Abstract
While cyclosporine (CsA) inhibits calcineurin and is highly effective in prolonging rejection for transplantation patients, the immunological mechanisms remain unknown. Herein, the role of calcineurin signaling was investigated in a mouse allogeneic skin transplantation model. The calcineurin inhibitor CsA significantly ameliorated allograft rejection. In CsA-treated allograft recipient mice, CD11b+ Gr1+ myeloid-derived suppressor cells (MDSCs) were functional suppressive immune modulators that resulted in fewer gamma interferon (IFN-γ)-producing CD8+ T cells and CD4+ T cells (TH1 T helper cells) and more interleukin 4 (IL-4)-producing CD4+ T cells (TH2) and prolonged allogeneic skin graft survival. Importantly, the expression of NFATc1 is significantly diminished in the CsA-induced MDSCs. Blocking NFAT (nuclear factor of activated T cells) with VIVIT phenocopied the CsA effects in MDSCs and increased the suppressive activities and recruitment of CD11b+ Gr1+ MDSCs in allograft recipient mice. Mechanistically, CsA treatment enhanced the expression of indoleamine 2,3-dioxygenase (IDO) and the suppressive activities of MDSCs in allograft recipients. Inhibition of IDO nearly completely recovered the increased MDSC suppressive activities and the effects on T cell differentiation. The results of this study indicate that MDSCs are an essential component in controlling allograft survival following CsA or VIVIT treatment, validating the calcineurin-NFAT-IDO signaling axis as a potential therapeutic target in transplantation.
INTRODUCTION
Calcineurin inhibitors, such as cyclosporine (CsA) and FK506, are drugs widely used to prevent the rejection of solid organ allograft (1–3). CsA is best characterized for its ability to inhibit T cell function, predominantly by preventing the activation of the NFAT (nuclear factor of activated T cells) transcription factors (4). Blocking the activation of NFATs prevents the transcription of many characteristic T cell effector cytokines, such as interleukin 2 (IL-2), in activated T cells (5, 6). All calcium-responsive members of the NFAT family are retained in an inactive state in the cytosol by phosphorylation of serines in an N-terminal serine-rich domain (7). Upon intracellular calcium influx, calmodulin displaces an autoinhibitory loop from the active site of the phosphatase calcineurin (8, 9). Calcineurin then removes the inhibitory phosphates, allowing NFATs to translocate to the nucleus where they collaborate with other transcription factors, such as activator protein 1 (AP-1), to effect changes in gene transcription (10–12). Although NFATs have been extensively studied in the context of T cells, relatively few studies have examined their function in myeloid lineages.
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous family of myeloid cells that suppress T cell immunity in tumor-bearing hosts (13–15). MDSCs have been detected in the blood of cancer patients, as well as the peripheral immunological organs of tumor-bearing mice (16, 17). In transplantation, MDSCs are beneficial for protecting against kidney and cardiovascular graft rejection (18, 19). A recent study showed that CsA may negatively impact regulatory T (Treg) cell proliferation when they receive strong allogeneic major histocompatibility complex (MHC)-mediated T cell receptor (TCR) signals (20). However, the MDSC regulatory mechanisms of the calcineurin pathway in transplantation remain unclear.
In the present study, our data showed that MDSCs are an essential immune component in allograft survival prolonged by a calcineurin inhibitor. Targeting the calcineurin-NFAT axis, CsA treatment significantly promoted the CD11b+ Gr1+ MDSC recruitment, potentiated their suppressive activities, and directed the T cell differentiation in ameliorating allograft immune rejection.
MATERIALS AND METHODS
Mice.
All animal experiments were performed in accordance with the approval of the Animal Ethics Committee of Fudan University, Shanghai, China. CD45.1+ C57BL/6 OTII and OTI mice were obtained from the Center of Model Animal Research at Nanjing University (Nanjing, China). BALB/c and C57BL/6 (CD45.2+) mice were obtained from the Fudan University Experimental Animal Center (Shanghai, China). All mice were bred and maintained in specific-pathogen-free conditions. Sex-matched littermates at 6 to 8 weeks of age were used in the experiments described in this study.
Skin transplantation and histopathological analysis.
Skin from BALB/c mice was transplanted into C57BL/6 recipients as previously described (21–24). Recipient mice were injected intraperitoneally (i.p.) with cyclosporine (CsA) (15 to 30 mg/kg body weight) daily starting on day 1 (6 h before the transplantation with allogeneic skin). For skin transplantation, erythema, edema, and hair loss were considered early signs of rejection, whereas ulceration, progressive shrinkage, and desquamation were considered the endpoints of rejection (25). Photographs were taken daily with a digital camera (Powershot A640; Canon, Japan) until the graft was rejected completely. The skin grafts were removed at the time points indicated in the figures and rinsed in cold phosphate-buffered saline (PBS), placed in OCT compound, and immediately frozen in liquid nitrogen for histopathological examination. Sections (4 to 6 μm) were fixed in 4% paraformaldehyde and stained with hematoxylin and eosin (H&E) for the assessment of infiltration of cells.
Monoclonal antibody (MAb) and flow cytometry.
For the flow cytometry method (FCM) of cell surface markers, cells were stained with antibodies in PBS containing 0.1% (wt/vol) bovine serum albumin (BSA) and 0.1% NaN3. The following antibodies were obtained from eBioscience: anti-CD11b (M1/70), anti-F4/80 (BM8), anti-Gr-1 (RB6-8C5), anti-CD4 (GK1.5), anti-CD44 (IM7), and anti-CD62L (MEL-4). The following antibodies were obtained from BD Biosciences: anti-CD45 (TU116), anti-CD8 (53-6.7), and antibody against chemokine (C-X-C motif) receptor 2 (anti-CXCR2) (242216). Anti-CD3 (145-2C11) and anti-CD19 (6D5) were obtained from Miltenyi Biotec, Bergisch Gladbach, Germany. Anti-indoleamine 2,3-dioxygenase (anti-IDO) (ab55305) was obtained from Abcam.
Intracellular staining was analyzed by FCM according to the manufacturer's instructions, with modifications as described previously (26). For cytokine expression analysis, C57BL/6 recipient cells isolated from the organs indicated in the figures were restimulated with lipopolysaccharide (LPS) (catalog no. L2630; Sigma) for 5 h for CD11b+ Gr1+ cell analysis or restimulated with alloantigen (mitomycin C-pretreated BALB/c splenocytes) or ovalbumin (OVA) antigen for 5 h for CD4+ or CD8+ T cell analysis, and GolgiStop (catalog no. 554724; BD Bioscience) was added for the last 2 h. After surface staining and washing, the cells were immediately fixed with cytofix/cytoperm solution (catalog no. 554714; BD Biosciences) and stained with anti-tumor necrosis factor alpha (anti-TNF-α) (MP6-XT22), anti-IL-10 (JES5-16E3), anti-IL-4 (11B11), and anti-IFN-γ (XMG1.2) from eBioscience. For Foxp3 expression analysis, after surface staining, the cells were fixed with fixation/permeabilization buffer (catalog no. 00-5523; eBioscience) and stained with anti-Foxp3 (FJK-16S; eBioscience). The FCM data were acquired on a FACSCalibur cell analyzer (Becton Dickinson, San Diego, CA) or an Epics XL bench-top flow cytometer (Beckman Coulter, CA), and data were analyzed with FlowJo (TreeStar, San Carlos, CA). The numbers of cells of the various populations were calculated by multiplying the percentage of cells of interest by the total cell number.
Cell isolation and purification.
For the isolation of skin graft cells, skin grafts were retrieved and minced into small pieces with a scalpel and then digested for about 1 h at 37°C in RPMI 1640 medium containing 30 U/ml of collagenase (type IV; Sigma) and 1 mM CaCl2. Collagenase-pretreated tissues were then ground with the plunger of a 5-ml disposable syringe and passed through a 70-μm nylon cell strainer. Cells were collected by centrifugation at 300 × g for 15 min and resuspended in fluorescence-activated cell sorting (FACS) staining buffer for cell marker staining.
Following cardiac perfusion with PBS, the spleen was aseptically removed and mechanically disrupted between sterile frosted microscope slides as described previously (27, 28). Splenic CD11b+ cells were isolated using anti-CD11b magnetic beads and positive-selection columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Gr1+ cells were isolated using phycoerythrin (PE)-labeled anti-Gr1 MAb (RB6-8C5; eBioscience) and positive immunomagnetic separation with a selection kit (Stem Cell Technologies, Vancouver, BC, Canada) or sorted on a FACSAria II instrument (Becton Dickinson, San Diego, CA). Lymphocytes were isolated from the lymph nodes and CD4+ or CD8+ T cells were sorted on a FACSAria II instrument as described previously (29). Flow cytometry verified that all of the isolated cells yielded a greater than 90% pure population.
Real-time PCR and immunoblot analysis.
Total RNA from the skin grafts or spleen of the allograft recipients was isolated using RNeasy columns, and contaminating DNA was removed by on-column treatment with RNase-free DNase (Qiagen). RNA was extracted with an RNeasy kit (Qiagen, Dusseldorf, Germany), and cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). An ABI 7900 real-time PCR system was used for quantitative PCR, with the primers and probe sets obtained from Applied Biosystems (Carlsbad, CA) (Table 1). Results were analyzed using SDS 2.1 software (Applied Biosystems). The cycle threshold (CT) value of the endogenous control gene (Hprt1, encoding hypoxanthine guanine phosphoribosyltransferase) was subtracted from the cycle threshold (ΔCT). The level of expression of each target gene is presented as the fold change relative to that of the control samples (2–ΔΔCT) as described previously (29). Immunoblot analysis was performed as described previously (30) using anti-NFATc1 (7A6; Thermo Scientific) and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) (ab9483; Abcam).
TABLE 1.
Primer sequences used for real-time PCR assays
| Protein | Primer sequence |
|
|---|---|---|
| Forward primer | Reverse primer | |
| IDO | 5′-TGTGGCTAGAAATCTGCCTGT-3′ | 5′-CTGCGATTTCCACCAATAGAG-3′ |
| IFN-γ | 5′-GAACTGGCAAAAGGATGGTGA-3′ | 5′-TGTGGGTTGTTGACCTCAAAC-3′ |
| IL-4 | 5′-TTGTCATCCTGCTCTTCTTTCTC-3′ | 5′-CAGGAAGTCTTTCAGTGATGTGG-3′ |
| Foxp3 | 5′-GGCCCTTCTCCAGGACAGA-3′ | 5′-GGCATGGGCATCCACAGT-3′ |
| RORγt | 5′-CCGCTGAGAGGGCTTCAC-3′ | 5′-TGCAGGAGTAGGCCACATTACA-3′ |
| T-bet | 5′-AGCAAGGACGGCGAATGTT-3′ | 5′-GGGTGGACATATAAGCGGTTC-3′ |
| GATA3 | 5′-GAATCCTCTGCATCAACAAGC-3′ | 5′-GGGCAAGGGTTCTGAGGT-3′ |
| HPRTa | 5′-CCTAAGATGAGCGCAAGTTGAA-3′ | 5′-CCACAGGACTAGAACACCTGCTAA-3′ |
HPRT, hypoxanthine phosphoribosyltransferase.
In vivo and in vitro functional assays of CD11b+ Gr1+ cells.
For depleting CD11b+ Gr1+ cells in vivo, 0.5 mg of depleting anti-Gr-1 MAb (RB6-8C5) was i.p. administered to recipients on day −1 before skin transplantation. For detection of the immunosuppression of CD11b+ Gr1+ cells in vivo, BALB/c skin grafts were transplanted into C57BL/6 mice. Seven days later, CD11b+ Gr1+ cells (CD8− cells; 1 × 106) from the spleens of PBS- and CsA-treated allograft recipient mice were sorted and intravenously (i.v.) injected into syngeneic C57BL/6 mice. One day after adoptive transfer, BALB/c skin was grafted onto these mice, and the skin graft was monitored daily.
For the suppression assay in vitro, CD11b+ Gr1+ cells sorted from the spleens of PBS- or CsA-treated allograft C57BL/6 recipients 7 days after allograft skin transplantation were added to a mixed lymphocyte reaction (MLR) system. The suppressive function of MDSCs was assessed by determining their ability to inhibit T cell activation as described previously (31). C57BL/6 CD4+ T cells (1 × 105 cells/well) were cocultured with 15 μg/ml mitomycin C-pretreated BALB/c splenocytes (1 × 105 cells/well), and CD11b+ Gr1+ cells (1 × 105 cells/well) were sorted at different ratios in a flat-bottom 96-well plate at 37°C in 5% CO2. Cell proliferation was determined 72 h after incubation with [3H]thymidine for the last 16 h of culture. 1-Methyl-tryptophan (1MT) (Sigma-Aldrich) (100 μM) was added at the beginning of the culture to block the IDO pathway.
Delayed-type hypersensitivity.
Approximately 2 weeks after the OTII or OTI mice were immunized with OVA (1 mg/ml), OTII CD4+ T cells, or OTI CD8+ T cells of the spleen and lymph nodes were enriched using the negative-selection magnetically activated cell sorting (MACS) kit for CD4+ T cells or CD8+ T cells (Miltenyi Biotec). CD11b+ Gr1+ cells (CD8− cells; 5 × 105 cells each) isolated from the spleens of C57BL/6 mice or PBS- or CsA-treated allograft recipient mice, were used as antigen-presenting cells (APCs) for OVA. Responder cells (OTII CD4+ T cells or OTI CD8+ T cells) and CD11b+ Gr1+ cells (5 × 105 cells each) with or without OVA were injected intradermally into the lateral part of the ears of naive CD45.1+ C57BL/6 mice. The changes in ear thickness were measured using an engineer's micrometer 48 h after challenge. The ear thickness changes were calculated by subtracting the thickness of the same ear before injection from that of the thickness after injection as described previously (32).
Cell proliferation and apoptosis assay.
To detect the proliferation of CD11b+ Gr1+ cells in vivo, 150 μl of a 10-mg/ml bromodeoxyuridine (BrdU) solution was injected into mice as described previously (30). Recipient splenic cells were isolated, and BrdU-positive (BrdU+) cells were visualized using the BrdU flow kit (BD Biosciences). Samples were also stained with CD11b and Gr1 to visualize MDSCs. Apoptosis of MDSCs in recipient mouse splenic cells was assayed by flow cytometry using annexin V (BD Pharmingen) staining with a gating strategy aligned to analyze CD11b+ Gr1+ cells as described previously (33). The FCM data were acquired on a FACSCalibur cell analyzer, and data were analyzed using FlowJo software.
Mitochondrial functional assay.
For mitochondrial studies, CD11b+ Gr1+ MDSCs were isolated from CsA- or PBS-treated allograft recipient mice and labeled with MitoTracker green (20 nM, 30 min at 37°C; Invitrogen) or labeled with JC1 (10 μg/ml, 20 min at 37°C; Invitrogen), as described before (34, 35). JC1 is a mitochondrial membrane potential-sensitive carbocyanine probe, monomeric green fluorescent JC1 is taken up into healthy higher membrane potential mitochondria, where it reversibly forms red-fluorescent aggregates, whereas with loss of mitochondrial membrane potential, JC1 remains in the cytoplasm or diffuses out of mitochondria and is green. The FCM data were acquired on a FACSCalibur cell analyzer, and data were analyzed with FlowJo software.
Statistical analyses.
All data are presented as means ± standard deviations (SDs). Student's unpaired t test for comparison of means was used to compare differences between groups. Comparison of the survival curves was performed using the log rank (Mantel-Cox) test. A P value (alpha value) of less than 0.05 was considered to be statistically significant.
RESULTS
Targeting calcineurin, CsA treatment prolongs allograft survival and increases CD11b+ Gr1+ cell local allograft infiltration.
We first investigated the effects of CsA on allograft skin survival. C57BL/6 recipient mice were treated daily with CsA (15 or 30 mg/kg body weight) or PBS (solvent) started 6 h before transplantation with allogeneic BALB/c skin graft. Compared with PBS-treated control groups, CsA treatment significantly prolonged allograft survival (Fig. 1A). Allograft photos consistently show that from days 5 to 10 after transplantation, the grafts on the PBS-treated control recipients showed progressive loss of hair, dermal necrosis, and scab formation, but the grafts on the CsA-treated recipients showed normal gross appearance and hair growth (Fig. 1B). In a histological examination of the skin allograft on day 10 after transplantation, CsA-treated allograft showed mild inflammatory immune cell infiltration (Fig. 1C). Finally, CsA-treated recipient mouse T cells displayed a lower autoimmune phenotype (lower TCR+ CD44high CD62Llow cells) (Fig. 1D). Allograft skin samples were then digested, and single-cell suspensions were prepared for analysis of the allograft-infiltrating inflammatory immune cell types. Compared to the PBS-treated controls, CsA-treated allografts showed significantly more CD11b+ Gr1+ cells but not CD11b+ F4/80+ macrophages, CD19+ B cells, or T (CD4+ T or CD8+ T) cell infiltrations (Fig. 1E). These data suggest that CsA-prolonged allograft skin survival is probably related to CD11b+ Gr1+ cells.
FIG 1.
CsA treatment prolonged allograft survival and promoted CD11b+ Gr1+ cell infiltration in allograft skin. Age-matched C57BL/6 mice were injected i.p. with PBS (solvent) or CsA (15 or 30 mg/kg body weight) daily starting 6 h before transplantation with allogeneic BALB/c skin. (A) CsA treatment significantly prolonged allograft skin graft (Allo) survival compared to that in the PBS-treated control. (B) Pictures of allograft skin grafts on days 5 to 10 after transplantation. (C) Pathological H&E staining photo of the allograft skin grafts on day 10 after transplantation. (D) The expression of CD44 and CD62L of CD4+ T cells and CD8+ T cells in blood was determined and CD44high CD62Llow cells were analyzed in the control or allograft recipient mice on day 10 after transplantation. A typical FCM figure is shown. The data are summarized in the graph to the right. (E) Infiltrating immune cell types were analyzed by FCM in allograft skin in the CsA-treated recipient mice, and the data were summarized. The data in panels B to E are representative of three independent experiments (n = 5). Values that are statistically significantly different are indicated by bars and asterisks as follows: **, P < 0.01; ***, P < 0.001. Values that are not statistically significantly different (n.s.) are also indicated.
CsA-induced CD11b+ Gr1+ cell recruitment prolonged allograft survival.
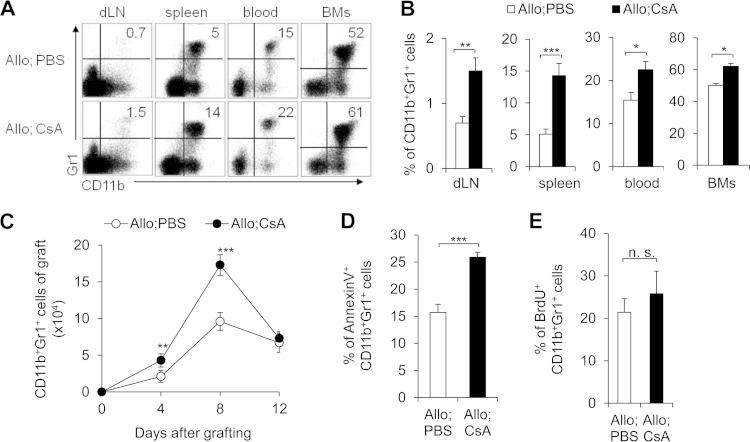
To fully investigate alterations in the absolute number of CD11b+ Gr1+ cells, we analyzed the inflammatory infiltrating cells in the recipient mouse draining lymph nodes (dLNs), spleen, blood, bone marrow cells (BMs), and allograft. There is consistently a significantly higher CD11b+ Gr1+ cell percentage from BMs to dLNs in the CsA-treated allograft recipients (Fig. 2A and B). Moreover, there is significantly more CD11b+ Gr1+ cell infiltration over time in the CsA-treated allograft skin than in the PBS-treated control (Fig. 2C). These data collectively indicate that increased CD11b+ Gr1+ cell infiltration is probably involved during the CsA-prolonged allograft survival.
FIG 2.
CsA treatment increases the number of CD11b+ Gr1+ cells in allograft recipients. PBS-treated control and CsA-treated allograft (Allo) recipient mice were studied. (A and B) At day 10 after allograft skin transplantation, representative FCM analysis of CD11b+ Gr1+ cells from the draining lymph nodes (dLN), spleen, blood, and bone marrow cells (BMs) is shown (A), and the data are summarized (B). (C) At the indicated time points, the absolute number of allograft skin local infiltrating CD11b+ Gr1+ cells was summarized. (D) Percentage of annexin V-positive cells in splenic CD11b+ Gr1+ cells isolated from the indicated mice. (E) BrdU incorporation in splenic CD11b+ Gr1+ cells from CsA-treated allograft recipient mice or PBS-treated controls were determined 48 h after injection of BrdU. The data in this figure are representative of four (A and B) and two (C to E) independent experiments (n = 3 to 5). Values that are statistically significantly different are indicated by bars and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Values that are not statistically significantly different (n.s.) are also indicated.
What can cause the increased number of CD11b+ Gr1+ cells in the CsA-treated recipients? It is probably caused by a decrease in cell death, an increase in differentiation from progenitors, or an enhanced recruitment into the allograft. Importantly, CsA treatment significantly promoted the cell death of graft CD11b+ Gr1+ cells (Fig. 2D). It is counterintuitive for CsA to both increase the number of CD11b+ Gr1+ cells from the BMs to inflammatory sites and also induce cell death. Furthermore, there are not significant differences in both the percentage (Fig. 2E) and absolute number of cells (data not shown) of BrdU+ CD11b+ Gr1+ cells between the CsA-treated group and PBS-treated control group. This suggests that CsA treatment does not alter the proliferation of CD11b+ Gr1+ cells. Thus, the change in survival of CsA-induced CD11b+ Gr1+ cells cannot contribute to the increased number of infiltrating CD11b+ Gr1+ cells. Meanwhile, this also suggests that increased cell death is probably a kind of physiological homeostasis regulatory mechanism for the recruited infiltrating CD11b+ Gr1+ cells in protecting against allograft survival.
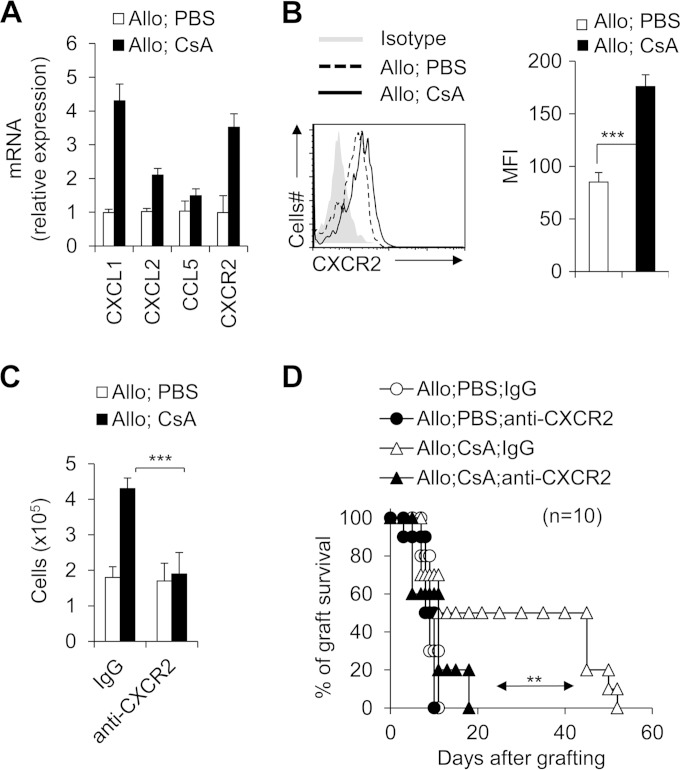
Does CsA treatment alter the CD11b+ Gr1+ cell migration in allograft recipient mice? The expression of chemoattractant chemokines and their receptors was investigated. CsA-treated CD11b+ Gr1+ cells showed increased expression of CXCL1 (chemokine [C-X-C motif] ligand 1) and CXCL2 but not CCL3 (chemokine [C-C motif] ligand 3) or CCL5 (Fig. 3A). These chemokines are critical for regulating CD11b+ Gr1+ cell migration (36–39). Furthermore, CsA treatment can significantly upregulate the expression of CXCR2 (chemokine [C-X-C motif] receptor 2), the receptor for chemokine CXCL1 and CXCL2 on the CD11b+ Gr1+ cells (Fig. 3B). Importantly, injection of an anti-CXCR2 antibody efficiently blocked the CD11b+ Gr1+ cell infiltration in the local allograft (Fig. 3C) and resulted in severe allograft rejection responses (Fig. 3D). These data collectively suggest that the CsA calcineurin inhibitor promoted the upregulation of CXCL1/2-CXCR2 and mediated the recruitment of CD11b+ Gr1+ cells in the allograft recipient mice.
FIG 3.
CXCR2 expression is required for CsA-mediated CD11b+ Gr1+ myeloid cell recruitment and allograft survival. PBS-treated control and CsA-treated allograft recipient mice were studied. (A and B) On day 10 after allograft skin transplantation, CXCL1, CXCL2, CCL3, and CXCR2 mRNA expression were analyzed by real-time PCR (A), and CXCR2 expression was determined by intracellular staining (B) in splenic CD11b+ Gr1+ cells isolated from the CsA-treated allograft recipient mice or PBS-treated control mice. The mean fluorescence intensity (MFI) of CXCR2 is summarized in the graph to the right. (C and D) Allograft recipient mice were administered 50 μg neutralizing antibody anti-CXCR2 MAb (catalog no. 242216; R&D Systems) or IgG isotype control (catalog no. 54447; R&D Systems) via i.v. injection 1 h before transplantation and PBS or CsA treatment. Mice were sacrificed on day 8 after allograft skin transplantation, the numbers of allograft local infiltrating CD11b+ Gr1+ cells were analyzed using flow cytometry (C), and graft survival was determined (D). Data (means plus standard deviations [SDs]) are representative of two (A to D) independent experiments (A and B, n = 3 to 5; C and D, n = 10). Values that are statistically significantly different are indicated by bars (or an arrow) and asterisks as follows: **, P < 0.01; ***, P < 0.001.
CD11b+ Gr1+ MDSCs are critical for CsA prolongation of allograft survival.
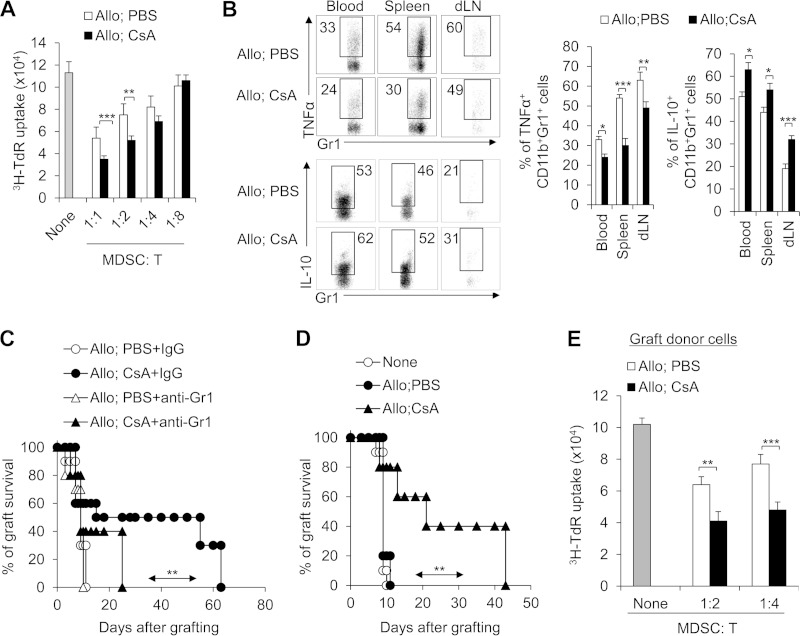
We then investigated whether recruited CD11b+ Gr1+ cells induced by CsA treatment have the feature of immune suppressors as reported previously for MDSCs (13, 14, 40). CD11b+ Gr1+ cells isolated from the CsA-treated allograft recipient spleen exhibited increased suppressive activities on the proliferation of T cells compared with PBS-treated control in vitro (Fig. 4A). Moreover, CsA-treated CD11b+ Gr1+ cells expressed lower tumor necrosis factor alpha (TNF-α) and higher IL-10 in the allograft recipient mice than the PBS-treated control (Fig. 4B). These findings suggest that CsA-induced CD11b+ Gr1+ cells had the characteristics of MDSCs. Furthermore, we investigated the relationship between these cells and CsA-prolonged allograft survival. We depleted CD11b+ Gr1+ MDSCs by injecting anti-Gr1 MAb as described previously (31). Strikingly, the depletion of Gr1+ cells significantly reduced CsA-prolonged allograft survival (Fig. 4C). However, adoptive transfer of sorted CD11b+ Gr1+ MDSCs (Gr1 also expressed on the subset of CD8+ T cells [41], so these cells were sorted with CD11b+ Gr1+ CD8− marker) from CsA-treated allograft recipient mice into C57BL/6 recipients significantly prolonged allograft survival (Fig. 4D). In addition, these donor CD11b+ Gr1+ cells isolated from grafts in the recipient mice also exhibited significantly enhanced suppressive activities than controls in vitro (Fig. 4E). Altogether these data consistently suggest that CD11b+ Gr1+ MDSCs are critical for calcineurin inhibitor CsA-prolonged allograft survival.
FIG 4.
CD11b+ Gr1+ MDSCs are required for CsA-prolonged allograft survival. (A) The suppressive activities of myeloid-derived CD11b+ Gr1+ cells were analyzed in vitro. The splenic CD11b+ Gr1+ cells treated with CsA in the allograft recipient mice showed increased suppressive activity. The ratio of MDSCs to T cells is shown below the graph. T cells were stimulated with allogeneic BALB/c splenocytes in the presence of splenic CD11b+ Gr1+ cells that were isolated from the allograft recipient mice. T cell proliferation was determined by [3H]thymidine (3H-TdR) incorporation. (B) TNF-α and IL-10 expression in splenic CD11b+ Gr1+ cells was analyzed by intracellular staining. A typical FCM figure is shown, and the data are summarized in the two graphs to the right of the FCM figure. (C) Depleting Gr1+ cells in CsA-treated allograft recipient mice with a MAb (RB6-8C5) resulted in severe allograft rejection responses in the recipient mice (n = 10). (D and E) Adoptive transfer of CD11b+ Gr1+ (CD8−) cells from CsA-treated allograft recipient mice significantly prolonged allograft survival (n = 10). The C57BL/6 recipient first underwent transplantation, and on days 7 and 8, a total of 1 × 106 CD11b+ Gr1+ cells were sorted from the spleens of CsA-treated or PBS-treated control mice and transferred into the C57BL/6 recipient mice via i.v. injection. After 10 to 12 h, both groups underwent allograft skin transplantation and the graft survival curve was plotted (n = 10) (D), and on days 7 and 8 after allograft skin transplantation, the local graft infiltrating donor CD45.1+ CD11b+ Gr1+ cells were isolated and their suppressive activities were analyzed in vitro (E). The data are representative of three (A and B) or two (C and D) independent experiments (for panels A, B, and E, n = 4 or 5). Values that are statistically significantly different are indicated by bars (or an arrow) and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Blocking the calcineurin-NFAT pathway in MDSCs prolonged allograft survival.
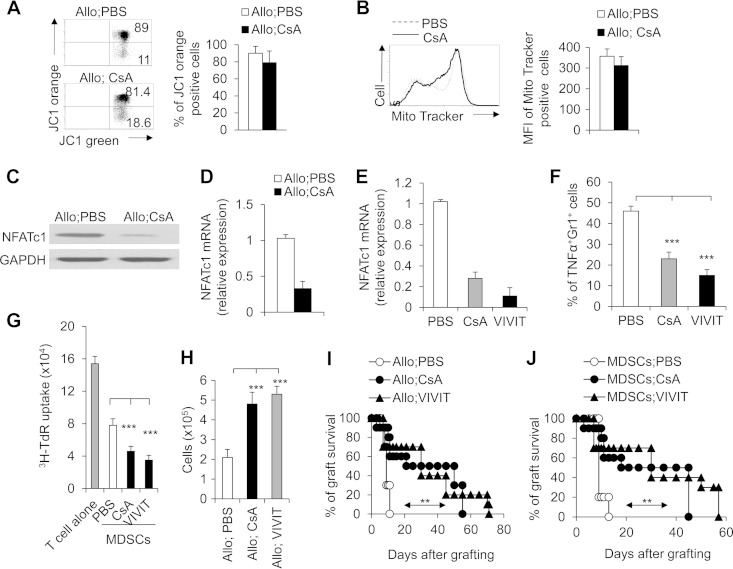
It is well-known that the calcineurin inhibitor CsA targets the mitochondrial permeability transition pores and takes effect on mitochondrial function (42, 43). We examined the mitochondrial functional changes of CD11b+ Gr1+ MDSCs in allograft recipients. CsA treatment cannot alter the mitochondrial membrane potential of MDSCs after allograft transplantation in mice (Fig. 5A and B). To ascertain how the calcineurin inhibitor CsA induces CD11b+ Gr1+ MDSCs and controls their activities in the allograft recipients, we examined the transcriptional effector mechanisms downstream of calcineurin in MDSCs. Because NFAT transcription factors are the best-characterized targets of calcineurin, we examined the expression of NFATc1, NFATc2, and NFATc3 in CD11b+ Gr1+ MDSCs. MDSCs expressed only NFATc1 and NFATc3. However, no detectable changes in NFATc3 expression were found in MDSCs in allograft recipient mice (data not shown). Substantial decreases at both the NFATc1 protein and mRNA levels in MDSCs were seen on day 10 after allograft transplantation in CsA-treated recipient mice (Fig. 5C and D). These results suggest that NFATc1 is probably involved in the CsA-induced MDSC effects. Blocking the NFAT signal with VIVIT (a NFAT inhibitor) efficiently downregulated NFAT expression in MDSCs in allograft recipient mice (Fig. 5E). Interestingly, VIVIT treatment also significantly reduced the production of TNF-α (Fig. 5F) and increased the suppressive activities of MDSCs (Fig. 5G), leading to the similar local levels of graft CD11b+ Gr1+ cell infiltration (Fig. 5H) and graft survival (Fig. 5I) compared to CsA-treated mice. Finally, adoptive transfer of sorted CD11b+ Gr1+ cells from VIVIT-treated allograft recipient mice but not the control group into C57BL/6 recipients significantly prolonged the allograft survival in a manner similar to CsA-induced MDSCs from allograft recipient mice (Fig. 5J). Taken together, these data suggest inhibition of calcineurin-NFAT signals potentiated both MDSC recruitment and functional activities in allograft recipients.
FIG 5.
Downregulation of NFATc1 is required for CsA-potentiated CD11b+ Gr1+ MDSC activities in prolonging allograft survival. PBS-treated control and CsA-treated allograft recipient mice were studied. (A and B) On day 10 after allograft skin transplantation, CD11b+ Gr1+ cells were isolated from CsA- or PBS-treated spleen in allograft recipient mice and incubated with JC1 or MitoTracker green after surface staining, and the JC1 (A) and MitoTracker green (B) expression on CD11b+ Gr1+ cells was analyzed by FCM. A representative FCM figure is shown to the left, and the data are summarized in the graph to the right. (C) NFATc1 expression in splenic CD11b+ Gr1+ cells was detected by immunoblotting. (D) The level of expression of NFATc1 mRNA was determined by real-time PCR (levels in PBS-treated control groups were set to 1), and the data are summarized. (E) The NFAT inhibitor VIVIT (10 mg/kg) (Calbiochem) efficiently diminished the expression of NFATc1 in the MDSCs isolated from the allograft recipient mice pretreated with PBS (control) or CsA. (F) VIVIT treatment significantly diminished the TNF-α expression of splenic CD11b+ Gr1+ MDSCs in allograft recipients. (G) VIVIT treatment (1 μM) (Calbiochem) enhanced the suppressive activities of splenic CD11b+ Gr1+ MDSCs in allograft recipients in vitro. (H and I) VIVIT treatment (10 mg/kg) significantly promoted local CD11b+ Gr1+ cell recruitment (H) and prolonged allograft survival (I) in the allograft recipient mice. (J) Adoptive transfer of CD11b+ Gr1+ cells from CsA- or VIVIT-treated allograft recipient mice significantly prolonged allograft survival (n = 10). The C57BL/6 recipient first underwent transplantation, and on days 7 and 8, a total of 1 × 106 CD11b+ Gr1+ (CD8−) cells were sorted from the spleens of CsA- or VIVIT-treated mice or PBS-treated control mice and transferred into the C57BL/6 recipient mice via i.v. injection. After 10 to 12 h, both groups underwent allograft skin transplantation, and the graft survival curve was plotted (n = 10). The data in this figure are representative of two (A and B and H to J) or three (C to G) independent experiments (n = 3 to 5 for panels A to G). Values that are statistically significantly different are indicated by bars (or an arrow) and asterisks as follows: **, P < 0.01; ***, P < 0.001. Values that are not statistically significantly different (n.s.) are also indicated.
CsA modulates T cell differentiation during allograft transplantation.
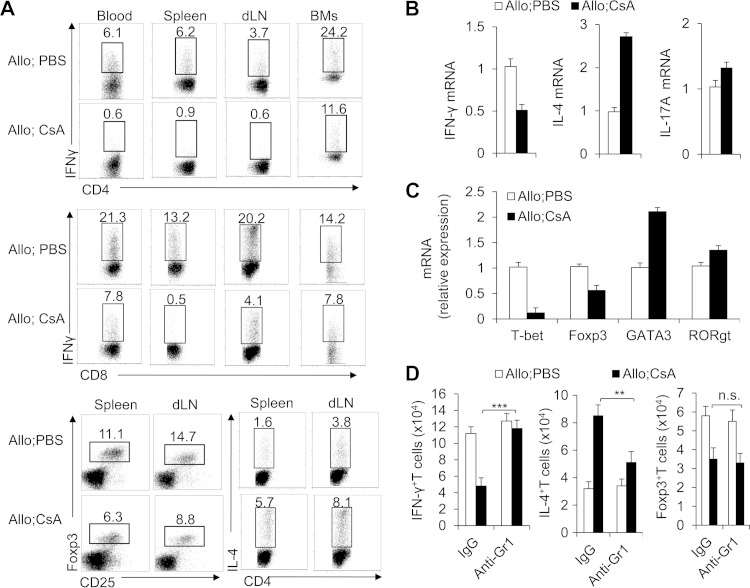
Furthermore, T cell functional alterations were investigated in the CsA-treated allograft recipient mice. CsA treatment significantly diminished the expression of IFN-γ of CD8+ T cells in blood, spleen, dLNs, and BMs (Fig. 6A). In CD4+ T cells, CsA treatment resulted in a reciprocal decrease in IFN-γ-producing TH1 T helper cells and increase in IL-4-producing TH2 cells but a significant decrease in Foxp3+ regulatory T cells (Treg cells) in the dLNs and spleens of allograft recipients (Fig. 6A). The CD4+ T cell mRNA level of IFN-γ (TH1 cells) and IL-4 (TH2 cells) consistently decreased and increased, but that of IL-17A (TH17 cells) did not, in the CsA-treated allograft recipient mice (Fig. 6B). In addition, specific T cell lineage transcriptional factor expression (T-bet for TH1, Foxp3 for Treg, GATA3 for TH2 and RORγt for TH17) exhibited consistent changes (Fig. 6C). These data collectively suggest that T cell differentiation is critical for CsA-prolonged allograft survival. However, depleting Gr1+ cells in the CsA-treated but not the PBS-treated control group clearly restored the IFN-γ level in T cells (CD4+ and CD8+ T cells) and the IL-4 level in TH2 cells but not Treg cells in the dLNs of the allograft recipient mice (Fig. 6D). Thus, these findings collectively indicate that MDSCs are critical for modulation of the reciprocal T cell differentiation in the CsA-prolonged allograft survival.
FIG 6.
CsA induced T cell differentiation dependent on CD11b+ Gr1+ MDSCs in allograft recipients. (A and B) On day 10, IFN-γ expression in the CD4+ T cells and CD8+ T cells in blood, spleen, dLN, or BMs and the IL-4 or Foxp3 expression in the CD4+ and CD8+ T cells in the dLN or spleen in the allograft recipient mice were detected by FCM, and representative results are shown. At the same time points, mRNA expression of IFN-γ, IL-4, and IL-17A of T cells isolated from the allograft was determined by real-time PCR (A) and the data are summarized (B). (C) The mRNA levels of T-bet, Foxp3, GATA3, and RORγt transcription factors in T cells isolated from the allograft were determined by real-time PCR, and data are summarized. (D) Absolute numbers of IFN-γ+ CD3+ T cells, IL-4+ CD3+ T cells, and Foxp3+ CD3+ T cells in dLN from indicated allograft recipient mice were determined by flow cytometry. Data (means plus SDs) in this figure are representative of three or four (A to C) or two (D) independent experiments (n = 3 to 5). Values that are statistically significantly different are indicated by bars and asterisks as follows: **, P < 0.01; ***, P < 0.001 compared with the indicated groups.
CsA-induced MDSCs promote reciprocal differentiation of T cells.
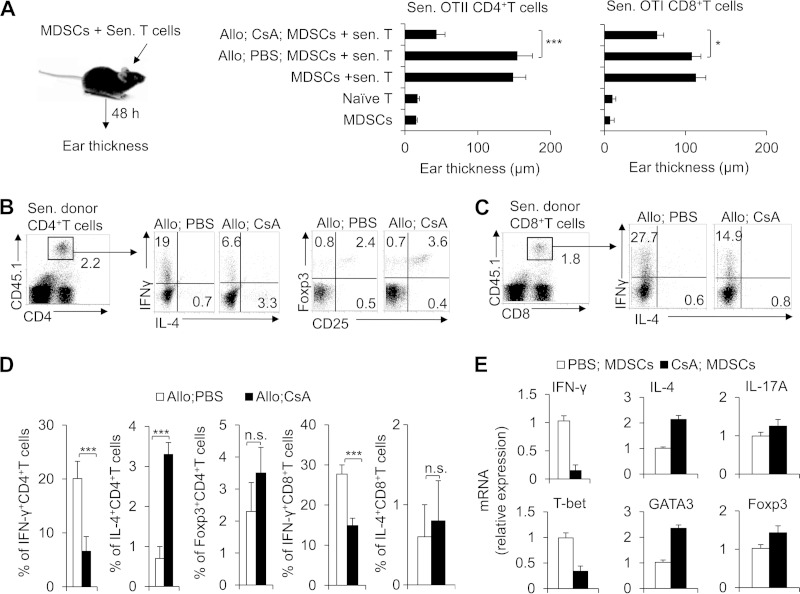
To investigate the relationship between MDSCs and T cell differentiation in the CsA-prolonged allograft recipients, we directly examined the effects of MDSCs on regulating the reciprocal T cell differentiation with delayed-type hypersensitivity (DTH) as described previously (32). The OTII or OTI mice were immunized in advance with the OVA peptide for 2 weeks, and CD4+ or CD8+ T cells as the responder cells were isolated from OTII or OTI mice, respectively. The CD11b+ Gr1+ MDSCs as the APCs were also isolated from CsA-treated allograft recipient mice or PBS-treated control mice, respectively. Responder cells (CD4+ T cells or CD8+ T cells) and APCs (CD11b+ Gr1+ MDSCs; 5 × 105 cells each) in the presence of antigen were injected intradermally into the lateral part of the ears of naive C57BL/6 mice, and the change in ear thickness was measured at 48 h after injection. As expected, groups with CsA-treated MDSCs and sensitized T cells cotransferred exhibited a diminished DTH response (Fig. 7A). Importantly, the donor CD4+ T cells of the dLNs were isolated and exhibited higher IL-4 and lower IFN-γ but not Foxp3 expression in the groups with CsA-treated MDSCs and sensitized T cells cotransferred (Fig. 7B and D). Similarly, the donor CD8+ T cells of the dLNs were isolated and exhibited lower IFN-γ but not IL-4 expression in the groups with CsA-treated MDSCs and sensitized T cells cotransferred (Fig. 7C and D). In addition, the donor CD4+ T cell mRNA expression of IL-4, IFN-γ, and IL-17A and the transcriptional factor expression of T-bet, GATA3, and Foxp3 were consistent (Fig. 7E). These data collectively suggest that CsA-induced MDSCs directly reciprocally regulate the differentiation of TH1 and TH2 in CD4+ T cells or inhibit the IFN-γ production in CD8+ T cells in prolonging allograft survival.
FIG 7.
CsA-induced CD11b+ Gr1+ MDSCs direct the differentiation of T cells. (A) Delayed-type hypersensitivity (DTH) responses were examined. Approximately 2 weeks after OTII or OTI mice had been immunized with OVA (1 mg/ml), OTII CD4+ T cells or OTI CD8+ T cells in the spleen and lymph nodes were shown to be enriched using the negative selecting MACS kit for CD4+ T cells or CD8+ T cells. CD11b+ Gr1+ cells (5 × 105 cells each) isolated from the spleens of C57BL/6 mice or from the PBS- or CsA-treated allograft recipient mice were used as APCs for OVA. Responder cells (OTII CD4+ T cells or OTI CD8+ T cells) and CD11b+ Gr1+ cells (5 × 105 cells each) with OVA were injected intradermally into the lateral part of the ears of naive CD45.1+ C57BL/6 mice. The changes in ear thickness were measured using an engineer's micrometer 48 h after the challenge. The ear thickness changes were calculated by subtracting the thickness of the same ear before injection from that after injection. Sen. T cells, sensitized T cells. (B to D) The dLN lymphocytes were isolated, and IFN-γ and IL-4 or Foxp3 expression in the donor CD4+ T cells (B) or CD8+ T cells (C) was analyzed with intracellular staining, a representative figure is shown, and the data are summarized (D). (E) The donor CD4+ T cell mRNA of IFN-γ, IL-4, IL-17A, T-bet, GATA3, and Foxp3 were analyzed by real-time PCR. The data in this figure are representative of three independent experiments (n = 3 to 6). Values that are statistically significantly different are indicated by bars and asterisks as follows: *, P < 0.05; ***, P < 0.001. Values that are not statistically significantly different (n.s.) are also indicated.
CsA-induced MDSC IDO expression suppresses T cell activation and directs T cell differentiation.
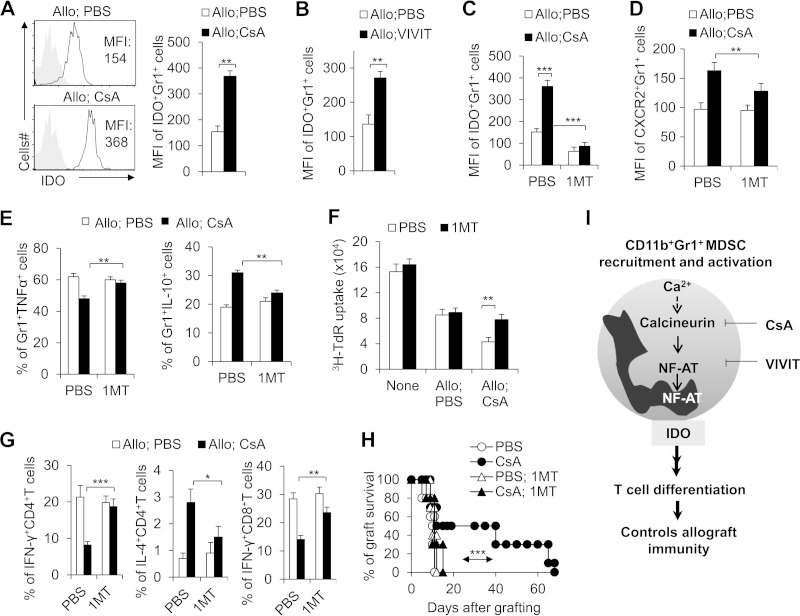
IDO has been known as a critical component of the immunosuppressive activity of MDSCs. We thus measured IDO expression in MDSCs in the allograft recipient mice. In agreement with the above findings, the expression of IDO in MDSCs was significantly higher in the CsA-treated allograft recipient mice than in the PBS-treated control mice (Fig. 8A). NFAT inhibitor VIVIT treatment consistently upregulated IDO expression of MDSCs (Fig. 8B). To ascertain whether IDO expression is a critical component in MDSC prolongation of allograft survival, we applied 1MT, an inhibitor of IDO, to the functional in vivo and in vitro assays. 1MT significantly reduced the expression of IDO (Fig. 8C) and CXCR2 (Fig. 8D) in MDSCs, recovered the decreased TNF-α and IL-10 secretion (Fig. 8E), and efficiently reduced the immunosuppressive activities in MDSCs isolated from CsA-treated allograft recipient mice (Fig. 8F). 1MT can also consistently significantly reverse the T cell differentiation induced by MDSCs isolated from CsA-treated allograft recipient mice along with T cells in DTH (Fig. 8G). Finally, the in vivo role of IDO was investigated in an adoptive transfer model using C57BL/6 recipients pretreated with an anti-Gr1 antibody to deplete Gr1+ cells. CD11b+ Gr1+ MDSCs were isolated from PBS- or CsA-treated allograft recipient mice or cotreated with either PBS or CsA and 1MT and then adoptively transferred into allograft recipients. 1MT cotreatment significantly reversed the effect of CsA on local infiltrating CD11b+ Gr1+ cell number (data not shown) and allograft survival (Fig. 8H). Thus, MDSC IDO is critical for CsA-prolonged allograft survival.
FIG 8.
IDO is required for the functional activities of CsA-induced CD11b+ Gr1+ MDSCs in prolonging allograft survival. (A) Significantly higher IDO expression in splenic CD11b+ Gr1+ cells from CsA-treated allograft recipient mice compared with PBS-treated control mice. MFI, mean fluorescence intensity. (B) Blocking NFAT activity significantly increased the IDO MFI expression in CD11b+ Gr1+ MDSCs. (C to F) Blocking IDO expression with 1MT (400 mg/kg, gavage daily) significantly diminished the MFI expression of IDO (C) and CXCR2 (D) and recovered the production of TNF-α and IL-10 (E) in CD11b+ Gr1+ MDSCs from CsA-treated allograft recipient mice compared with PBS-treated control group and diminished the suppressive activity of CsA-treated CD11b+ Gr1+ MDSCs in vitro (F). (G) DTH responses were examined. Approximately 2 weeks after OTII or OTI mice had been immunized with OVA (1 mg/ml), OTII CD4+ T cells or OTI CD8+ T cells in the spleen and lymph nodes were shown to be enriched using the negative selecting MACS kit for CD4+T cells or CD8+ T cells. CD11b+ Gr1+ cells (CD8− cells; 5 × 105 cells each) isolated from the spleens of C57BL/6 mice from the indicated treatment allograft recipient mice were used as APCs for OVA. Responder cells (OTII CD4+ T cells or OTI CD8+ T cells) and CD11b+ Gr1+ cells (5 × 105 cells each) with OVA were injected intradermally into the lateral part of the ears of naive CD45.1+ C57BL/6 mice. At 48 h after the challenge, the dLN lymphocytes were isolated, and IFN-γ and/or IL-4 expression in the donor (CD45.2+) CD4+ T cells or CD8+ T cells was analyzed by intracellular staining, and the data were summarized. (H) The indicated allograft recipient mice were treated with 1MT (400 mg/kg, gavage daily) or without IMT. The mice were sacrificed 8 days later, and 1 × 106 CD11b+ Gr1+ cells were sorted from the spleen and transferred into the same C57BL/6 recipient mice pretreated with an anti-Gr1 MAb (0.5 mg) 1 day earlier. Allograft recipient mice underwent skin transplantation 12 h after cell transfer, and the graft survival curve was plotted (n = 10). (I) Model summarizing the role of calcineurin-NFAT axis in controlling allograft immunity in CD11b+ Gr1+ MDSCs. Blocking the calcineurin-NFAT axis with CsA and VIVIT promotes CD11b+ Gr1+ MDSC activity, recruitment, and IDO expression. IDO is critical for the MDSC suppressive activities and directing reciprocal differentiation in CD4+ T cells between TH1 and TH2 or differentiation of CD8+ T cells in prolonging allograft survival. The data in this figure are representative of three (A, F, and G) or two (B to E and H) independent experiments (for panels A to G, n = 3 to 5). Values that are statistically significantly different are indicated by bars (or an arrow) and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Calcineurin inhibitors, such as CsA, are potent immunosuppressants used clinically for the treatment of ranges of immune-mediated diseases, including the rejection of solid-organ allograft or autoimmune diseases. The impressive characteristic of CsA in the immune-mediated diseases is its immunoinhibitory effects on T cell function, but the innate immune mechanism remains unclear. Here, we show that MDSCs are essential for CsA to prolong allograft survival and ameliorate the allograft immune response. Mechanistically, targeting the calcineurin signaling pathway, CsA promotes MDSC recruitment and functional activities as well as promoting the reciprocal differentiation of TH2 and TH1 and diminishing the production of IFN-γ of CD8+ T cells in alleviating allograft immunity (Fig. 8I). This is consistent with the previously established role of MDSCs in limiting immune rejection inflammatory injury in the course of clinical transplantation (44). However, this study reveals a previously unknown functional role for MDSCs in reprogramming T cell differentiation from TH1 to TH2 or CD8+ T cell differentiation by targeting the calcineurin-NFAT-IDO signaling pathway in modulating allograft immunity.
Calcineurin inhibitors have been widely used in treating inflammatory disorders, including amelioration of the transplant rejection responses and autoimmune diseases. However, the mechanisms by which CsA could alter the innate immune system and thereby decrease allograft immune inflammatory injuries remain largely unknown. Calcineurin signaling was shown to negatively regulate myeloid lineage development (45, 46). The susceptibility to fungal infection caused by CsA was also shown to be the consequence of an innate immune pathway regulating antifungal resistance in neutrophils (47). CsA can also impair nucleotide binding oligomerization domain 1 (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients, and these results confirmed that inhibition of Nod1-mediated innate immune response together with the decrease in Toll-like receptor 4 (TLR4)-mediated production of chemoattractant chemokines caused by CsA may contribute to sensitizing kidney grafts to acute pyelonephritis (48). However, the immune regulatory effect and important role in transplant immunology of calcineurin inhibitors is highly complex. In the present study, typical immunological mouse allograft models were used to investigate the effect of the calcineurin signaling pathway in modulating transplant rejection. We showed that MDSCs are essential for CsA to prolong allograft survival and alleviate the allograft immune rejection. CsA, a classic calcineurin inhibitor, directly modulated MDSC IDO expression, which consequently directs the reciprocal differentiation of CD4+ T cell lineages and CD8+ T cell differentiation in the allograft immune inflammatory injuries. Meanwhile, we found that the effect of depleting MDSCs with Gr1 antibody treatment on skin allograft survival seems to be only partial (Fig. 4C). These data are consistent with previous studies (1, 3), which showed that CsA can efficiently inhibit T cell function in preventing allograft rejection, and also suggest that graft tolerance can be achieved through direct T cell-dependent mechanisms without Gr1+ MDSCs.
Calcium-responsive members of the NFAT family are retained in an inactive state in the cytosol. Upon intracellular calcium influx, calmodulin displaces an autoinhibitory loop from the active site of the phosphatase calcineurin. Calcineurin then removes the inhibitory phosphates, allowing NFATs to translocate to the nucleus and take effect (3, 49, 50). Although NFATs have been extensively studied in the context of T cells, relatively few studies have examined their function in myeloid lineages. However, several recent studies about calcineurin signaling in innate immune effects have showed their NFAT-dependent regulatory effects (45–48). In the present study, we demonstrate the intrinsic regulatory effects of MDSCs in prolonging allograft survival and show a previously unknown feature of MDSC function in immunohomeostasis, i.e., the reprogramming of T cell differentiation from TH1 to TH2 and CD8+ T cell differentiation, which represents a novel mechanism of calcineurin amelioration of transplant immunological rejection by targeting the NFAT signaling pathway.
Regulation of tryptophan metabolism by IDO in adaptive and innate immune cells is a highly versatile modulator of immunity. IDO can promote the differentiation and activation of Treg cells, resulting in fewer tumor-targeting T cells, and promote immune tolerance (51, 52). Blocking IDO thus should result in the production of more tumor-targeting T cells. In inflammation, IFN-γ is the main inducer of IDO for the prevention of hyperinflammatory responses, yet IDO is also responsible for self-tolerance effects in the longer term. These results confirmed that IDO was responsible for the self-amplification and maintenance of a stably regulatory phenotype in plasmacytoid dendritic cells (53). Therefore, IDO has a nonenzymic function that contributes to immune tolerance in inflammatory or noninflammatory contexts.
IDO enzyme inhibitors have entered clinical trials for cancer treatment based on preclinical studies, indicating that they can defeat immune escape and broadly enhance other therapeutic modalities. IDO deficiency resulted in reduced lung tumor burden and improved survival. During lung tumor and metastasis outgrowth, inflammatory cytokines were greatly attenuated in conjunction with the loss of IDO. Biologically, this resulted in a consequential impairment of protumorigenic MDSCs, as restoration of inflammatory cytokines recovered both MDSC suppressive activities and metastasis susceptibility in IDO1-nullizygous mice (54). These results indicate that IDO is a prototypical integrative modifier that bridges inflammation and immune escape to license primary and metastatic tumor outgrowth. Actually, IDO in MDSCs can directly regulate tumor-related immunity (55, 56). MDSCs suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer and confirmed that activation of the noncanonical NF-κB pathway mediated IDO gene transcription (57, 58). 1MT is a well-studied IDO inhibitor that acts as a competitive inhibitor of l-tryptophan binding to and metabolization by IDO, and a phase III clinical trial of IDO has been initiated (59, 60). However, the IDO mechanism of MDSCs in allograft immunity remains unclear. In the present study, we showed that MDSC IDO is critical for CsA-induced allograft immunity. CsA can increase MDSC IDO expression, and blocking IDO can significantly reverse MDSC inflammatory cytokine secretion, CXCR2 expression, and its suppressive activities on T cell proliferation, which consequently results in the reprogramming of T cell differentiation and alteration of allograft inflammatory injuries.
In summary, calcineurin inhibitors (such as CsA), one of the important immunosuppressants for clinical transplant patients, play a great role in treating transplant rejection by targeting NFAT signaling. Our studies suggest that MDSCs contribute to the modulation of allograft immunity of calcineurin signals. The calcineurin-NFAT-IDO pathway in MDSCs is promising for the development of new treatment strategies for transplantation.
ACKNOWLEDGMENTS
Our research is supported by grants from the National Natural Science Foundation for General Programs of China (31171407 and 81273201 [G.L.]; 81271907 [Y.B.]), National Natural Science Foundation for Young Programs of China (81401740 [H.Y.]), Key Basic Research Project of the Science and Technology Commission of Shanghai Municipality (12JC1400900 [G.L.]), Innovation Program of Shanghai Municipal Education Commission (14Z Z009 [G.L.]), Shanghai City Health Committee of planning of key projects (C704688 [G.L.]), and Excellent Youth Foundation of the Chinese Academy of Sciences (KSCX2-EW-Q-7 [G.L.]).
X.W. and Y.B. designed and conducted the experiments with cells and mice and analyzed data. L.X. and H.Y. conducted the experiments with cells and contributed to the writing of the manuscript. J.L. conducted the mouse allograft skin transplantation and analyzed data. X.C., Y.L., Z.Z., J.W., and H.L. participated in discussions. G.L. developed the concept, designed and conducted the experiments with cells and mice, analyzed data, wrote the manuscript, and provided overall direction.
We declare that we have no competing financial interests.
REFERENCES
- 1.Clipstone NA, Crabtree GR. 1992. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 2.Kelly P, Kahan BD. 2002. Review: metabolism of immunosuppressant drugs. Curr Drug Metab 3:275–287. doi: 10.2174/1389200023337630. [DOI] [PubMed] [Google Scholar]
- 3.Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, Hofbauer GF, Dotto GP. 2010. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan PG, Chen L, Nardone J, Rao A. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 5.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. 1999. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 6.Serfling E, Klein-Hessling S, Palmetshofer A, Bopp T, Stassen M, Schmitt E. 2006. NFAT transcription factors in control of peripheral T cell tolerance. Eur J Immunol 36:2837–2843. doi: 10.1002/eji.200536618. [DOI] [PubMed] [Google Scholar]
- 7.Rao A, Luo C, Hogan PG. 1997. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 8.Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A. 2006. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 9.Hogan PG, Rao A. 1999. Transcriptional regulation. Modification by nuclear export? Nature 398:200–201. [DOI] [PubMed] [Google Scholar]
- 10.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. 2000. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell 6:539–550. doi: 10.1016/S1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 11.Roehrl MH, Kang S, Aramburu J, Wagner G, Rao A, Hogan PG. 2004. Selective inhibition of calcineurin-NFAT signaling by blocking protein-protein interaction with small organic molecules. Proc Natl Acad Sci U S A 101:7554–7559. doi: 10.1073/pnas.0401835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. 2006. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. 2007. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrand-Rosenberg S, Sinha P. 2009. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray P, Arora M, Poe SL, Ray A. 2011. Lung myeloid-derived suppressor cells and regulation of inflammation. Immunol Res 50:153–158. doi: 10.1007/s12026-011-8230-1. [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, Cannan D, Ramacher M, Kato M, Overwijk WW, Chen SH, Umansky VY, Sikora AG. 2012. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol 188:5365–5376. doi: 10.4049/jimmunol.1103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Rompaey N, Le Moine A. 2011. Myeloid-derived suppressor cells: characterization and expansion in models of endotoxemia and transplantation. Methods Mol Biol 677:169–180. doi: 10.1007/978-1-60761-869-0_12. [DOI] [PubMed] [Google Scholar]
- 18.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B. 2008. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 180:7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 19.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, Grisotto M, van Rooijen N, Matesanz R, Tacke F, Ginhoux F, Ding Y, Chen SH, Randolph G, Merad M, Bromberg JS, Ochando JC. 2010. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest 120:2486–2496. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satake A, Schmidt AM, Nomura S, Kambayashi T. 2014. Inhibition of calcineurin abrogates while inhibition of mTOR promotes regulatory T cell expansion and graft-versus-host disease protection by IL-2 in allogeneic bone marrow transplantation. PLoS One 9:e92888. doi: 10.1371/journal.pone.0092888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corry RJ, Winn HJ, Russell PS. 1973. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16:343–350. [DOI] [PubMed] [Google Scholar]
- 22.Mayumi H, Nomoto K, Good RA. 1988. A surgical technique for experimental free skin grafting in mice. Jpn J Surg 18:548–557. doi: 10.1007/BF02471489. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg AS, Mizuochi T, Singer A. 1988. Evidence for involvement of dual-function T cells in rejection of MHC class I disparate skin grafts. Assessment of MHC class I alloantigens as in vivo helper determinants. J Exp Med 168:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg AS, Munitz TI, Maniero TG, Singer A. 1991. Cellular basis of skin allograft rejection across a class I major histocompatibility barrier in mice depleted of CD8+ T cells in vivo. J Exp Med 173:1463–1471. doi: 10.1084/jem.173.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siemionow M, Demir Y, Mukherjee A, Klimczak A. 2005. Development and maintenance of donor-specific chimerism in semi-allogenic and fully major histocompatibility complex mismatched facial allograft transplants. Transplantation 79:558–567. doi: 10.1097/01.TP.0000152799.16035.B7. [DOI] [PubMed] [Google Scholar]
- 26.Sun B, Hu X, Liu G, Ma B, Xu Y, Yang T, Shi J, Yang F, Li H, Zhang L, Zhao Y. 2014. Phosphatase wip1 negatively regulates neutrophil migration and inflammation. J Immunol 192:1184–1195. doi: 10.4049/jimmunol.1300656. [DOI] [PubMed] [Google Scholar]
- 27.Cripps JG, Wang J, Maria A, Blumenthal I, Gorham JD. 2010. Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology 52:1350–1359. doi: 10.1002/hep.23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. 2003. Silibinin protects mice from T cell-dependent liver injury. J Hepatol 39:333–340. doi: 10.1016/S0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 29.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. 2009. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol 10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Hu X, Sun B, Yang T, Shi J, Zhang L, Zhao Y. 2013. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood 121:519–529. doi: 10.1182/blood-2012-05-432674. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Sun C, Chen Z, Zhen Y, Peng J, Qi Z, Yang X, Zhao Y. 2012. Smad3-deficient CD11b(+)Gr1(+) myeloid-derived suppressor cells prevent allograft rejection via the nitric oxide pathway. J Immunol 189:4989–5000. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. 2011. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol 89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Bi Y, Wang R, Shen B, Zhang Y, Yang H, Wang X, Liu H, Lu Y, Han F. 2013. Kinase AKT1 negatively controls neutrophil recruitment and function in mice. J Immunol 191:2680–2690. doi: 10.4049/jimmunol.1300736. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Huang G, Zeng H, Yang K, Lamb RF, Chi H. 2013. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci U S A 110:E4894–E4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Q, Bao W, Ge Y, Rikihisa Y. 2008. Ehrlichia ewingii infection delays spontaneous neutrophil apoptosis through stabilization of mitochondria. J Infect Dis 197:1110–1118. doi: 10.1086/533457. [DOI] [PubMed] [Google Scholar]
- 36.Chen HW, Chen HY, Wang LT, Wang FH, Fang LW, Lai HY, Chen HH, Lu J, Hung MS, Cheng Y, Chen MY, Liu SJ, Chong P, Lee OK, Hsu SC. 2013. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol 190:5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 37.Dilek N, Poirier N, Usal C, Martinet B, Blancho G, Vanhove B. 2012. Control of transplant tolerance and intragraft regulatory T cell localization by myeloid-derived suppressor cells and CCL5. J Immunol 188:4209–4216. doi: 10.4049/jimmunol.1101512. [DOI] [PubMed] [Google Scholar]
- 38.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, Blander JM, Tacke F, Trautwein C. 2010. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 207:1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. 2012. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol 189:5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 40.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. 2012. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol 189:5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuzaki J, Tsuji T, Chamoto K, Takeshima T, Sendo F, Nishimura T. 2003. Successful elimination of memory-type CD8+ T cell subsets by the administration of anti-Gr-1 monoclonal antibody in vivo. Cell Immunol 224:98–105. doi: 10.1016/j.cellimm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Roy MK, Takenaka M, Kobori M, Nakahara K, Isobe S, Tsushida T. 2006. Apoptosis, necrosis and cell proliferation-inhibition by cyclosporine A in U937 cells (a human monocytic cell line). Pharmacol Res 53:293–302. doi: 10.1016/j.phrs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Sovcikova A, Tulinska J, Kubova J, Liskova A, Syrova D, Horakova K. 2002. Effect of cyclosporin A in Lewis rats in vivo and HeLa cells in vitro. J Appl Toxicol 22:153–160. doi: 10.1002/jat.842. [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Akbar SM, Abe M, Hiasa Y, Onji M. 2011. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 166:134–142. doi: 10.1111/j.1365-2249.2011.04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fric J, Lim CX, Koh EG, Hofmann B, Chen J, Tay HS, Mohammad Isa SA, Mortellaro A, Ruedl C, Ricciardi-Castagnoli P. 2012. Calcineurin/NFAT signalling inhibits myeloid haematopoiesis. EMBO Mol Med 4:269–282. doi: 10.1002/emmm.201100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallo EM, Ho L, Winslow MM, Staton TL, Crabtree GR. 2008. Selective role of calcineurin in haematopoiesis and lymphopoiesis. EMBO Rep 9:1141–1148. doi: 10.1038/embor.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. 2010. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med 207:923–931. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tourneur E, Ben Mkaddem S, Chassin C, Bens M, Goujon JM, Charles N, Pellefigues C, Aloulou M, Hertig A, Monteiro RC, Girardin SE, Philpott DJ, Rondeau E, Elbim C, Werts C, Vandewalle A. 2013. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog 9:e1003152. doi: 10.1371/journal.ppat.1003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolfe SA, Zhou P, Dotsch V, Chen L, You A, Ho SN, Crabtree GR, Wagner G, Verdine GL. 1997. Unusual Rel-like architecture in the DNA-binding domain of the transcription factor NFATc. Nature 385:172–176. doi: 10.1038/385172a0. [DOI] [PubMed] [Google Scholar]
- 50.Yeo H, Beck LH, McDonald JM, Zayzafoon M. 2007. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone 40:1502–1516. doi: 10.1016/j.bone.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. 2006. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother 55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tawara I, Shlomchik WD, Jones A, Zou W, Nieves E, Liu C, Toubai T, Duran-Struuck R, Sun Y, Clouthier SG, Evers R, Lowler KP, Levy RB, Reddy P. 2010. A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease. J Immunol 185:3866–3872. doi: 10.4049/jimmunol.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. 2011. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 54.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ. 2012. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alizadeh D, Larmonier N. 2014. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res 74:2663–2668. doi: 10.1158/0008-5472.CAN-14-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, Mackall CL. 2013. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 122:1105–1113. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mougiakakos D, Jitschin R, von Bahr L, Poschke I, Gary R, Sundberg B, Gerbitz A, Ljungman P, Le Blanc K. 2013. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia 27:377–388. doi: 10.1038/leu.2012.215. [DOI] [PubMed] [Google Scholar]
- 58.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. 2013. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 59.Katz JB, Muller AJ, Prendergast GC. 2008. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 60.Vaccari M, Boasso A, Fenizia C, Fuchs D, Hryniewicz A, Morgan T, Weiss D, Doster MN, Heraud JM, Shearer GM, Franchini G. 2012. Fatal pancreatitis in simian immunodeficiency virus SIVmac251-infected macaques treated with 2′,3′-dideoxyinosine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol 86:108–113. doi: 10.1128/JVI.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]