Abstract
The spatial organization of eukaryotic genomes is linked to their functions. However, how individual features of the global spatial structure contribute to nuclear function remains largely unknown. We previously identified a high-frequency interchromosomal interaction within the Saccharomyces cerevisiae genome that occurs between the intergenic spacer of the ribosomal DNA (rDNA) repeats and the intergenic sequence between the locus encoding the second largest RNA polymerase I subunit and a lysine tRNA gene [i.e., RPA135-tK(CUU)P]. Here, we used quantitative chromosome conformation capture in combination with replacement mapping to identify a 75-bp sequence within the RPA135-tK(CUU)P intergenic region that is involved in the interaction. We demonstrate that the RPA135-IGS1 interaction is dependent on the rDNA copy number and the Msn2 protein. Surprisingly, we found that the interaction does not govern RPA135 transcription. Instead, replacement of a 605-bp region within the RPA135-tK(CUU)P intergenic region results in a reduction in the RPA135-IGS1 interaction level and fluctuations in rDNA copy number. We conclude that the chromosomal interaction that occurs between the RPA135-tK(CUU)P and rDNA IGS1 loci stabilizes rDNA repeat number and contributes to the maintenance of nucleolar stability. Our results provide evidence that the DNA loci involved in chromosomal interactions are composite elements, sections of which function in stabilizing the interaction or mediating a functional outcome.
INTRODUCTION
There are strong links between the spatial organization of a genome and its function (reviewed in references 1 to 3). This spatial organization is dynamic and specific for particular cell types and stages of the cell cycle (4–6). Genome spatial organization has been proposed to result from the physical properties of the chromosomes (e.g., volume and flexibility), the proteins (e.g., DNA binding and aggregation), and the nucleic acids (e.g., ability to form triplexes) that are present within the limited volume of the nucleus (7–10). While global genome structure contributes to cell identity (5) and differentiation and cycling (6), the contributions that individual contacts (i.e., interactions) between chromosomal loci make to genome function remain poorly understood. Here, we characterize a high-frequency interaction between the yeast rRNA genes (rDNA) and a locus encoding an RNA polymerase I subunit, RPA135 (Fig. 1A).
FIG 1.
RPA135-IGS1 interaction is sequence specific. (A) Cartoon illustrating the interaction between the RPA135-tK(CUU)P intergenic region and IGS1 of the rDNA in Saccharomyces cerevisiae (24). The RPA135-IGS1 interaction was originally identified as occurring with the IGS1 locus (Chr XII; SGD coordinates 460025 to 460609) (24). However, here we used HindIII to standardize the detection of the RPA135-IGS1 interaction in the replacement strains, which means the captured rDNA fragment encompasses the IGS2 and 5S regions. However, we continue to refer to the interaction as occurring between the RPA135-tK intergenic region and IGS1 loci based on the previously published information (24). (B) Schematic illustration of the homologous recombination-based replacement mapping within the RPA135-tK intergenic region. The Roman numerals correspond to the seven replacement strains created. (C) Quantitative real-time PCR (qPCR) was used to measure the RPA135-IGS1 interaction frequency in 3C libraries generated from the exponentially growing isogenic replacement strains illustrated in panel B. Replacement of region III significantly (P < 0.05, two-tailed, Student t test) decreased the RPA135-IGS1 interaction. Interaction frequencies were normalized by RPA135 copy number for interstrain comparison (Materials and Methods). Error bars denote standard errors of the means. n = 3 biological replicates; *, P < 0.05 (two-tailed, Student t test). WT, wild type.
In Saccharomyces cerevisiae, the rDNA is arranged as 150 to 200 tandem repeats on the long arm of chromosome XII (Chr XII) (11). Each of these tandemly arranged rDNA repeats consists of genes that encode the 35S and 5S rRNAs and two intergenic sequences, IGS1 and IGS2 (Fig. 1A). The rDNA is transcribed by two RNA polymerases: RNA polymerase I (RNAP I), which transcribes the 35S rRNA precursor, and RNAP III, which transcribes the 5S rRNA. Transcription of the rDNA by RNAP I is responsible for the majority of transcription in a cell (reviewed in reference 12).
The high rate of transcription and the repetitive structure of the rDNA genes contribute to the recombinogenic nature of the rDNA locus (13). This rDNA recombination results in fluctuations (i.e., increases and decreases) in the number of rDNA copies within populations of yeast cells (14, 15). Maintenance of copy number is achieved through the use of a dedicated rDNA maintenance system (reviewed in references 16 and 17) and involves the action of various nuclear factors, including the histone deacetylase Sir2 (18–20). Fluctuations in rDNA repeat copy number affect a variety of cellular processes and nuclear functions, including aging (21), telomere silencing (22), and cell sensitivity to DNA damage (16).
The S. cerevisiae nucleolus is directly linked to other nuclear loci through a large number of chromosomal contacts (here called interactions) that involve the rDNA, both in cis (intrachromosomal) (23) and in trans (interchromosomal) (24, 25). Functional roles for subsets of the connections between the rDNA and other genomic loci have been proposed (25) but have yet to be experimentally tested. Additionally, the clustering of dispersed tRNA genes at the nucleolar boundary has been well characterized and is hypothesized to relate to tRNA gene activity and pre-tRNA processing in yeast (26–28). This nucleolar clustering silences RNA polymerase II (RNAP II)-transcribed genes that are located in the vicinity of the tRNA genes, resulting in tRNA gene-mediated silencing (TGM) (27). Nevertheless, the roles of the majority of rDNA interactions are not well understood, and it is currently unknown whether nucleolar structure contributes to global nuclear regulation or whether nucleolar structure is regulated by global nuclear organization.
We have previously characterized one high-frequency, interchromosomal connection between the intergenic spacer 1 region (IGS1) of the rDNA and a locus on chromosome XVI that is flanked by the gene encoding the second largest RNAP I subunit, RPA135, and a lysine tRNA gene, tK(CUU)P (24). This interaction was shown not to depend on the presence of the lysine tRNA gene (24). The high frequency of this interaction coupled with the striking functional connection between the two interacting partners, one being the rDNA and the other being involved in rDNA transcription, led us to hypothesize that the interaction provides a feedback loop connecting the regulation of RNAP I production with nucleolar structure and function.
Here, we used quantitative chromosome conformation capture (q3C) in combination with replacement mapping of the RPA135-tK(CUU)P intergenic region to characterize the RPA135-IGS1 interaction. First, we identified a 75-bp sequence that mediates the interaction. The protein Msn2 binds to the boundary of this 75-bp sequence and affects the RPA135-IGS1 interaction. Second, we identified a second region within the RPA135-tK intergenic region that acts to stabilize rDNA copy number even during prolonged culturing. Finally, our results provide evidence that the DNA loci involved in chromosomal interactions are composite elements, sections of which function to stabilize the interaction or mediate a functional outcome.
MATERIALS AND METHODS
Strains and growth conditions.
All mutants generated in this study were derived from S. cerevisiae BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), except where stated. The full list of yeast strains that were used and generated in this study is provided in Table S1 in the supplemental material. Yeast strains were stored at −80°C and cultured in yeast peptone dextrose (YPD; 2% [wt/vol] glucose, 1% [wt/vol] yeast extract, 2% [wt/vol] bacteriological peptone) at 30°C with shaking (160 to 200 rpm). Where necessary, media were supplemented with agarose (2%, wt/vol) and G-418 (200 μg ml−1). Where necessary, yeast strains were grown on synthetic complete (SC) medium (0.17% [wt/vol] yeast nitrogen base without amino acids, 0.5% [wt/vol] ammonium sulfate, pH 7.0, 2% [wt/vol] glucose) supplemented with appropriate amino acids.
Escherichia coli was grown in LB (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 1% [wt/vol] NaCl). Where necessary, media were supplemented with 1.5% (wt/vol) agar and 100 μg ml−1 ampicillin for selection of transformants.
Generation of plasmids used in this study.
RPA135 was cloned by PCR amplification in two steps. RPA135-part 1 (Chr XVI; SGD coordinates 577585 to 579512) was PCR amplified (primers AC1015 and AC1016; see Table S2 in the supplemental material). The RPA135-part 1 amplicon was sequentially digested with ClaI (Fermentas; 7.5 U, 37°C, 18 h) and EcoRI (Invitrogen; 7.5 U, 37°C, 6.5 h). Prior to ligation, pRS416 was digested sequentially with ClaI (7.5 U, 37°C, 18 h) and EcoRI (7.5 U, 37°C, 6.5 h), dephosphorylated with calf intestinal alkaline phosphatase (CIAP; 1 U, 37°C, 5 min), and heat inactivated (15 min, 85°C). The ClaI- and EcoRI-digested RPA135-part 1 and linearized pRS416 were mixed at a 3:1 molar ratio, ligated (T4 DNA ligase [Invitrogen], 2.5 U, 16°C, overnight), and transformed into E. coli DH5α cells (29). Transformed colonies were selected on LB-ampicillin (100 μg ml−1) plates. Plasmid pIC101 was isolated, and the RPA135-part 1 insertion was checked by independent restriction digestion of an ∼6.8-kb pRS416-RPA135 fragment using EcoRI and NcoI.
RPA135-part 2 (Chr XVI; SGD coordinates 579513 to 582046) was PCR amplified (primers AC1017 and AC1018 [see Table S2 in the supplemental material]). The RPA135-part 2 amplicon was sequentially digested with BamHI (Fermentas; 7.5 U, 37°C, overnight) and EcoRI (Invitrogen; 7.5 U, 37°C, 6.5 h). Plasmid pIC101 was similarly digested with BamHI and EcoRI and dephosphorylated (CIAP; 1 U, 37°C, 5 min) before heat inactivation (15 min, 85°C). BamHI- and EcoRI-digested RPA135-part 2 and pIC101 were mixed at a 3:1 molar ratio, ligated (T4 DNA ligase [Invitrogen], 2.5 U, 16°C, overnight), and transformed into E. coli DH5α. Clones were selected on LB-ampicillin (100 μg ml−1), pIC112 was isolated, and the insertion was checked by PCR (see Table S2) and restriction digestion (single digestion with BamHI and double digestion with BamHI and EcoRI).
Lambda DNA (30) (nucleotide positions 29879 to 31279) was PCR amplified (primers shown in Table S2 in the supplemental material) and digested with NdeI (20 U, 37°C, 2 h). Plasmid pFA6-KanMX2 was also digested with NdeI (20 U, 37°C, 2 h) and dephosphorylated (CIAP; 1 U, 37°C, 5 min) before heat inactivation (15 min, 85°C). NdeI-digested lambda DNA amplicon and pFA6-KanMX2 were mixed in a 3:1 molar ratio, ligated (T4 DNA ligase [Invitrogen], 2.5 U, 16°C, 18 h), and transformed into E. coli DH5α. Clones were selected on LB-ampicillin (100 μg ml−1) plates, pFA6-KanMX2-λD4 (see Table S4 in the supplemental material) was isolated, and the insertion was checked by independent restriction digestion by NdeI and ClaI.
Generation of deletion-replacement strains.
PCR amplicons for homologous recombination were prepared using pFA6-KanMX2-λD4 and pIC112 (see Table S2 in the supplemental material for primers).
The S. cerevisiae BY4741 tK(CUU)P coding sequence (Chr XVI; SGD coordinates 582062 to 582134) formed the distal boundary of the incrementing replacement regions of the RPA135-tK(CUU)P intergenic region (Chr XVI; SGD coordinates 581196 to 582061) (Fig. 1B). Replacements of the RPA135-tK(CUU)P intergenic region with the λ-DNA:KanMX2 fusion sequence (amplified from pFA6-KanMX2-λD4) were precisely achieved using PCR-based homologous recombination in S. cerevisiae BY4741 according to the method of Orr-Weaver et al. (31). Briefly, replacement regions were PCR amplified (primer pairs are shown in Table S2 in the supplemental material), purified, and transformed into S. cerevisiae BY4741 cells by heat shock (32). Transformants were selected on YPD medium containing G-418 (200 μg ml−1). Insertion sites were confirmed by PCR (see Table S2). Seven viable mutant strains (rpa135::λ-DNA:KanMX2), each with an incremental λ-DNA sequence replacement, were produced (details are shown in Table S3): replacements I, II, III, IV, V, VI, and VII.
Generation of control replacement strains and double-RPA135-copy strain.
The region upstream of the LEU1 open reading frame (ORF) (Chr VII; SGD coordinates 479081 to 479270 or 478918 to 479270) was precisely replaced by homologous recombination using the λ-DNA:KanMX2 fusions corresponding to replacements VI and VII (see Table S3 in the supplemental material) to generate S. cerevisiae control VI and S. cerevisiae control VII, respectively (see Tables S1 and S2). Transformants were selected on YPD medium containing G-418 (200 μg ml−1), and insertion sites were confirmed by PCR (primers are shown in Table S2).
Homologous recombination was used to precisely replace the LEU1 coding sequence (Chr VII; SGD coordinates 476394 to 478652) in S. cerevisiae BY4741 with a full-length RPA135:URA3 fusion, generating the S. cerevisiae 2RPA strain (see Table S1 in the supplemental material). The RPA135:URA3 recombination template was prepared by PCR amplification using Phusion high-fidelity DNA polymerase (2 U μl−1; Thermo Scientific; primers in Table S2) from plasmid pIC112. Transformants were selected on dropout synthetic dextrose (SD; SC without amino acids) medium supplemented with histidine (300 μM), leucine (2 mM), and methionine (1 mM). Correct insertion was confirmed by PCR (primers are shown in Table S2).
q3C.
Quantitative chromosome conformation capture (q3C) was performed using a modified 3C protocol (4, 33). Briefly, overnight cultures of S. cerevisiae mutants were grown (30°C, 180 rpm), used to inoculate a YPD (50-ml) culture to an optical density at 600 nm (OD600) of 0.002, and grown to a final OD600 of 0.6 (i.e., 8 doubling times). Cells were cross-linked with formaldehyde (final concentration, 1%; 10 min) and quenched with glycine (final concentration, 125 mM) (24). Cells were then lysed with glass beads (Sigma-Aldrich) and vortexing, and the sedimented chromatin was suspended in chromatin digestion buffer (24). Cross-linked chromatin (24) was digested with HindIII (100 U; New England Biolabs). Ligation was achieved using T4 ligase (20 U; Invitrogen) (24). Cross-links were reversed and protein was digested before phenol-chloroform extraction. 3C libraries were precipitated using ethanol-sodium acetate (−20°C, overnight) in the presence of linearized polyacrylamide (0.25% [wt/vol]) and suspended in 60 μl H2O.
The RPA135-IGS1 interaction was measured using a probe-based quantitative real-time PCR (qPCR) assay. qPCR analyses were performed on a LightCycler 480 II real-time machine using LightCycler 480 Probes master mix (Roche Applied Science) and dedicated standards (24). PCRs (reaction mixtures, 15-μl final volume) were performed in triplicate with 2-μl samples of diluted (1:5 with H2O) 3C libraries using the rDNAHindIIIF and RT-RPA135Frag1_R primers in combination with the 5′–6-carboxyfluorescein (6-FAM)-labeled double-quenched rDNAHindIII ZEN probe (IDT) (see Table S2 in the supplemental material). Real-time PCR analyses were performed using the monocolor hydrolysis probe/Universal Probe Library (UPL) program with the following conditions: 95°C for 10 min and 50 cycles of 95°C for 13 s, 60°C for 30 s, and 72°C for 1 s. Electrophoresis of qPCR products following completion of the q3C resulted in the detection of a single band, supporting the specificity of the assay (see Fig. S1 in the supplemental material).
Cross-strain and interexperiment comparisons of the RPA135-IGS1 interaction required internal normalization. Unless otherwise stated, all RPA135-IGS1 interaction data were normalized by quantifying the copy number of the RPA135 coding region (see Table S2 in the supplemental material) in each sample by real-time PCR. Theoretically, this reduces the RPA135-IGS1 interaction frequency to interactions per genome unit. All standardization reactions were performed in triplicate using 3C libraries, dedicated primers (see Table S2), LightCycler 480 SYBR green I master mix (Roche Applied Science), and a SYBR green I/high-resolution melting (HRM) dye program—95°C for 5 min and 50 cycles of 95°C for 15 s, 58°C for 10 s, and 72°C for 10 s—that included the default dissociation analysis.
Total RNA isolation and quantitation.
RNA was isolated from S. cerevisiae cells using a modification of the method in reference 34. Briefly, cells were sedimented (2,200 × g, 2 min, room temperature) from 10 ml of each culture that was used for the 3C assay. Cells were washed and suspended in AE buffer (50 mM sodium acetate, 10 mM EDTA, pH 5.3). RNA was isolated from the cell samples by glass bead lysis and three rounds of phenol-chloroform-isoamyl alcohol (PCA; 25:24:1 [vol/vol/vol]) extraction and RNA precipitation (2.5× volume absolute ethanol and 10% [vol/vol] 3 M sodium acetate, −20°C, overnight). RNA was sedimented (maximum speed, 4°C, 5 min), and the pellets were suspended in RNase-free water before measurement of the RNA concentration and purity (NanoDrop). Total RNA (1.5 μg) integrity was confirmed by electrophoresis through a 1% agarose gel (80 V, 1.5 h). Nondegraded RNA samples (5 μg) were treated with the Turbo DNA-free kit (Ambion) according to the manufacturer's instructions before dilution to 25 ng μl−1 (final concentration).
One-step real-time reverse transcription-PCRs (RT-PCRs) (15-μl final volume) were performed using dedicated primers (see Table S2 in the supplemental material) and a one-step SYBR RT-PCR kit (TaKaRa RR086A), according to the manufacturer's instructions. Control reactions (minus reverse transcriptase) were performed simultaneously to control for genomic DNA contamination. Quantification was achieved by quantitative PCR on an ABI 7900HT machine under the conditions of 42°C for 5 min, 95°C for 10 s, and 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 15 s and the default dissociation assay.
Activation of GAL1/10 promoter in GAL-pro strain.
Starter cultures of S. cerevisiae were grown for ∼12 h in YPD. The starter culture was then used to inoculate 50 ml YP-galactose (glucose was replaced with 2% [wt/vol] galactose) test culture to a final OD600 of 0.002. The test culture was grown (30°C, 180 rpm) to a final OD600 of 0.6 (approximately 8 doubling times) for formaldehyde fixation as in q3C.
Prolonged culturing and genomic DNA extractions.
Starter cultures were grown overnight in YPD. SC medium (15 ml) was inoculated with 150 μl of each starter culture and grown (30°C, 200 rpm) for 15 days. At days 2, 4, and 15, cells were pelleted (2,200 × g, 5 min, room temperature) from 1 ml of culture. Cells were washed in solution A (2% Triton X-100, 1% SDS, 0.1 M NaCl, 10 mM Tris, pH 8.0, 1 mM EDTA) and pelleted. Cells were lysed with glass beads (Sigma-Aldrich) and vortexing. Genomic DNA was isolated by three rounds of phenol-chloroform (1:1) extraction followed by precipitation (2.5× volume absolute ethanol and 10% [vol/vol] 3 M sodium acetate, −20°C, overnight) and was then sedimented (maximum speed, 4°C, 10 min). DNA pellets were suspended in water (50 μl) before the genomic DNA concentration was measured (NanoDrop) and diluted to 10 ng μl−1 (final concentration).
Southern blotting.
Cultures were grown in YPD to an OD600 of 0.6 (as for the 3C assay) and harvested. Genomic DNA samples were extracted and analyzed for rDNA copy number by Southern blotting (35). Briefly, genomic DNA (1.5 μg) was digested with BglII (15 U; New England Biolabs) at 37°C overnight, separated on a 1% (wt/vol) agarose gel (80 V, 2.5 h), and transferred to a nylon membrane (Amersham RPN303N) by capillary transfer. rDNA repeats were detected on the membrane by hybridization with a digoxigenin (DIG)-labeled IGS1 probe (see Table S2 in the supplemental material; Roche PCR DIG probe synthesis kit). Bound probe was detected by anti-DIG-AP antibody (1.5 U; Roche) with CSPD [disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro)tricyclo [3.3.1.13,7]decan}-4-yl)phenyl phosphate] as the chemiluminescent substrate (Roche) before exposure on an Image Reader LAS-4000 (Fujifilm). For normalization, the blot was stripped with 0.1% SDS (90 to 100°C) and reprobed with a DIG-labeled RPA135 probe (see Table S2) (Roche PCR DIG probe synthesis kit).
Quantitation of rDNA repeat number was achieved using the Multi Gauge software (version 2.0; Fujifilm). All signals were normalized to RPA135 levels measured for the same genomic DNA sample.
RESULTS
A short region near RPA135 mediates the high-frequency RPA135-IGS1 interaction.
Our earlier work revealed the presence of a high-frequency interaction between the IGS1 region of the rDNA (Chr XII; SGD coordinates 460025 to 460609) and an intergenic region (Chr XVI; SGD coordinates 581196 to 582061) located between the start of the RPA135 gene and a tRNA lysine gene [RPA135-tK(CUU)P, here referred to as RPA135-tK] (Fig. 1A) (24). This interaction is 3 orders of magnitude higher than rDNA interactions that involve neighboring restriction fragments (Chr XVI; SGD coordinates 585884 to 589137 and 549477 to 580469) (24). We demonstrated that this interaction is not solely dependent on the presence of the tRNA gene (24), suggesting that the interaction may involve the RPA135 promoter. The striking potential functional link between the two partners prompted us to investigate this interaction further.
First, we wanted to test whether the RPA135-IGS1 interaction is dependent on a specific sequence within the RPA135-tK intergenic region. To achieve this, we employed a sequence-replacement strategy that replaced parts of the RPA135-tK intergenic region. In total, seven replacement strains were generated by homologous recombination (Fig. 1B). Replacement I replaced only the tK gene, without altering any of the RPA135-tK intergenic region; replacements II to VII replaced the tK gene and sequentially larger regions of the RPA135-tK intergenic region (Fig. 1B). All replacement strains maintained the length of the RPA135-tK intergenic region and were viable (did not require exogenously expressed Rpa135).
We employed q3C (24) to determine the effect of these replacements on the RPA135-IGS1 interaction. The RPA135-IGS1 interaction frequency became more variable following replacement of the tK gene (replacement I) and the first 75 bp (replacement II) of the RPA135-tK intergenic region (Fig. 1C). Replacement of the first 150 bp (replacement III) resulted in a significant reduction (P < 0.05, two-tailed, Student t test) in RPA135-IGS1 interaction frequency, and this reduction was maintained through all subsequent replacements (Fig. 1C). These results indicate that replacement of the lysine tRNA gene destabilizes the RPA135-IGS1 interaction, while replacement of the second 75 bp of the intergenic region (in replacement III) disrupts the interaction. Therefore, the 75-bp region that is unique to replacement III (compared to replacement II) appears to be involved in the formation and/or maintenance of the RPA135-IGS1 interaction, suggesting that the interaction is mediated in a sequence-specific manner.
The RPA135-IGS1 interaction is altered in strains with reduced rDNA copy number.
The RPA135-IGS1 interaction occurs between the single-copy RPA135 locus and the intergenic spacer region of the rDNA repeats. It is unknown if this interaction occurs with a single rDNA repeat or many, or if the interacting IGS1 sequences are from active or inactive rDNA repeats. To investigate this, we made use of yeast strains (YAG33 and YAG99 [36]) that contain only 20 and 80 copies, respectively, of the ribosomal DNA repeats. We measured the RPA135-IGS1 interaction levels in these strains and found a significant reduction in interaction frequency in the 20-copy strain when normalized to the RPA135 copy number (see Fig. S2 in the supplemental material). Interestingly, however, the 80-copy strain did not exhibit a reduction in interaction frequency when normalized to the RPA135 copy number (see Fig. S2). These results suggest that the RPA135-IGS1 interaction is dependent upon rDNA repeat number but that the effect is not linear. Instead, it seems that there is a threshold repeat number (80 copies or fewer) below which copy number affects the interaction frequency.
RPA135-IGS1 interaction frequency is reduced in the msn2Δ mutant.
Our observations suggest that the RPA135-IGS1 interaction requires region III of the RPA135-tK intergenic region (Fig. 1C). This raises the possibility that specific motifs within this region are recognized by protein factors that mediate the interaction.
We looked for candidate proteins that may be involved in the interaction by searching a comprehensive genome binding map of gene and chromatin regulatory proteins (37) for proteins that bind to this region. We selected five nonessential proteins (i.e., Msn2, Rph1, Stp1, Xbp1, and Yap5) that have been shown to bind (see Table S5 in the supplemental material) (37) and have a recognized DNA motif (identified using JASPAR [38]) within the RPA135-tK region (Fig. 2A). Sir2 was also included in the analyses because it (i) has recognized roles in nucleolar structure (18, 19, 39) and (ii) binds at the boundary of region VII, albeit in the absence of a known DNA motif (see Table S5) (37).
FIG 2.
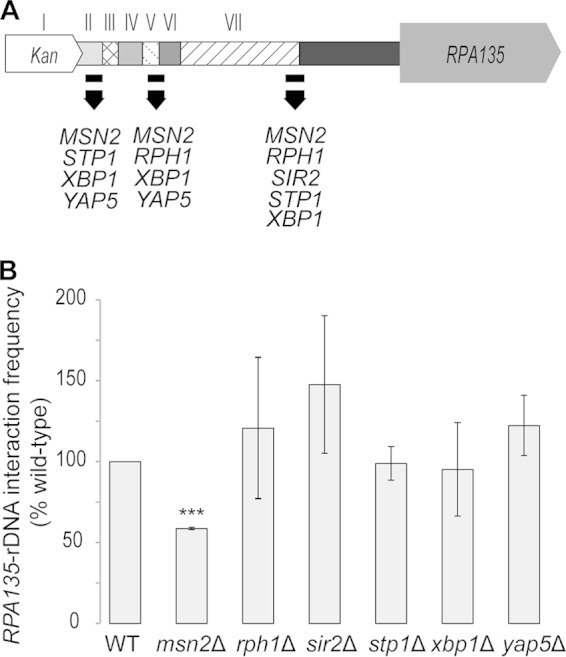
The RPA135-IGS1 interaction is reduced in the msn2Δ mutant. (A) Cartoon illustrating the characterized protein binding sites within the RPA135-tK intergenic region, as determined by Venters et al. using chromatin immunoprecipitation sequencing (37). (B) Deletion strains for the proteins illustrated in panel A were obtained from the S. cerevisiae genome deletion project (52) (see Table S1 in the supplemental material). RPA135-IGS1 interaction frequency (normalized to RPA135 copy number) was measured in exponentially growing S. cerevisiae deletion mutants. RPA135-IGS1 interaction frequency was significantly (P < 0.001, two-tailed, Student t test) decreased in the msn2Δ strain. Error bars denote standard errors of the means. n = 3 biological replicates; ***, P < 0.001 (two-tailed, Student t test). WT, wild type.
Deletion strains for these six proteins were obtained from the yeast deletion project, and the deletions were confirmed by PCR (data not shown). We tested the RPA135-IGS1 interaction frequencies in these six deletion strains (Fig. 2B). Only the msn2Δ strain showed a significant reduction in the RPA135-IGS1 interaction frequency when normalized by RPA135 copy number (P < 0.001, two-tailed, Student t test) (Fig. 2B). Collectively, these observations are consistent with a scenario where Msn2 binding to the boundary of regions II and III (Fig. 2A) contributes to the region III-mediated formation or maintenance of the RPA135-IGS1 interaction (Fig. 1B and C). However, it remains unclear whether the Msn2 protein binds to both the RPA135-tK and IGS1 regions to directly mediate this connection or facilitates the interaction indirectly.
The RPA135-IGS1 interaction is not correlated with RPA135 transcriptional output.
We hypothesized that the RPA135-IGS1 interaction would serve as a feedback loop to regulate RPA135 transcription and thus help to coordinate rRNA transcriptional output. To test this, we measured RPA135 and 25S rRNA transcript levels in all the strains used in the study. 25S rRNA levels were affected only in the sir2Δ, rph1Δ, and 20-copy strains (see Fig. S3 in the supplemental material), while RPA135 transcript levels were generally upregulated in the msn2Δ, rph1Δ, sir2Δ, stp1Δ, xbp1Δ, and yap5Δ strains (Fig. 3). This upregulation is consistent with the known roles of these proteins in the regulation of RNAP II transcription (37, 40, 41). In contrast, the 20-copy rDNA strain showed a significant reduction in RPA135 transcript levels (P < 0.05, two-tailed, Student t test) (Fig. 3). Surprisingly, the replacement of regions within the RPA135-tK intergenic region did not change the RPA135 transcript level, with the exception of replacement I, which resulted in a reduction in RPA135 transcript level (P < 0.05, two-tailed, Student's t test) (Fig. 3). Overall, there was no correlation between the RPA135-IGS1 interaction frequency and either the RPA135 transcript or 25S rRNA levels (see Fig. S4A and B). Therefore, contrary to our prediction, the RPA135-IGS1 interaction does not regulate RPA135 or 25S rRNA transcription.
FIG 3.
Interruption of the RPA135-IGS1 interaction does not correlate with altered RPA135 transcript levels. RPA135 transcript levels were measured by RT-qPCR from total RNA isolated from the exponentially growing S. cerevisiae cultures. RPA135 expression levels were normalized to the ACT1, ALG9, and UBC6 genes (53). Replacement of the tK tRNA gene with KanMX2 correlated with a small but significant reduction (P < 0.05, two-tailed, Student t test) in RPA135 transcript levels. However, replacement of increasingly larger regions within the RPA135-tK intergenic region resulted in no significant changes in RPA135 transcript levels compared to the wild type (left set of bars). RPA135 transcript level was significantly (P < 0.05, two-tailed, Student t test) decreased in the 20-copy (YAG33) rDNA strain (middle set of bars). Except for the sir2Δ and yap5Δ strains, RPA135 transcripts were significantly (P < 0.05 and < 0.01, two-tailed, Student t test) upregulated in deletion strains (right set of bars) whose proteins bind within the RPA135-tK intergenic region. Error bars denote standard errors of the means. n = 3 biological replicates; *, P < 0.05; **, P < 0.01 (two-tailed, Student t test). WT, wild type.
Reduction of the RPA135-IGS1 interaction frequency affects rDNA copy number.
Given that the interaction does not regulate RPA135 transcription, we wondered whether it might be playing some other role. The apparent dependence of the RPA135-IGS1 interaction frequency on the number of rDNA repeats (see Fig. S2 in the supplemental material) suggests that the changes in RPA135-IGS1 interaction frequency that we observed in the replacement strains may result from changes in rDNA copy number in these strains. qPCR analyses of IGS1 copy number showed that replacement strains I to VI do not have (P = 0.063 for replacement III, two-tailed, Student t test) IGS1 copy numbers significantly different from those of the wild-type (BY4741) strain (Fig. 4A). In contrast, the rDNA copy number in replacement VII was approximately half of that observed in the wild-type strain (P < 0.05, two-tailed, Student t test) (Fig. 4A). Interestingly, the IGS1 copy number was not significantly different in the msn2Δ strain (see Fig. S5). Thus, replacement of region VII somehow results in reduced rDNA copy number.
FIG 4.
rDNA copy number fluctuates in replacement VII strains. Ribosomal DNA levels were measured in different clones of the replacement VII strain during exponential growth and over prolonged culturing. (A) IGS1 rDNA copy number was measured in the 3C libraries by qPCR and normalized to RPA135 copy number. Replacement strain VII exhibited a significant reduction in IGS1 copy number (P < 0.05, two-tailed, Student t test). (B) Wild-type, replacement III, and replacement VII strains were grown (30°C, 230 rpm) in SC medium for 15 days. IGS1 copy number was measured by qPCR on genomic DNA samples isolated from cultures after 2, 4, and 15 days of culturing. Replacement III maintained the IGS1 rDNA sequence at wild-type copy number. In contrast, significant fluctuations in rDNA copy number were observed in replacement VII over the course of the experiment. (C) Southern blotting of BglII-digested genomic DNA extracted from biological replicates of cells grown (30°C, 180 rpm) in YPD medium to an OD600 of 0.6. DNA was extracted from cultures grown on two different days (i.e., preparations 1 and 2). Southern blots were consecutively probed by IGS1 and RPA135. (D) IGS1 levels detected by Southern blotting were normalized to RPA135 levels and expressed as the percentage of the measured wild-type rDNA levels. IGS1 levels for replacement VII showed significant variation in rDNA copy number for cultures grown on different occasions (i.e., preparation 1 versus preparation 2). Between-clone variation in IGS1 levels was also significant (P < 0.05, two-tailed, Student t test). Values are the means ± standard errors of the means. n = 3 biological replicates (or 4 in preparation 1); *, P < 0.05; **, P < 0.01;***, P < 0.001 (two-tailed, Student t test). WT, wild type.
rDNA copy number fluctuates in the replacement VII strain.
Reductions in rDNA copy number can be a manifestation of decreased stability of the rDNA (16, 42). Therefore, we hypothesized that the observed reduction in rDNA copy number in the replacement VII strain reflects increased rDNA instability in this strain. To test for increased rDNA instability, we quantified the rDNA copy number in different clones and over prolonged culturing.
The IGS1 copy number showed small nonsignificant variations in the wild-type strain over the course of the prolonged culturing experiment (see Fig. S6 in the supplemental material). Similarly, the replacement III strain maintained an rDNA copy number that was similar to that of the wild type during prolonged culturing (Fig. 4B). In contrast, replacement VII exhibited large and significant fluctuations in rDNA copy number at 4 and 15 days of prolonged culturing (Fig. 4B).
To further confirm instability in rDNA copy number within the replacement VII strain, rDNA levels were determined by Southern blotting using genomic DNA isolated from exponentially growing cells (Fig. 4C and D). We observed that the rDNA copy number fluctuated up to 2-fold (P < 0.05, two-tailed, Student t test) over the wild-type level in replicate batches of cells, and independent clones, which were independently grown to the same cell density under identical conditions (Fig. 4C and D).
The RPA135-IGS1 interaction is independent of RPA135 copy number.
The asymmetric nature of the RPA135-IGS1 interaction, in which a single-copy locus interacts with a multicopy locus, suggests that the interaction level may be limited by the single-copy nature of the RPA135-tK intergenic region. To test this, we replaced the LEU1 locus (Chr VII; SGD coordinates 476394 to 478552) with the complete RPA135 coding sequence and the RPA135-tK intergenic region to create a strain with two copies of RPA135 and its upstream sequence (strain 2RPA) (see Fig. S7A in the supplemental material). The RPA135 transcript level in this strain is double that of the wild type (see Fig. S7C), consistent with both copies being functional. However, the RPA135-IGS1 interaction level in this strain is not significantly different from that of the wild type (see Fig. S7B). While it is unclear from the assay whether one or both RPA135 loci are interacting, this result suggests that the interaction does not depend on RPA135 copy number.
The RPA135-IGS1 interaction depends on noncoding RNAP II transcriptional activity at the IGS1 locus.
The replacement VII strain exhibited a reduced RPA135-IGS1 interaction frequency and increased rDNA copy number instability. rDNA stability is known to be regulated by a noncoding bidirectional RNAP II promoter, E-pro, that is present within IGS1 (36). Transcription from E-pro stimulates rDNA repeat recombination and thus rDNA instability, while conversely, E-pro silencing results in rDNA stabilization (15, 36). Therefore, we hypothesized that noncoding transcription from the IGS1 region would affect the RPA135-IGS1 interaction.
To test this, we quantified the RPA135-IGS1 interaction in a strain, GAL-pro, where the E-pro sequence has been replaced by a galactose-inducible bidirectional GAL1/10 promoter (Fig. 5A) (36). The noncoding IGS1 transcription can be controlled in this strain by carbon source such that growth in glucose represses transcription while growth in galactose activates transcription from the GAL1/10 promoter (15).
FIG 5.
RPA135-IGS1 interaction frequency is affected by bidirectional RNAP II promoter activity within the IGS1 region. (A) The GAL-pro strain (36) has the bidirectional IGS1 RNAP II promoter (E-pro) replaced with a GAL1/10 bidirectional promoter as illustrated. (B) RPA135-IGS1 interaction levels were measured in wild-type and GAL-pro mutant strains grown in YP-glucose or -galactose. RPA135-IGS1 interaction data were normalized by RPA135 copy number. The RPA135-IGS1 interaction frequency was significantly reduced (P < 0.01, two-tailed, Student t test) when the GAL1/10 promoter was activated by growth in galactose-supplemented medium. (C) 25S rDNA copy number was measured in the strains from panel B. 25S rDNA copy number was normalized to RPA135 copy number for interstrain comparison. 25S rDNA copy number was significantly reduced (P < 0.05, two-tailed, Student t test) in the GAL-pro mutant strain following galactose induction of the GAL1/10 promoter. Error bars denote standard errors of the means. n = 4 biological replicates; *, P < 0.05; **, P < 0.01 (two-tailed, Student t test). WT, wild type.
Repression of the GAL1/10 promoter by growth in glucose altered neither the interaction frequency (Fig. 5B) nor the rDNA copy number (Fig. 5C) compared to the wild type. Therefore, replacing the E-pro region with the GAL1/10 promoter does not prevent the interaction, suggesting either that the IGS1 component of the interaction is sequence independent or that the region responsible for mediating the interaction falls outside the E-pro region (Chr XII; SGD coordinates 460110 to 460245) (36).
Activation of the GAL1/10 promoter by growth of the GAL-pro strain in galactose medium resulted in the RPA135-IGS1 interaction being reduced to 40% of the wild-type level (Fig. 5B) (P < 0.01, two-tailed, Student t test). Galactose-induced reductions in the RPA135-IGS1 interaction frequency did not alter the RPA135 transcript level (see Fig. S8 in the supplemental material). It is tempting to ascribe the observed reduction in the levels of the RPA135-IGS1 interaction to the process of transcription itself. However, we also observed a significant reduction in rDNA copy number following activation of the GAL1/10 promoter (Fig. 5C) (P < 0.05, two-tailed, Student t test), consistent with previous observations of increased rDNA instability in this strain (15, 36). These results are consistent with the RPA135-IGS1 interaction being dependent on the rDNA copy number.
DISCUSSION
The RPA135-IGS1 interaction requires a specific sequence within the RPA135-tK intergenic region and is affected by the Msn2 protein.
In this study, we have empirically identified two functional regions within the RPA135-tK intergenic region. One, region III (Chr XVI; SGD coordinates 581912 to 581986), is involved in the formation and/or maintenance of a high-frequency interaction between the upstream region of RPA135, encoding a subunit of RNAP I, and the IGS1 region of the rDNA. The second region that we identified, region VII (Chr XVI; SGD coordinates 581457 to 581684), affects rDNA copy number stability. Together, these regions form the first example of a nonlinked locus, on another chromosome, that affects the stability of the rDNA array through the formation of an interchromosomal connection. Moreover, they provide evidence that the DNA loci involved in chromosomal interactions can be composite elements.
The RPA135-tK intergenic region contains binding sites for numerous RNAP II gene regulatory and chromatin-modifying factors (37). Deletion of the MSN2 gene, an RNAP II transcription factor that has been shown to bind the RPA135-tK intergenic region (Fig. 2A), reduced the RPA135-IGS1 interaction frequency to approximately the same level as replacement of the region III-specific 75-bp sequence. One of the Msn2p binding sites is in the vicinity of region III (Fig. 2A); thus, we suggest that binding of Msn2 to this region contributes directly to the formation or maintenance of the RPA135-IGS1 interaction. Furthermore, there are predicted Msn2p binding sites within the IGS1 and IGS2 regions (JASPAR [38]) and known Msn2p binding sites that border the left and right sides of the intact rDNA array (37). Therefore, although it is not known whether Msn2p directly contacts both the RPA135-tK intergenic region and the rDNA, these results are consistent with a model whereby Msn2p bridges, either by itself or as part of a larger complex, the two interacting loci. The other Msn2 protein binding sites within the RPA135-tK intergenic region, located in regions V-VI and VII (Fig. 2A), do not appear to contribute to interaction formation, as their deletion does not further reduce the frequency of the RPA135-IGS1 interaction (Fig. 1C).
Deletion of the MSN2 gene resulted in an increase in RPA135 transcript levels. However, we suggest that Msn2 regulation of RPA135 transcription is independent of its role in the RPA135-IGS1 interaction, as we show that the interaction does not affect RPA135 transcription (Fig. 3). It is possible that the Msn2 protein binds to the RPA135-tK intergenic region and contributes to the regulation of RPA135 transcription while simultaneously facilitating the RPA135-IGS1 interaction. This model of independent RPA135 transcriptional regulation and RPA135-IGS1 interaction formation by Msn2p is supported by the results of the deletion of the Stp1 and Xbp1 transcription factors. Both bind to regions II and III, and deletion of Stp1p and Xbp1p significantly increases RPA135 transcript levels without altering rDNA copy number, 25S rRNA levels, or RPA135-IGS1 interaction frequencies.
Biological role of the RPA135-IGS1 interchromosomal interaction.
Our results suggest that the RPA135-IGS1 interaction stabilizes rDNA repeat number. Here, we present two models (i.e., binary and multiplex) proposing how this stabilization is achieved. In both of these models, Msn2 protein binding at region III helps to mediate a physical interaction that localizes region VII in the spatial vicinity of the IGS1 region(s). This enables region VII to stabilize the Sir2 complex that forms over IGS1 and controls rDNA recombination (18). In the binary model, the RPA135-IGS1 interaction occurs with just a single IGS1 repeat at any one moment in time. In contrast, in the multiplex model the interaction involves two or more IGS1 repeats (Fig. 6).
FIG 6.
Model for the organization of the RPA135-IGS1 interchromosomal interaction The RPA135-IGS1 interaction is mediated by Msn2p bound to the RPA135-tK intergenic region and possibly to the IGS. This interaction localizes a second region of the RPA135-tK intergenic region in the spatial vicinity of the rDNA, enabling it to mediate rDNA stability. Disruption of the RPA135-IGS1 interaction destabilizes the complex and leads to fluctuations in rDNA copy number. In the binary model, only one IGS1 repeat interacts at any one time. In contrast, in the multiplex model, more than one IGS1 repeat is directly involved in the interaction. For illustrative purposes, the Msn2 protein complex is shown interacting with three IGS1 repeats in the multiplex model. However, theoretically the interaction could involve more IGS1 regions. tK, tK(CUU)P; III, region III; VII, region VII; Chr, chromosome; figure not to scale.
The multicopy nature of the rDNA genes means that the RPA135-IGS1 interaction could concurrently, or consecutively, occur with one or many repeats. However, the 3C assay that we used can detect and measure only one IGS1 region interacting with the RPA135-tK intergenic region at any given moment within a single haploid cell. As such, our data are unable to distinguish between the RPA135-IGS1 interaction occurring with just a single IGS1 repeat (the binary model) and that occurring with many IGS1 repeats (the multiplex model) (Fig. 6). Nevertheless, the dramatic reduction in interaction frequency that we observed in the 20-copy strain but not in the 80-copy strain suggests that there is an rDNA copy number threshold for the interaction. The distinguishing feature of the 20-copy strain is that all the rDNA repeats are transcriptionally active, unlike the wild-type and 80-copy strains (43). Therefore, this copy number threshold could be explained if the RPA135-IGS1 interaction occurs primarily with inactive rDNA repeats that are present in the 80-copy strain but not the 20-copy strain (44, 45). We propose that one role of this interaction is to produce an rDNA organization in which at least some inactive repeats are spatially segregated from the active repeats within the nucleolus (16). It will be interesting to determine whether this interaction contributes to the protection from DNA damage that the inactive rDNA repeats provide (42).
There are a number of different implications that arise from the binary and multiplex models of the RPA135-IGS1 interaction. One implication of the binary model is that a single interaction acts as a master regulator of rDNA copy number. The one-to-one interaction in the binary model also implies a rigid structure that does not conform well to our current understanding of the dynamic nature of genome spatial organization. However, the interacting rDNA unit need not be fixed within the binary model; instead, the interaction could occur by a one-at-a-time mechanism that is both robust and dynamic. In the one-at-a-time mechanism, the rDNA repeats that are involved in the RPA135-IGS1 interaction interchange, such that all or a large number of the rDNA repeats are sequentially involved in the connection. In contrast, in the multiplex model at least two IGS1 repeats, the identity of which could be fixed or interchanging, are simultaneously involved in the RPA135-IGS1 interaction. The repeats that are involved in this multiplex interaction need not be adjacent, and the overall structure of the interaction resembles the replication-factory model proposed by Cook (46).
Nucleolar organization and tDNA clustering are both facilitated by proteins, including cohesin, that are involved in mediating chromosome structure (47, 48). Interestingly, we found that replacement of the tK gene on the boundary of the RPA135 intergenic region also contributed to increased variation in the RPA135-IGS1 interaction frequency. This raises the possibility that the RPA135-IGS1 interaction is facilitated in part by tDNA clustering at the nucleolar periphery. Therefore, the chromosome structuring mechanisms that contribute to tDNA clustering (28, 49) and nucleolar organization may also work in conjunction with the factors that we identify here (Msn2 and elements III and VII) to promote the RPA135-IGS1 interaction.
In conclusion, we have identified a role for the RPA135-IGS1 interchromosomal interaction in rDNA stability. This functional role is mediated by a composite element, consisting of two separate regions within the RPA135-tK intergenic region, and involves the Msn2 protein. The significance of this finding lies in the demonstration that nongenetically linked loci can affect the stability of repetitive loci—in much the same way as an enhancer contributes to transcriptional control. This finding adds another level to our understanding of the regulation of rDNA stability and is important given the central and pleomorphic roles that rDNA instability plays in eukaryotic cells (15, 36, 42, 50, 51).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Marsden Fund (UOA1023 to J.M.O. and 10-MAU-072 to A.R.D.G.), the National Institutes of Health (R01GM082875 to D.R.E.), and a Liggins Institute Scholarship (I.C.).
We thank Evelyn Sattlegger (Massey University) for the transcription factor deletion strains, Daniela Quintana-James (Massey University) for assistance with Southern blotting, Ralph S. Grand for transcription factor analyses and methodological inputs, and Yannan Jiang and Beatrix Jones for statistical advice.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01249-14.
REFERENCES
- 1.Parada L, Misteli T. 2002. Chromosome positioning in the interphase nucleus. Trends Cell Biol 12:425–432. doi: 10.1016/S0962-8924(02)02351-6. [DOI] [PubMed] [Google Scholar]
- 2.Branco MR, Pombo A. 2007. Chromosome organization: new facts, new models. Trends Cell Biol 17:127–134. doi: 10.1016/j.tcb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Bickmore WA, van Steensel B. 2013. Genome architecture: domain organization of interphase chromosomes. Cell 152:1270–1284. doi: 10.1016/j.cell.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Dekker J, Rippe K, Dekker M, Kleckner N. 2002. Capturing chromosome conformation. Science 295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 5.De Wit E, Bouwman BAM, Zhu Y, Klous P, Splinter E, Verstegen MJAM, Krijger PHL, Festuccia N, Nora EP, Welling M, Heard E, Geijsen N, Poot RA, Chambers I, de Laat W. 2013. The pluripotent genome in three dimensions is shaped around pluripotency factors. Nature 501:227–231. doi: 10.1038/nature12420. [DOI] [PubMed] [Google Scholar]
- 6.Naumova N, Imakaev M, Fudenberg G, Zhan Y, Lajoie BR, Mirny LA, Dekker J. 2013. Organization of the mitotic chromosome. Science 342:948–953. doi: 10.1126/science.1236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjong H, Gong K, Chen L, Alber F. 2012. Physical tethering and volume exclusion determine higher-order genome organization in budding yeast. Genome Res 22:1295–1305. doi: 10.1101/gr.129437.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock R. 2004. A role for macromolecular crowding effects in the assembly and function of compartments in the nucleus. J Struct Biol 146:281–290. doi: 10.1016/j.jsb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Bancaud A, Huet S, Daigle N, Mozziconacci J, Beaudouin J, Ellenberg J. 2009. Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J 28:3785–3798. doi: 10.1038/emboj.2009.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz K-M, Mayer C, Postepska A, Grummt I. 2010. Interaction of noncoding RNA with the rDNA promoter mediates recruitment of DNMT3b and silencing of rRNA genes. Genes Dev 24:2264–2269. doi: 10.1101/gad.590910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petes TD. 1979. Yeast ribosomal DNA genes are located on chromosome XII. Proc Natl Acad Sci U S A 76:410–414. doi: 10.1073/pnas.76.1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer C, Grummt I. 2006. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25:6384–6391. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- 13.Hawley RS, Marcus CH. 1989. Recombinational controls of rDNA redundancy in Drosophila. Annu Rev Genet 23:87–120. doi: 10.1146/annurev.ge.23.120189.000511. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Horiuchi T, Tongaonkar P, Vu L, Nomura M. 2004. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 117:441–453. doi: 10.1016/S0092-8674(04)00414-3. [DOI] [PubMed] [Google Scholar]
- 15.Saka K, Ide S, Ganley ARD, Kobayashi T. 2013. Cellular senescence in yeast is regulated by rDNA noncoding transcription. Curr Biol 23:1794–1798. doi: 10.1016/j.cub.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T. 2011. Regulation of ribosomal RNA gene copy number and its role in modulating genome integrity and evolutionary adaptability in yeast. Cell Mol Life Sci 68:1395–1403. doi: 10.1007/s00018-010-0613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang E, Carr AM. 2008. Replication fork arrest, recombination and the maintenance of ribosomal DNA stability. DNA Repair (Amst) 7:1613–1623. doi: 10.1016/j.dnarep.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb S, Esposito RE. 1989. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 19.Fritze CE, Verschueren K, Strich R, Easton Esposito R. 1997. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J 16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Zhou BO, Lenzmeier BA, Zhou J-Q. 2009. Histone deacetylase Rpd3 antagonizes Sir2-dependent silent chromatin propagation. Nucleic Acids Res 37:3699–3713. doi: 10.1093/nar/gkp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganley ARD, Kobayashi T. 2014. Ribosomal DNA and cellular senescence: new evidence supporting the connection between rDNA and aging. FEMS Yeast Res 14:49–59. doi: 10.1111/1567-1364.12133. [DOI] [PubMed] [Google Scholar]
- 22.Michel AH, Kornmann B, Dubrana K, Shore D. 2005. Spontaneous rDNA copy number variation modulates Sir2 levels and epigenetic gene silencing. Genes Dev 19:1199–1210. doi: 10.1101/gad.340205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayan M, Aragon L. 2010. Cis-interactions between noncoding ribosomal spacers dependent on RNAP-II separate RNAP-I and RNAP-III transcription domains. Cell Cycle 9:4328–4337. doi: 10.4161/cc.9.21.13591. [DOI] [PubMed] [Google Scholar]
- 24.Rodley CDM, Pai DA, Mills TA, Engelke DR, O'Sullivan JM. 2011. tRNA gene identity affects nuclear positioning. PLoS One 6:e29267. doi: 10.1371/journal.pone.0029267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Sullivan JM, Sontam DM, Grierson R, Jones B. 2009. Repeated elements coordinate the spatial organization of the yeast genome. Yeast 26:125–138. doi: 10.1002/yea.1657. [DOI] [PubMed] [Google Scholar]
- 26.Thompson M, Haeusler RA, Good PD, Engelke DR. 2003. Nucleolar clustering of dispersed tRNA genes. Science 302:1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Haeusler RA, Good PD, Thompson M, Nagar S, Engelke DR. 2005. Silencing near tRNA genes requires nucleolar localization. J Biol Chem 280:8637–8639. doi: 10.1074/jbc.C500017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haeusler RA, Pratt-Hyatt M, Good PD, Gipson TA, Engelke DR. 2008. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev 22:2204–2214. doi: 10.1101/gad.1675908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue H, Nojima H, Okayama H. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23–28. doi: 10.1016/0378-1119(90)90336-P. [DOI] [PubMed] [Google Scholar]
- 30.Sanger F, Coulson A, Hong G, Hill D, Petersen G. 1982. Sequence of bacteriophage. J Mol Biol 162:729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- 31.Orr-Weaver TL, Szostak JW, Rothstein RJ. 1981. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A 78:6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gietz RD, Schiestl RH. 2007. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2:38–41. doi: 10.1038/nprot.2007.15. [DOI] [PubMed] [Google Scholar]
- 33.Rodley CDM, Bertels F, Jones B, O'Sullivan JM. 2009. Global identification of yeast chromosome interactions using genome conformation capture. Fungal Genet Biol 46:879–886. doi: 10.1016/j.fgb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Ares M. 2012. Isolation of total RNA from yeast cell cultures. Cold Spring Harb Protoc 2012:1082–1086. doi: 10.1101/pdb.prot071456. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Heck DJ, Nomura M, Horiuchi T. 1998. Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev 12:3821–3830. doi: 10.1101/gad.12.24.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi T, Ganley ARD. 2005. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309:1581–1584. doi: 10.1126/science.1116102. [DOI] [PubMed] [Google Scholar]
- 37.Venters BJ, Wachi S, Mavrich TN, Andersen BE, Jena P, Sinnamon AJ, Jain P, Rolleri NS, Jiang C, Hemeryck-Walsh C, Pugh BF. 2011. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol Cell 41:480–492. doi: 10.1016/j.molcel.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryne JC, Valen E, Tang M-HE, Marstrand T, Winther O, da Piedade I, Krogh A, Lenhard B, Sandelin A. 2008. JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update. Nucleic Acids Res 36:D102–D106. doi: 10.1093/nar/gkm955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Mueller JE, Bryk M. 2006. Sir2 represses endogenous polymerase II transcription units in the ribosomal DNA nontranscribed spacer. Mol Biol Cell 17:3848–3859. doi: 10.1091/mbc.E06-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt AP, McEntee K. 1996. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orzechowski Westholm J, Tronnersjö S, Nordberg N, Olsson I, Komorowski J, Ronne H. 2012. Gis1 and Rph1 regulate glycerol and acetate metabolism in glucose depleted yeast cells. PLoS One 7:e31577. doi: 10.1371/journal.pone.0031577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ide S, Miyazaki T, Maki H, Kobayashi T. 2010. Abundance of ribosomal RNA gene copies maintains genome integrity. Science 327:693–696. doi: 10.1126/science.1179044. [DOI] [PubMed] [Google Scholar]
- 43.French SL, Osheim YN, Cioci F, Nomura M, Beyer AL. 2003. In exponentially growing Saccharomyces cerevisiae cells, rRNA synthesis is determined by the summed RNA polymerase I loading rate rather than by the number of active genes. Mol Cell Biol 23:1558–1568. doi: 10.1128/MCB.23.5.1558-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dammann R, Lucchini R, Koller T, Sogo JM. 1993. Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res 21:2331–2338. doi: 10.1093/nar/21.10.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dammann R, Lucchini R, Koller T, Sogo JM. 1995. Transcription in the yeast rRNA gene locus: distribution of the active gene copies and chromatin structure of their flanking regulatory sequences. Mol Cell Biol 15:5294–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook PR. 1999. The organization of replication and transcription. Science 284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- 47.Bose T, Lee KK, Lu S, Xu B, Harris B, Slaughter B, Unruh J, Garrett A, McDowell W, Box A, Li H, Peak A, Ramachandran S, Seidel C, Gerton JL. 2012. Cohesin proteins promote ribosomal RNA production and protein translation in yeast and human cells. PLoS Genet 8:e1002749. doi: 10.1371/journal.pgen.1002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris B, Bose T, Lee KK, Wang F, Lu S, Ross RT, Zhang Y, French SL, Beyer AL, Slaughter BD, Unruh JR, Gerton JL. 2014. Cohesion promotes nucleolar structure and function. Mol Biol Cell 25:337–346. doi: 10.1091/mbc.E13-07-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gard S, Light W, Xiong B, Bose T, McNairn AJ, Harris B, Fleharty B, Seidel C, Brickner JH, Gerton JL. 2009. Cohesinopathy mutations disrupt the subnuclear organization of chromatin. J Cell Biol 187:455–462. doi: 10.1083/jcb.200906075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganley ARD, Kobayashi T. 2007. Highly efficient concerted evolution in the ribosomal DNA repeats: total rDNA repeat variation revealed by whole-genome shotgun sequence data. Genome Res 17:184–191. doi: 10.1101/gr.5457707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paredes S, Branco AT, Hartl DL, Maggert KA, Lemos B. 2011. Ribosomal DNA deletions modulate genome-wide gene expression: “rDNA-sensitive” genes and natural variation. PLoS Genet 7:1–10. doi: 10.1371/journal.pgen.1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Véronneau S, Dow S, Lucau-Danila A, Anderson K, André B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian K-D, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Güldener U, Hegemann JH, Hempel S, Herman Z, Jaramillo DF, Kelly DE, Kelly SL, Kötter P, LaBonte D, Lamb DC, Lan N, Liang H, Liao H, Liu L, Luo C, Lussier M, Mao R, Menard P, Ooi SL, Revuelta JL, Roberts CJ, Rose M, Ross-Macdonald P, Scherens B, Schimmack G, Shafer B, Shoemaker DD, Sookhai-Mahadeo S, Storms RK, Strathern JN, Valle G, Voet M, Volckaert G, Wang C, Ward TR, Wilhelmy J, Winzeler EA, Yang Y, Yen G, Youngman E, Yu K, Bussey H, Boeke JD, Snyder M, Philippsen P, Davis RW, Johnston M. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 53.Teste M-A, Duquenne M, François JM, Parrou J-L. 2009. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol 10:99. doi: 10.1186/1471-2199-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







