research. 1997;114:463–496. doi: 10.1016/s0079-6123(08)63381-6. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Smith IM, Eastwood D. Obstetrical suboptimality in autistic children. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(4):418–422. doi: 10.1097/00004583-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparen P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124(5):e817–e825. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]
- Burd L, Severud R, Kerbeshian J, Klug MG. Prenatal and perinatal risk factors for autism. Journal of Perinatal Medicine. 1999;27(6):441–450. doi: 10.1515/JPM.1999.059. [DOI] [PubMed] [Google Scholar]
- Cashore WJ, Oh W. Unbound bilirubin and kernicterus in low-birth-weight infants. Pediatrics. 1982;69(4):481–485. [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. Jama. 2001;285(24):3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neuroanatomic imaging in autism. Pediatrics. 1991;87(5 Pt 2):781–790. [PubMed] [Google Scholar]
- Courchesne E. New evidence of cerebellar and brainstem hypoplasia in autistic infants, children and adolescents: The MR imaging study by Hashimoto and colleagues. Journal of Austism and Developmental Disorders. 1995;25(1):19–22. doi: 10.1007/BF02178164. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. The New England Journal of Medicine. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Croen LA, Yoshida CK, Odouli R, Newman TB. Neonatal hyperbilirubinemia and risk of autism spectrum disorders. Pediatrics. 2005;115(2):e135–e138. doi: 10.1542/peds.2004-1870. [DOI] [PubMed] [Google Scholar]
- Deb S, Prasad KB, Seth H, Eagles JM. A comparison of obstetric and neonatal complications between children with autistic disorder and their siblings. Journal of Intellectual Disablity Research. 1997;41(Pt 1):81–86. doi: 10.1111/j.1365-2788.1997.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Deykin EY, MacMahon B. Pregnancy, delivery, and neonatal complications among autistic children. American Journal of Diseases of Children. 1980;134(9):860–864. doi: 10.1001/archpedi.1980.02130210044012. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Mortensen PB, Thomsen PH, Frydenberg M. Obstetric complications and risk for severe psychopathology in childhood. Journal of Austism and Developmental Disorders. 2001;31(3):279–285. doi: 10.1023/a:1010743203048. [DOI] [PubMed] [Google Scholar]
- Finegan JA, Quarrington B. Pre-, peri-, and neonatal factors and infantile autism. Journal of Child Psychology and Psychiatry. 1979;20(2):119–128. doi: 10.1111/j.1469-7610.1979.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Funato M, Tamai H, Shimada S, Nakamura H. Vigintiphobia, unbound bilirubin, and auditory brainstem responses. Pediatrics. 1994;93(1):50–53. [PubMed] [Google Scholar]
- Funato M, Teraoka S, Tamai H, Shimida S. Follow-up study of auditory brainstem responses in hyperbilirubinemic newborns treated with exchange transfusion. Acta Paediatrica Japonica. 1996;38(1):17–21. doi: 10.1111/j.1442-200x.1996.tb03428.x. [DOI] [PubMed] [Google Scholar]
- Ganz ML. The lifetime distribution of the incremental societal costs of autism. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- Gartner LM, Snyder RN, Chabon RS, Bernstein J. Kernicterus: High incidence in premature infants with low serum bilirubin concentrations. Pediatrics. 1970;45(6):906–917. [PubMed] [Google Scholar]
- Gillberg C, Gillberg IC. Infantile autism: A total population study of reduced optimality in the pre-, peri-, and neonatal period. Journal of Austism and Developmental Disorders. 1983;13(2):153–166. doi: 10.1007/BF01531816. [DOI] [PubMed] [Google Scholar]
- Jangaard KA, Fell DB, Dodds L, Allen AC. Outcomes in a population of healthy term and near-term infants with serum bilirubin levels of > or = 325 micromol/L (>or = 19 mg/dL) who were born in Nova Scotia, Canada, between 1994 and 2000. Pediatrics. 2008;122(1):119–124. doi: 10.1542/peds.2007-0967. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism Spectrum disorders in extremely preterm children. Journal of Pediatrics. 2010;156:525–31.e2. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107(4):E63. doi: 10.1542/peds.107.4.e63. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurology Clinic. 1993;11(1):175–187. [PubMed] [Google Scholar]
- Kern JK. The possible role of the cerebellum in autism/PDD: Disruption of a multisensory feedback loop. Medical Hypotheses. 2002;59(3):255–260. doi: 10.1016/s0306-9877(02)00212-8. [DOI] [PubMed] [Google Scholar]
- Kern JK. Purkinje cell vulnerability and autism: A possible etiological connection. Brain and Development. 2003;25(6):377–382. doi: 10.1016/s0387-7604(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. CDC: Autism spectrum disorders common. Jama. 2007;297(9):940. doi: 10.1001/jama.297.9.940. [DOI] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161(10):916–925. doi: 10.1093/aje/kwi123. discussion 926–918. [DOI] [PubMed] [Google Scholar]
- Leslie DL, Martin A. Health care expenditures associated with autism spectrum disorders. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):350–355. doi: 10.1001/archpedi.161.4.350. [DOI] [PubMed] [Google Scholar]
- Levy S, Zoltak B, Saelens T. A comparison of obstetrical records of autistic and nonautistic referrals for psychoeducational evaluations. Journal of Austism and Developmental Disorders. 1988;18(4):573–581. doi: 10.1007/BF02211875. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C. Autism spectrum disorders in survivors of extreme prematurity. Clinical Perinatology. 2009;36(4):791–805. vi. doi: 10.1016/j.clp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- Lord C, Mulloy C, Wendelboe M, Schopler E. Pre- and perinatal factors in high-functioning females and males with autism. Journal of Austism and Developmental Disorders. 1991;21(2):197–209. doi: 10.1007/BF02284760. [DOI] [PubMed] [Google Scholar]
- Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatrica Scandinavica. 2006;114(4):257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Maimburg RD, Vaeth M, Schendel DE, Bech BH, Olsen J, Thorsen P. Neonatal jaundice: A risk factor for infantile autism? Paediatric and Perinatal Epidemiology. 2008;22(6):562–568. doi: 10.1111/j.1365-3016.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- Mason-Brothers A, Ritvo ER, Pingree C, Petersen PB, Jenson WR, McMahon WM, et al. The UCLA-University of Utah epidemiologic survey of autism: Prenatal, perinatal, and postnatal factors. Pediatrics. 1990;86(4):514–519. [PubMed] [Google Scholar]
- Matsuishi T, Yamashita Y, Ohtani Y, Ornitz E, Kuriya N, Murakami Y, et al. Brief report: Incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. Journal of Austism and Developmental Disorders. 1999;29(2):161–166. doi: 10.1023/a:1023048812202. [DOI] [PubMed] [Google Scholar]
- Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. The New England Journal of Medicine. 2008;359(3):262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Takada S, Shimabuku R, Matsuo M, Matsuo T, Negishi H. Auditory nerve and brainstem responses in newborn infants with hyperbilirubinemia. Pediatrics. 1985;75(4):703–708. [PubMed] [Google Scholar]
- Nakamura H, Yonetani M, Uetani Y, Funato M, Lee Y. Determination of serum unbound bilirubin for prediction of kernicterus in low birthweight infants. Acta Paediatrica Japonica. 1992;34(6):642–647. doi: 10.1111/j.1442-200x.1992.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127(Pt 12):2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Perlstein M. The late clinical syndrome of posticteric encephalopathy. Pediatric Clinics of North America. 1960;7:665–687. [Google Scholar]
- Petitti DB. Statistical methods in meta-analysis. In: Petitti DB, editor. Meta-analysis, decision analysis, and cost-effectiveness analysis: Methods for qunatitative synthesis in medicine. 2. New York: Oxford University Press; 2000. pp. 94–116. [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry. 2001;49(8):655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Piven J, Simon J, Chase GA, Wzorek M, Landa R, Gayle J, et al. The etiology of autism: Pre-, peri- and neonatal factors. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(6):1256–1263. doi: 10.1097/00004583-199311000-00021. [DOI] [PubMed] [Google Scholar]
- Ritter DA, Kenny JD, Norton HJ, Rudolph AJ. A prospective study of free bilirubin and other risk factors in the development of kernicterus in premature infants. Pediatrics. 1982;69(3):260–266. [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: Initial findings of the UCLA-NSAC Autopsy Research Report. American Journal of Psychiatry. 1986;143(7):862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Brantberg K, Gillberg C. Autism and auditory brain stem responses. Ear and Hearing. 2003;24(3):206–214. doi: 10.1097/01.AUD.0000069326.11466.7E. [DOI] [PubMed] [Google Scholar]
- Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, et al. Deficient brainstem encoding of pitch in children with Autism Spectrum Disorders. Clinical Neurophysiology. 2008;119(8):1720–1731. doi: 10.1016/j.clinph.2008.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked M, Yirmiya N. Matching procedures in autism research: Evidence from meta-analytic studies. Journal of Austism and Developmental Disorders. 2004;34(1):35–40. doi: 10.1023/b:jadd.0000018072.42845.83. [DOI] [PubMed] [Google Scholar]
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatric Clinics of North America. 2009;56(3):631–646. doi: 10.1016/j.pcl.2009.03.005. Table of Contents. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Sugie Y, Sugie H. Perinatal and neonatal risk factors for autism spectrum disorders. Seishin Shinkeigaku Zasshi. 2009;111(11):1397–1403. [PubMed] [Google Scholar]
- Sugie Y, Sugie H, Fukuda T, Ito M. Neonatal factors in infants with Autistic Disorder and typically developing infants. Autism. 2005;9(5):487–494. doi: 10.1177/1362361305057877. [DOI] [PubMed] [Google Scholar]
- Thivierge J, Bedard C, Cote R, Maziade M. Brainstem auditory evoked response and subcortical abnormalities in autism. Amican Journal of Psychiatry. 1990;147(12):1609–1613. doi: 10.1176/ajp.147.12.1609. [DOI] [PubMed] [Google Scholar]
- Valaes T, Petmezaki S, Henschke C, Drummond GS, Kappas A. Control of jaundice in preterm newborns by an inhibitor of bilirubin production: Studies with tin-mesoporphyrin. Pediatrics. 1994;93(1):1–11. [PubMed] [Google Scholar]
- Watchko JF, Maisels MJ. Enduring controversies in the management of hyperbilirubinemia in preterm neonates. Seminars in Fetal and Neonatal Medicine. 2010;15(3):136–140. doi: 10.1016/j.siny.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Wennberg RP, Ahlfors CE, Bhutani VK, Johnson LH, Shapiro SM. Toward understanding kernicterus: A challenge to improve the management of jaundiced newborns. Pediatrics. 2006;117(2):474–485. doi: 10.1542/peds.2005-0395. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV. Do children with autism ‘switch off’ to speech sounds? An investigation using event-related potentials. Developmental Science. 2008;11(4):516–524. doi: 10.1111/j.1467-7687.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- Wong V, Wong SN. Brainstem auditory evoked potential study in children with autistic disorder. Journal of Austism and Developmental Disorders. 1991;21(3):329–340. doi: 10.1007/BF02207329. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. Jama. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: Pathophysiological implications. Acta Neuropathologica. 2007;113(5):559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]


