Abstract
Recurrent breast cancer is typically an incurable disease and, as such, is disproportionately responsible for deaths from this disease. Recurrent breast cancers arise from the pool of disseminated tumor cells (DTCs) that survive adjuvant or neoadjuvant therapy, and patients with detectable DTCs following therapy are at substantially increased risk for recurrence. Consequently, the identification of pathways that contribute to the survival of breast cancer cells following therapy could aid in the development of more effective therapies that decrease the burden of residual disease and thereby reduce the risk of breast cancer recurrence. We now report that Ceramide Kinase (Cerk) is required for mammary tumor recurrence following HER2/neu pathway inhibition and is spontaneously up-regulated during tumor recurrence in multiple genetically engineered mouse models for breast cancer. We find that Cerk is rapidly up-regulated in tumor cells following HER2/neu down-regulation or treatment with Adriamycin and that Cerk is required for tumor cell survival following HER2/neu down-regulation. Consistent with our observations in mouse models, analysis of gene expression profiles from over 2,200 patients revealed that elevated CERK expression is associated with an increased risk of recurrence in women with breast cancer. Additionally, although CERK expression is associated with aggressive subtypes of breast cancer, including those that are ER–, HER2+, basal-like, or high grade, its association with poor clinical outcome is independent of these clinicopathological variables. Together, our findings identify a functional role for Cerk in breast cancer recurrence and suggest the clinical utility of agents targeted against this pro-survival pathway.
Introduction
Breast cancer is the most common cancer and the leading cause of cancer-related mortality among women worldwide (1). In general, primary tumors can be effectively treated by the combination of surgery, radiotherapy, and adjuvant treatment with hormonal, chemotherapeutic and/or targeted therapies, as reflected in 5-year survival rates for this disease that approach 90% (1). Despite this favorable short-term prognosis, however, many breast cancer survivors relapse with recurrent disease, sometimes up to 20 years after their initial diagnosis and treatment. Since recurrent breast cancers can be treated, but not cured, the re-emergence of tumors following therapy is principally responsible for the morbidity and mortality associated with this disease.
Recurrent breast cancers arise from the pool of disseminated tumor cells (DTCs), termed minimal residual disease, that survive therapy in their host and DTCs can be identified in a substantial fraction of breast cancer patients at the time of diagnosis, even those with early stage disease (2). Nevertheless, despite the clinical importance of DTCs the mechanisms that underlie their survival and recurrence following therapy are largely unknown.
In order to dissect the mechanisms contributing to breast cancer recurrence, we have employed inducible transgenic mouse models of breast cancer that recapitulate critical features of disease progression as it occurs in patients. In this system, the doxycycline-dependent, mammary-specific expression of oncogenes relevant to human breast cancer, including HER2/neu, c-MYC, Wnt1 and Akt1, results in the formation of invasive mammary adenocarcinomas (3–7). Furthermore, in a manner analogous to the treatment of cancers with targeted therapies such as trastuzumab, mammary tumors regress to a non-palpable state following oncogene down-regulation induced by doxycycline withdrawal. Similar to breast cancer patients, however, mice bearing primary tumors that have spontaneously regressed often develop recurrent tumors following a variable period of dormancy. Moreover, since recurrent tumors typically arise in the absence of re-expression of the initiating oncogene, the survival and growth of recurrent tumor cells is ostensibly driven by the activation of alternate escape pathways, as is commonly observed in cancer patients treated with targeted therapies. As such, genetically engineered mouse models can provide a platform with which to investigate the cellular and molecular mechanisms that contribute to the survival and recurrence of residual tumor cells, thereby identifying essential pathways that may be amenable to therapeutic targeting.
In recent years, sphingolipids have emerged as important regulators of tumor cell survival as well as a number of other pathophysiological processes relevant to cancer, including proliferation, migration and inflammatory signaling. Ceramide is a central molecule in sphingolipid signaling and its accumulation has been demonstrated to play an important role in mediating apoptosis in tumor cells in response chemotherapy, radiotherapy and, more recently, targeted therapy (8–18).
Ceramide can mediate apoptosis in cancer cells both through the extrinsic and intrinsic pathways with structural and signaling roles. Ceramide-enriched membrane platforms in the plasma membrane facilitate the clustering of death receptors and increase formation of death-inducing signaling complexes (DISCs), resulting in apoptotic signaling (19,20). Ceramide can also induce mitochondrial outer membrane permeabilization – a hallmark of the intrinsic pathway of apoptotic signaling – by forming ceramide channels (21,22). Many signaling pathways downstream of ceramide, involving BAX, p38 MAPK, AKT, PP2A, GSK3β, and PKCδ can also enhance apoptotic signaling (reviewed in (23)). Conversely, decreased ceramide levels – either through decreased production or accelerated metabolism – have been described as a mechanism of resistance to cancer therapies (16,24). To this end, a number of ceramide mimetics and inhibitors of enzymes that metabolize ceramide are currently under development as antineoplastic agents (23,25,26).
In light of the importance of ceramide in apoptosis induced by anti-neoplastic therapies, ceramide metabolites such as glucosylceramide, sphingosine-1-phosphate (S1P), and ceramide-1-phosphate (C1P) have been the focus of increasing attention (26,27). In addition to decreasing levels of pro-apoptotic ceramide by virtue of their generation, ceramide metabolites exert anti-apoptotic functions of their own (24).
Glucosylceramide is synthesized by glucosylceramide synthase (GCS), which catalyzes the transfer of a glucose molecule to ceramide, the first step in glycolipid biosynthesis. GCS has been shown to impart resistance to apoptosis to cancer cell lines in the context of cancer therapy in vitro (reviewed in (28)). However, it has not been shown to be explicitly involved in tumorigenesis or tumor progression in vivo.
S1P is generated by the cleavage of ceramide into sphingosine by ceramidase followed by phosphorylation by sphingosine kinase (SphK). The lysosomal ceramidase, acid ceramidase (AC), is overexpressed in many cancers including prostate and head and neck cancers, and has been implicated in resistance to apoptosis induced by treatment with chemotherapeutic agents and C6-ceramide in vitro (29). However, its role in breast cancer remains unclear, as high AC expression has been shown to correlate with better recurrence-free survival in ER-positive breast cancer patients (30). S1P and SphK have been implicated in many cellular processes including cell growth, proliferation, survival and migration. The functions of S1P, AC and SphK in cancer, including in tumorigenesis and resistance to therapy, have been extensively characterized and have led to the development of a number of inhibitors of that pathway (reviewed in (29,31,32)). These findings suggest the potential utility of targeting ceramide metabolism in cancer therapy.
C1P and its synthetic enzyme Ceramide Kinase (Cerk) have been implicated in a number of pro-tumorigenic cellular processes, including inflammation, proliferation and cell survival. Several studies have shown that Cerk is cytoprotective by virtue of its generation of C1P, which signals through the PI3K-Akt pathway (33,34). C1P has also been reported to activate cPLA2, recruiting it to Golgi and plasma membranes, resulting in the cleavage of arachidonic acid that is then converted into a variety of prostaglandins. Prostaglandins have, in turn, been associated with many inflammatory processes as well as regulation of cell growth (35) and cancer (reviewed in (36)). High CERK expression has been reported to be associated with poor recurrence-free survival in women with ER-negative breast cancer (37). Consistent with this, CERK expression has been reported to be associated with aggressive subtypes of breast cancer, including ER-negative status, HER2-positive status, and high tumor grade (37).
Although ceramide and its metabolites have been demonstrated to contribute to cell death following therapy in vitro, whether this pathway plays a functional role in tumor progression in vivo has not been addressed. Moreover, despite being cytoprotective in tumor cell lines in vitro and predicting poor recurrence-free survival in ER-negative breast cancer patients, functional roles for Cerk and C1P have not been established in tumorigenesis or tumor recurrence.
In this study, we identify a functional role for Cerk in breast cancer recurrence. We demonstrate that Cerk is spontaneously up-regulated during tumor recurrence in mice and is rapidly up-regulated in response to HER2/neu pathway inhibition or treatment with Adriamycin. We further establish that Cerk is required for tumor cell survival following HER2/neu down-regulation and is sufficient to promote mammary tumor recurrence. Consistent with observations in mice and with an analogous role for this enzyme in human breast cancer, we find that elevated CERK expression in primary tumors is strongly associated with an increased risk of relapse in breast cancer patients. Taken together, these observations provide the first functional evidence for a role for Cerk in cancer and identify a potential target for the treatment and prevention of breast cancer recurrence.
Materials and Methods
Animals and Orthotopic Recurrence Assays
All animal experiments were performed in accordance with guidelines of the Institutional Animal Care and Use Committee at the University of Pennsylvania. MTB/TAN, MTB/TOM, MTB/TWNT;p53+/− and MTB/TAKT mice were bred and tumors were generated as previously described (3–6). Orthotopic recurrence assays and competition assays were performed as described (7,38). Athymic (nu/nu) mice were obtained from Taconic (Germantown, NY).
Tissue Culture and Reagents
Primary MTB/TAN tumor cells were cultured as described in the presence of doxycycline (7,38). Primary tumor cells were transduced with retrovirally-packaged mCerk cDNA or shRNAs targeting mCerk.
BT474, SKBR3 and BT20 cells were purchased from ATCC. All cells were cultured at 37°C with 5% CO2. BT474 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin and 1% L-glutamine. SKBR3 cells were cultured in McCoy’s 5A modified medium supplemented with 10% FBS, 1% Penicillin/Streptomycin and 1% L-glutamine. BT20 cells were cultured in EMEM medium supplemented with 10% FBS, 1% Penicillin/Streptomycin and 1% L-glutamine.
Plasmids and RNAi
A cDNA for mCerk (MMM1013-202798251, Open Biosystems) was subcloned into the pk1 vector. shRNAs targeting mCerk were designed using RNAi Central (http://katahdin.cshl.org/siRNA/RNAi.cgi?type=shRNA) and purchased from Open Biosystems. The following sequences were used: shCerk1 TGCTGTTGACAGTGAGC GCGCACTTGCTGGTATTCATCAATAGTGAAGCCACAGATGTATTGATGAATACCAG CAAGTGCTTGCCTACTGCCTCGGA; shCerk2 TGCTGTTGACAGTGAGCGAAGATTG TGTGTGTTACTCAACTAGTGAAGCCACAGATGTAGTTGAGTAACACACACAATCTG TGCCTACTGCCTCGGA. Oligonucleotides were cloned into the LMP vector (Open Biosystems) as described (39).
cDNA and shRNA plasmids were virally packaged using Plat-E cells (40), which were transfected with retroviral constructs using Lipofectamine® 2000 (Life Technologies). Supernatant containing viral particles was collected 48 hr post-transfection, filtered and used to infect primary tumor cell lines. Transduction was performed in the presence of 4 µg/ml polybrene (Millipore).
Antibodies
The following antibodies were used as indicated throughout the text: cleaved caspase-3 (Cell Signaling), cleaved PARP (Cell Signaling), Ki67 (Dako), and β-Tubulin (Biogenex).
Immunoblotting
Protein lysates were generated by homogenizing cells in RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP40, 0.5% NaDOC, 0.1% SDS) supplemented with HALT™ Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific).
Secondary antibodies conjugated to IRDye 800CW (LI-COR Biosciences) were used. Odyssey V3.0 system (LI-COR Biosciences) was used to visualize and quantify proteins of interest. Quantification was performed using ImageStudio software (LI-COR Biosciences).
Immunofluorescence and Microscopy
For immunofluorescence studies of tumor tissue from the fluorescent cell competition assay, tumors were snap-frozen in OCT (TissueTek) and cut into 8 µm frozen sections. Sections were fixed in 4% PFA for 10 min then stained with Hoechst 33258 (Sigma). For cleaved caspase-3 staining, tumors were fixed in 4% PFA in PBS overnight then embedded in paraffin. Sections were prepared using a standard xylene-based de-waxing procedure, then subjected to antigen retrieval and stained with appropriate primary and secondary antibodies.
For cell culture experiments, cells were seeded on 4-chamber slides, fixed in 4% PFA for 10 min then permeabilized with 0.5% Triton in PBS for 20 min. Slides were then stained with corresponding primary and secondary antibodies. Fluorescence and immunofluorescence microscopy were performed on a DM 5000B Automated Upright Microscope (Leica). Images were captured with a DFC350 FX monochrome digital camera (Leica).
RNA Isolation and Quantitative RT-PCR
RNA was isolated from tumors using Trizol (Invitrogen) followed by RNeasy® RNA Mini Kit (Qiagen). RNA was isolated from cells using the RNeasy® RNA Mini Kit (Qiagen). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to manufacturer’s instructions. qPCR was performed on the Viia™ 7 Real-Time PCR System (Life Technologies) using 6-carboxyfluorescein-labeled Taqman probes for mCerk, hCerk, mTBP and hTBP (Applied Biosystems). Relative expression levels were calculated using the comparative CT method.
Statistical Analyses
Unpaired Student’s t-tests were utilized to analyze normally distributed data. Mann-Whitney U tests were used when data were not normally distributed. Two-way ANOVAs were used to compare paired data. Log-rank tests were used to analyze survival curves. p-values < 0.05 were considered statistically significant. Hazard ratios with 95% confidence intervals were calculated for all survival curves.
Human Breast Cancer Microarray Data Analysis
We obtained publicly available microarray data and the corresponding clinical annotations from 14 data sets encompassing 2,224 breast cancer patients (41–54). Normalized data were downloaded from NCBI GEO or original authors’ websites. Microarray data were converted to base-2 log scale where necessary. Affymetrix microarray data were re-normalized using Robust Multi-array Average when .CEL files were available.
Association between Cerk mRNA expression and 5-year relapse-free survival was estimated using meta-analysis of univariate Cox proportional hazards regression as previously described (38). Meta-analyses were performed in subsets of patients stratified by ER status, PR status, HER2 status, molecular subtype and tumor grade as described (38). Influence analyses were performed on all significant findings derived by meta-analysis to determine whether the significant result was independent of any single dataset.
The association between Cerk mRNA expression and known prognostic factors of human breast cancer such as ER status, PR status, HER2 status, lymph node status, tumor size, tumor grade and molecular subtype was assessed by ANOVA in pooled microarray datasets as previously described (38).
For each prognostic factor significantly associated with Cerk mRNA expression, we re-assessed the association between Cerk mRNA expression and relapse-free survival after adjusting for the prognostic factor in a multivariate Cox proportional hazards model. The adjusted hazard ratios and confidence intervals for Cerk mRNA expression were aggregated across multiple data sets and assigned an overall significance using meta-analysis as described above.
Results
Cerk is spontaneously up-regulated in recurrent mammary tumors in mice
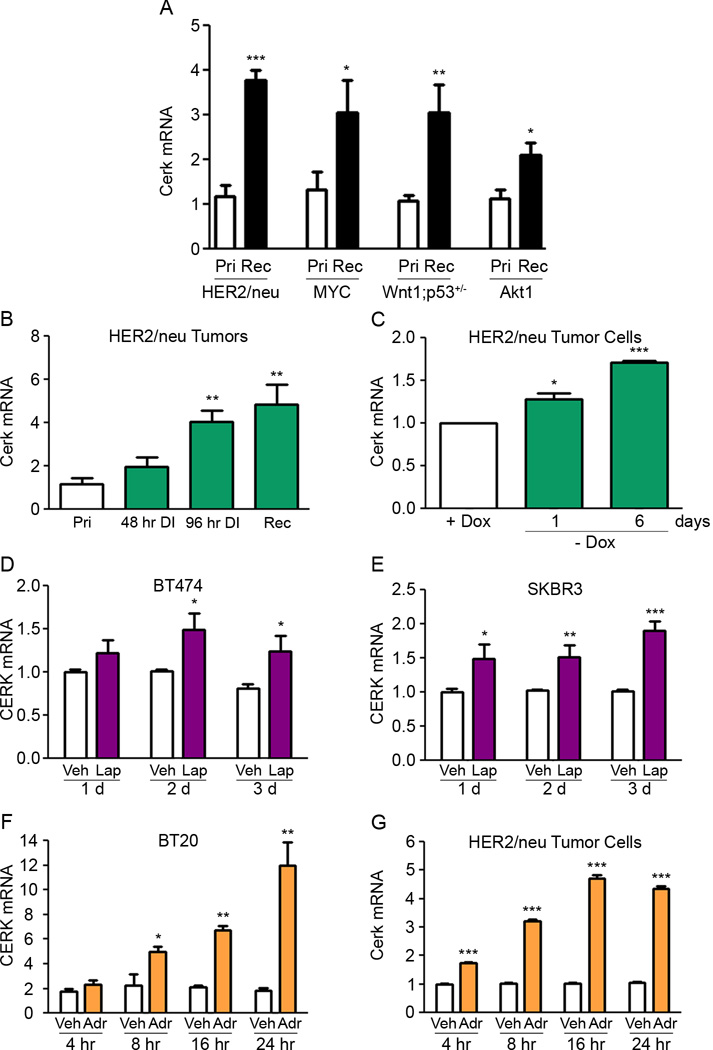
As an initial approach to investigating the potential role of sphingolipid metabolites in tumor progression, we evaluated the expression of enzymes involved in sphingolipid metabolism in primary and recurrent mammary tumors induced by the inducible expression – and subsequent down-regulation – of HER2/neu, c-MYC, Wnt1, and Akt1 in MMTV-rtTA;TetO-HER2/neu, MMTV-rtTA;TetO-MYC, MMTV-rtTA;TetO-Wnt1;p53+/−, and MMTV-rtTA;TetO-Akt1 transgenic mice (3–7). Expression of sphingosine kinase 1, which generates S1P from sphingosine, and glucosylceramide synthase, which generates glucosylceramide from ceramide, were not consistently altered in recurrent compared to primary tumors (data not shown). In contrast, expression of Cerk, which generates C1P from ceramide, was markedly up-regulated in recurrent tumors compared to primary tumors generated by induction of each of the four oncogenes tested (Figure 1A).
Figure 1. Cerk is spontaneously up-regulated in recurrent mammary tumors and following acute HER2/neu down-regulation.
(A) qRT-PCR analysis of Cerk mRNA expression in primary and recurrent HER2/neu, MYC, Wnt1, and Akt1-driven tumors. (B) qRT-PCR analysis of Cerk mRNA expression from MTB/TAN tumors at primary, 48 hr deinduced, 96 hr deinduced, and recurrent time points. (C) qRT-PCR analysis of Cerk mRNA expression in primary MTB/TAN tumor cell line in vitro removed from doxycycline 1 or 6 days. (D) qRT-PCR analysis of CERK mRNA expression in BT474 cells and (E) SKBR3 cells treated with 100 nM Lapatinib or DMSO for 1, 2 or 3 days. qRT-PCR analysis of CERK mRNA expression in (F) BT20 cells and (G) primary MTB/TAN cells treated with 10 µM Adriamycin or vehicle for 4, 8, 16 or 24 hr. *p<0.05, **p<0.01, ***p<0.001.
Cerk is up-regulated following HER2/neu pathway inhibition
To identify the stage of tumor progression at which Cerk up-regulation occurs, tumors were harvested from doxycycline-induced MMTV-rtTA;TetO-HER2/neu (MTB/TAN) mice bearing primary tumors, MTB/TAN mice bearing primary tumors in which the HER2/neu transgene had been de-induced for 48 hr or 96 hr, or MTB/TAN mice bearing primary tumors that had fully regressed and then spontaneously recurred following doxycycline withdrawal and HER2/neu down-regulation.
Quantitative RT-PCR analysis demonstrated Cerk up-regulation in mammary tumors within 48 hr following doxycycline withdrawal, which reached statistical significance by 96 hr and remained up-regulated 4.1-fold in recurrent tumors (Figure 1B). To determine whether alterations in Cerk expression occur in tumor cells subjected to HER2/neu down-regulation, we next evaluated Cerk mRNA levels following oncogene down-regulation in vitro in doxycycline-dependent primary tumor cells derived from a tumor-bearing MTB/TAN mouse. Doxycycline withdrawal resulted in Cerk up-regulation within 24 hr that became more pronounced over time (Figure 1C). This finding suggests that Cerk may be acutely up-regulated in primary tumor cells in response to HER2/neu pathway down-regulation.
To determine whether CERK up-regulation is an evolutionarily conserved response to HER2/neu pathway inhibition in breast cancer cells, we treated two HER2/neu-amplified human breast cancer cell lines, BT474 and SKBR3, with the dual inhibitor of HER2/neu and EGFR, Lapatinib. Treatment with Lapatinib resulted in up-regulation of CERK in each of the HER2/neu amplified cell lines relative to controls within 24 hr (Figure 1D, E), demonstrating that CERK up-regulation is conserved in human and mouse breast cancer cells following HER2/neu pathway inhibition.
To determine whether CERK up-regulation is also seen in response to other types of anti-neoplastic therapy, we treated BT20 human breast cancer cells with the chemotherapeutic agent Adriamycin for increasing periods of time. Adriamycin treatment resulted in marked up-regulation of CERK within 8 hr, peaking at 6.5-fold at 24 hr relative to vehicle-treated controls (Figure 1F). Consistent with this, Cerk was up-regulated within 4 hr in primary MTB/TAN mouse tumor cells treated with Adriamycin, with levels peaking at 4.5-fold at 16 hr (Figure 1G). Together, these data indicate that CERK is rapidly up-regulated in mouse and human mammary tumor cells following HER2/neu pathway inhibition or treatment with Adriamycin.
Cerk promotes tumor cell survival in vitro following HER2/neu down-regulation
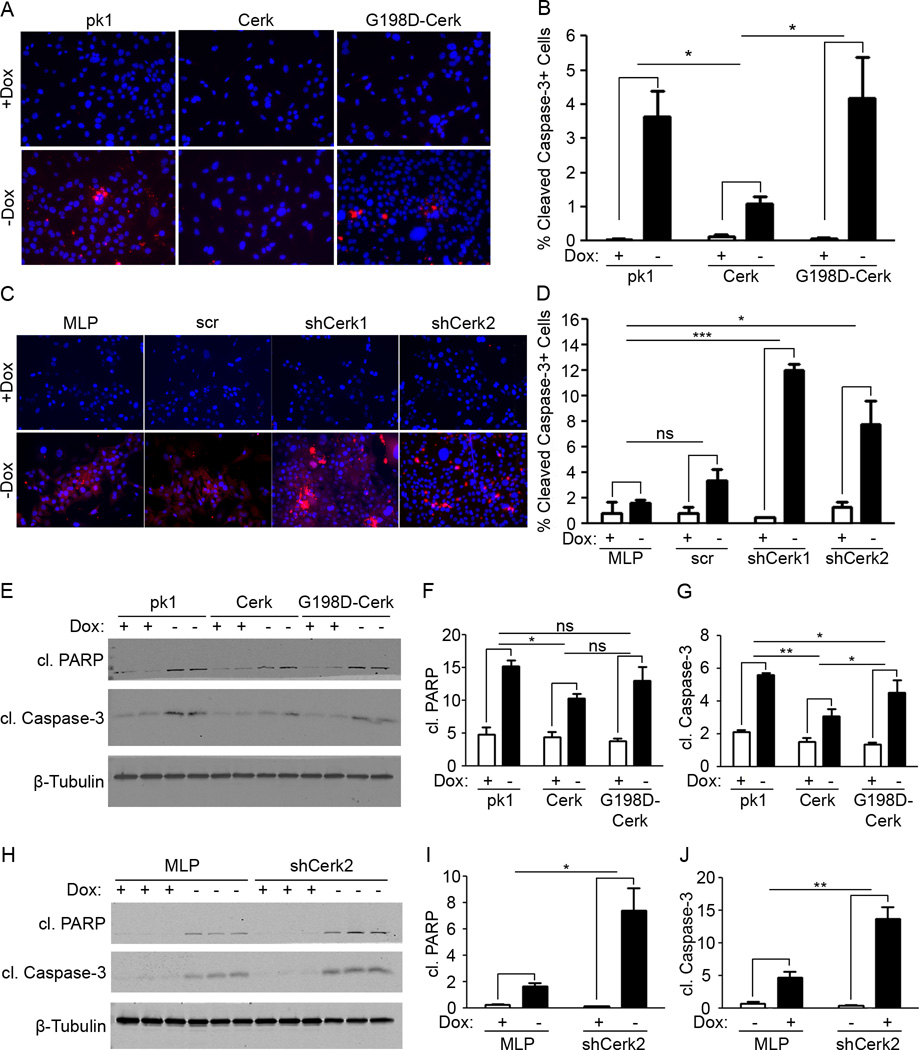
Having observed acute Cerk up-regulation following HER2/neu pathway inhibition in vivo and in vitro, we hypothesized that Cerk up-regulation might contribute to the survival of tumor cells following HER2/neu pathway inhibition. Doxycycline was removed from the culture media of primary MTB/TAN tumor cell lines transduced with expression constructs for wild type Cerk or the kinase dead mutant G198D–Cerk, which are expressed at comparable levels (data not shown), or a control vector. Cells were then stained for cleaved caspase-3 and Ki67.
Cerk overexpressing MTB/TAN cells exhibited markedly reduced staining for cleaved caspase-3 compared to control cells following HER2/neu down-regulation induced by doxycycline withdrawal (Figure 2A, B). In contrast, MTB/TAN cells transduced with kinase dead G198D-Cerk exhibited an increase in cleaved caspase-3 staining following HER2/neu down-regulation that was comparable to control cells (Figure 2A, B). As anticipated, proliferation rates as measured by Ki67 staining fell dramatically upon HER2/neu down-regulation, but did not differ between Cerk, G198D-Cerk, and empty vector controls (Supplementary Figure 1A). These findings suggest that Cerk overexpression is sufficient to suppress apoptosis induced by HER2/neu pathway inhibition and that it does so in a kinase-dependent manner.
Figure 2. Cerk protects tumor cells from apoptosis upon HER2/neu down-regulation in vitro.
(A) Representative fluorescence images and (B) quantification of MTB/TAN primary tumor cell lines expressing empty vector, Cerk or G198D-Cerk withdrawn from doxycycline 72 hr then stained for cleaved caspase-3 (red; Hoechst = blue). (C) Representative fluorescence images and (D) quantification of MTB/TAN primary tumor cell lines expressing empty vector, a control scrambled shRNA, shCerk1 or shCerk2 withdrawn from doxycycline 72 hr then stained for cleaved caspase-3. (E) Western blot of MTB/TAN primary tumor cell lines expressing empty vector, Cerk or G198D-Cerk withdrawn from doxycycline 48 hr, blotted for cleaved PARP, quantified in (F), and cleaved caspase-3, quantified in (G). (H) Western blot of MTB/TAN primary tumor cell lines expressing empty vector, a control scrambled shRNA, or shCerk2 withdrawn from doxycycline 48 hr, blotted for cleaved PARP, quantified in (I), and cleaved caspase-3, quantified in (J).
To determine whether loss of function of Cerk would sensitize cells to apoptosis following HER2/neu down-regulation, we performed an analogous set of experiments in primary MTB/TAN tumor cells expressing either of two Cerk shRNAs, a scrambled control shRNA or an empty vector control. Cerk knockdown cells exhibited markedly increased staining for cleaved caspase-3 compared to control cells following doxycycline withdrawal (Figure 2C, D). Proliferation rates fell dramatically upon HER2/neu down-regulation, but were not significantly different between control and Cerk knockdown cells (Supplementary Figure 1B). These findings suggest that Cerk is required for the suppression of apoptosis following HER2/neu down-regulation.
To confirm the observed increase in apoptosis, we performed Western blots for cleaved caspase-3 and cleaved PARP under the same culture conditions. Consistent with immunofluorescence results, Cerk overexpression reduced levels of cleaved PARP and cleaved caspase-3 following doxycycline withdrawal (Figure 2E, F, G). Kinase dead G198D-Cerk exhibited comparable levels of cleaved PARP and cleaved caspase-3 as controls, indicating that kinase activity is required for Cerk to protect cells from apoptosis. Similarly, Cerk knockdown cell lines exhibited significantly higher levels of cleaved PARP and cleaved caspase-3 than controls following doxycycline withdrawal (Figure 2H, I, J).
Cerk is required for tumor cell survival following HER2/neu down-regulation
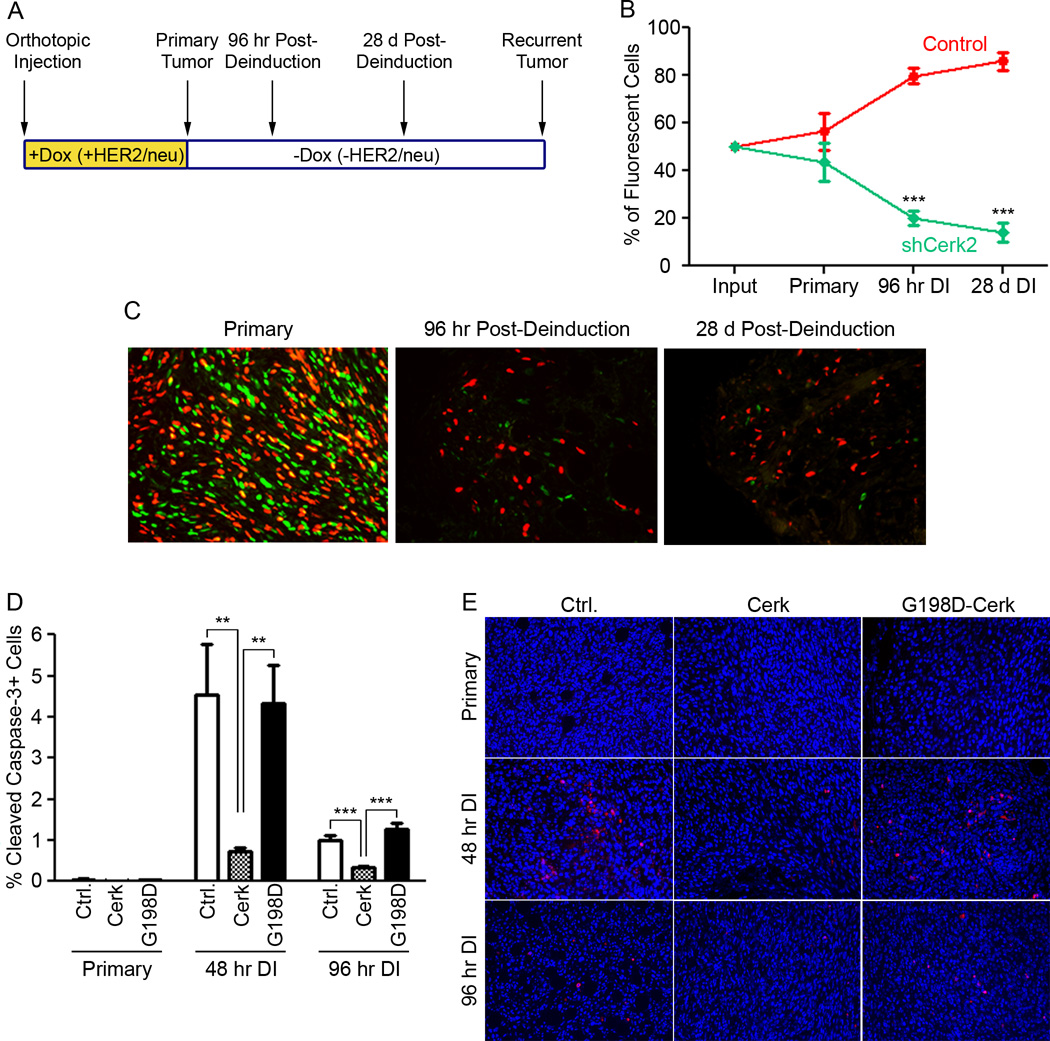
To expand our in vitro findings that Cerk protects tumor cells from apoptosis following HER2/neu pathway inhibition, we sought to test this in vivo. To determine whether Cerk is required for the survival of tumor cells following acute HER2/neu down-regulation, we performed an orthotopic fluorescent cell competition assay using an isogenic pair of doxycycline-dependent MTB/TAN tumor cell lines that differed only in Cerk expression. MTB/TAN primary tumor cells expressing one of two shRNAs targeting Cerk were labeled with an H2B-eGFP reporter, whereas MTB/TAN cells transduced with a control vector were labeled with an H2B-mCherry reporter. These two fluorescent populations of cells were injected into the mammary glands of nu/nu mice at a 1:1 ratio and allowed to form orthotopic primary tumors in the presence of doxycycline and HER2/neu expression. Doxycycline was then withdrawn to initiate tumor regression and the ratio of eGFP-labeled Cerk knockdown cells to mCherry-labeled control cells was determined in primary tumors, residual tumor cells 96 hr following HER2/neu down-regulation, and residual tumor cells 28 days following HER2/neu down-regulation by fluorescence microscopy performed on histological sections (Figure 3A).
Figure 3. Cerk protects tumor cells from apoptosis upon HER2/neu down-regulation in vivo.
(A) Schematic of competition assay and timing of doxycycline treatment and tumor harvest. (B) Percentage of eGFP-positive and mCherry-positive cells was determined at timepoints in (A) up to 28 days post-deinduction. Data represent mean ± SEM. (C) Representative fluorescence images of primary tumors, 96 hr deinduced tumors and residual lesions 28 days post-deinduction. (D) Quantification of cleaved caspase-3 staining in primary orthotopic empty vector, Cerk and G198D-Cerk tumors at 48 and 96 hr post-deinduction. (E) Representative fluorescence images of primary tumors, 48 hr and 96 hr deinduced tumors stained for cleaved caspase-3 (red; Hoechst = blue).
Cerk shRNA-expressing cells were neither selected for, nor against, during the outgrowth of primary orthotopic tumors (Figure 3B, C, Supplementary Figure 2C), indicating that Cerk knockdown does not confer a selective advantage or disadvantage during primary tumorigenesis in the presence of HER2/neu expression. In contrast, eGFP-labeled Cerk knockdown cells were strongly selected against within 96 hr following doxycycline withdrawal and HER2/neu down-regulation, as these cells comprised only 20–25% of the fluorescent tumor cells at this time point (Figure 3B, C, Supplementary Figure 2C). By 28 days following doxycycline withdrawal, Cerk knockdown cells comprised only 14–20% of fluorescent cells, indicating modest further negative selection against Cerk knockdown cells (Figure 3B, C, Supplementary Figure 2C). Tumors containing control mCherry-labeled cells and control eGFP-labeled cells maintained an approximately 1:1 ratio throughout tumor progression through all these time points (Supplementary Figure 2B). These findings indicate that cells in which Cerk has been knocked down are rapidly and persistently selected against following HER2/neu down-regulation, but not in actively growing primary tumor cells expressing HER2/neu.
Cerk promotes tumor cell survival in vivo following HER2/neu down-regulation
One mechanism by which Cerk-expressing cells could be selected for following HER2/neu down-regulation would be if Cerk provides a cell-autonomous survival advantage in this context. To address this possibility, we generated mice bearing orthotopic tumors derived from MTB/TAN primary tumor cells overexpressing wild type Cerk, kinase dead G198D–Cerk, or a control vector. We then performed immunofluorescence staining for cleaved caspase-3 on histological sections from primary tumors on doxycycline or in which HER2/neu had been acutely down-regulated for 48 or 96 hr.
Primary tumors from control, Cerk and G198D-Cerk overexpressing cohorts exhibited comparable, low levels of cleaved caspase-3 staining in the presence of HER2/neu expression, and a marked increase in cleaved caspase-3 staining was observed 48 hr following doxycycline withdrawal (Figure 3D). Strikingly, however, tumors overexpressing Cerk displayed a significantly blunted apoptotic response to HER2/neu down-regulation at 48 hr post-deinduction and cleaved caspase-3 levels remained lower in Cerk overexpressing tumors compared to controls at 96 hr (Figure 3D). In contrast, tumors overexpressing the kinase dead mutant G198D-Cerk did not blunt the apoptotic response and exhibited high levels of cleaved caspase-3 comparable to control tumors following HER2/neu down-regulation (Figure 3D). These findings indicate that Cerk up-regulation protects tumor cells from apoptosis following HER2/neu pathway inhibition, suggesting a cellular mechanism for the observation that cells with Cerk knockdown are selected against following oncogene down-regulation in vivo.
Cerk promotes mammary tumor recurrence in vivo
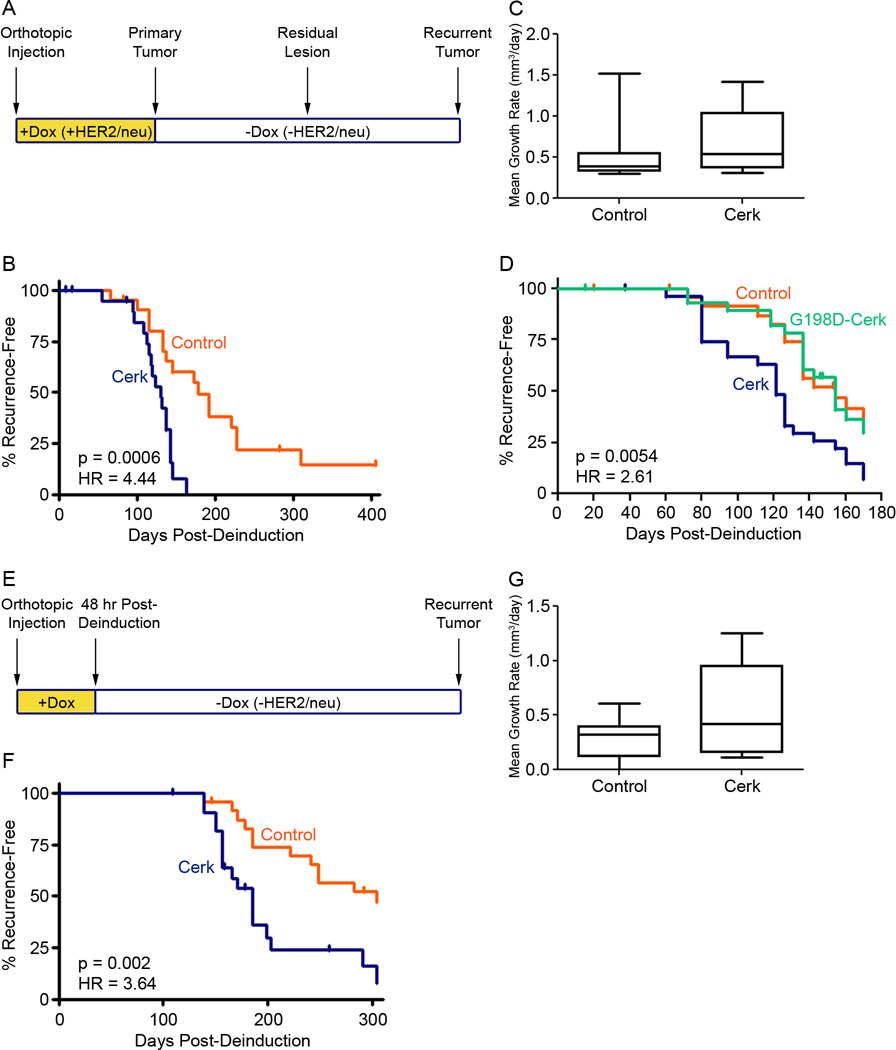
Cerk’s ability to inhibit apoptosis following HER2/neu down-regulation in tumor cells in vivo and in vitro, coupled with its spontaneous up-regulation in recurrent mammary tumors in multiple genetically engineered mouse models, suggested the possibility that Cerk might promote breast cancer recurrence by enhancing the survival of tumor cells following therapy. To test this hypothesis, we injected primary MTB/TAN tumor cells transduced with a Cerk expression vector or a control vector into the mammary fat pads of nu/nu mice maintained on doxycycline. Following primary tumor formation, mice were withdrawn from doxycycline to induce oncogene down-regulation and tumor regression (Figure 4A). All tumors regressed to a non-palpable state, irrespective of Cerk expression status. Mice were then monitored for tumor recurrence.
Figure 4. Cerk promotes mammary tumor recurrence in the MTB/TAN mouse model.
(A) Schematic of recurrence assay and timing of doxycycline treatment. (B) Recurrence-free survival of nu/nu mice harboring primary orthotopic tumors from MTB/TAN primary cell lines expressing empty vector or Cerk induced to regress by doxycycline withdrawal. (C) Mean growth rate of control and Cerk overexpressing recurrent tumors. (D) Recurrence-free survival of nu/nu mice harboring primary orthotopic tumors from MTB/TAN cell lines expressing empty vector, wildtype Cerk or the kinase dead mutant G198D-Cerk induced to regress by doxycycline withdrawal. (E) Schematic of modified recurrence assay with no primary tumor formation. (F) Recurrence-free survival of nu/nu mice harboring MTB/TAN primary cell lines expressing empty vector, Cerk or G198D-Cerk withdrawn from doxycycline 48 hr post-injection. (G) Mean growth rate of control and Cerk overexpressing recurrent tumors.
Consistent with our hypothesis, Cerk expression in MTB/TAN tumor cells markedly accelerated tumor recurrence, with median time to recurrence decreasing from 178 days for control tumors to 130 days in Cerk overexpressing tumors (Figure 4B, HR = 4.44, p = 0.0006). However, mean growth rate of recurrent tumors was not found to be different between control and Cerk overexpressing cohorts, indicating increased rates of proliferation are unlikely to be responsible for the observed difference in latency to tumor recurrence (Figure 4C).
To determine whether the ability of Cerk to promote tumor recurrence was dependent upon Cerk kinase activity, we repeated this experiment with the addition of a cohort of mice injected with primary tumor cells expressing the G198D kinase dead allele of Cerk. As before, tumors overexpressing wild type Cerk exhibited significantly accelerated tumor recurrence with median latency at 121 days (Figure 4D, HR = 2.61, p = 0.0054). However, in contrast to the effects of overexpressing wild type Cerk, and consistent with our in vitro findings indicating that Cerk kinase activity is required for its ability to promote tumor cell survival following HER2/neu down-regulation, overexpression of G198D-Cerk did not accelerate the rate of mammary tumor recurrence compared to control tumors, with a median time to recurrence of 154 days in each cohort.
Though suggestive, the above experiments could not rule out the possibility that the increased rate of relapse observed for wild type Cerk overexpressing tumors was due to effects of Cerk expression during primary tumor formation. That is, if Cerk expression resulted in the formation of primary tumors with different properties than control tumors, this could explain the reduced time to recurrence, rather than effects of Cerk following HER2/neu down-regulation. To address the potential confounding effects of Cerk expression during primary tumor formation, we modified the orthotopic recurrence assay to eliminate primary tumor formation, thereby isolating the effects of Cerk on tumor cell survival and recurrence.
Recipient mice pretreated with doxycycline were injected with control or Cerk overexpressing cells and then withdrawn from doxycycline after 48 hr, before primary tumors had formed (Figure 4E). Consistent with our prior results, Cerk overexpression accelerated the rate of mammary tumor recurrence and reduced the median time to recurrence from 304 days in controls to 185 days in the presence of Cerk expression (Figure 4F, HR = 3.64, p = 0.002). Again, mean growth rate was not found to be different between control and Cerk overexpressing cohorts (Figure 4G). These results indicate that Cerk can promote mammary tumor recurrence following HER2/neu down-regulation and that this effect is unlikely to be due to Cerk-induced alterations in primary tumor formation.
Elevated CERK expression is associated with an increased risk of recurrence in women with breast cancer
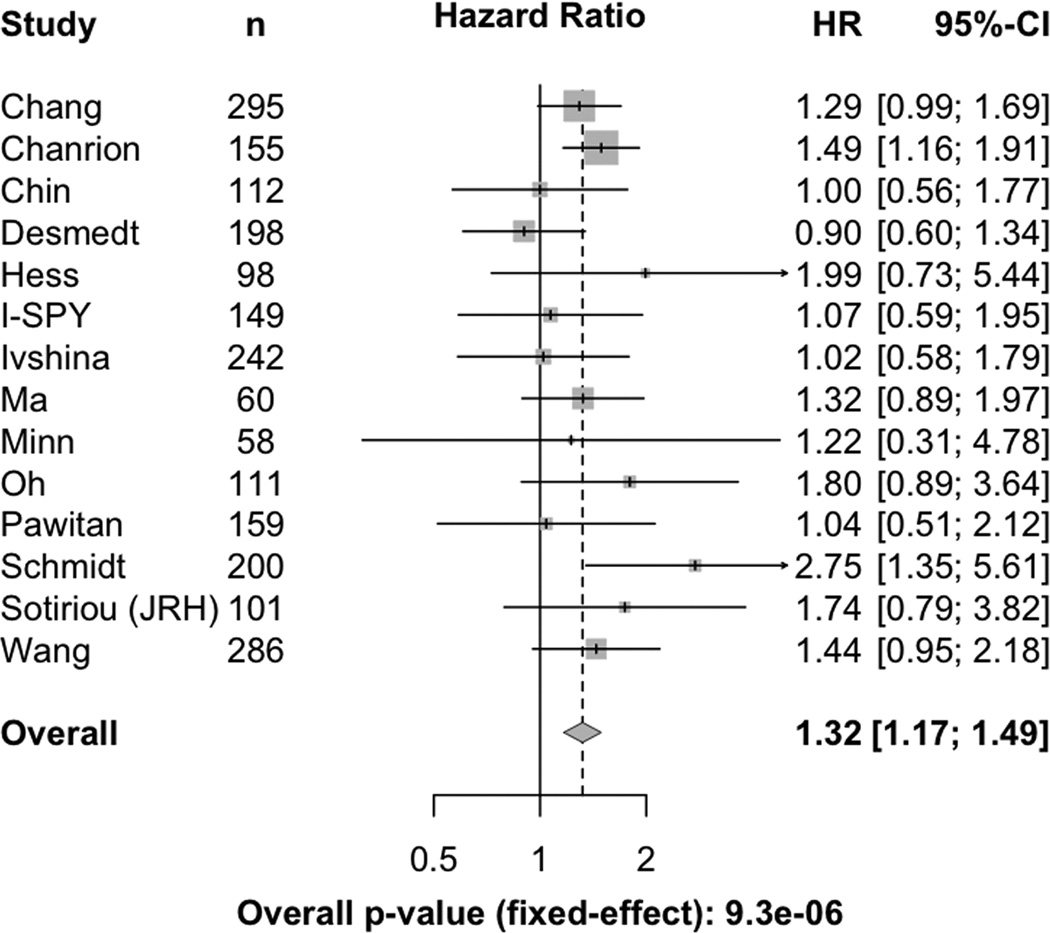
A prior report suggested that elevated CERK expression is associated with poor prognosis in ER-negative breast cancer patients (37). To confirm and extend this analysis, we interrogated publicly available human breast cancer expression datasets corresponding to 2,224 patients with tumors of mixed ER status in order to evaluate the association of CERK expression with recurrence-free survival in a meta-analysis. Using a Cox proportional hazards (PH) model, we found that women with primary tumors expressing high levels of CERK exhibited an increased risk of tumor recurrence within five years of diagnosis (Figure 5, HR = 1.32 [1.17 – 1.49]).
Figure 5. High CERK expression predicts decreased relapse-free survival in women with breast cancer.
Forest plot representation of 5-year survival estimates and hazard ratios for relapse-free survival of individual datasets of human breast cancer patients.
We next asked whether CERK expression is associated with aggressive subtypes of human breast cancer. Association studies revealed that CERK is expressed at higher levels in ER-negative compared to ER-positive, and PR-negative compared to PR-positive, tumors. CERK expression was also associated with HER2-positive status as assessed by immunohistochemistry (Supplementary Figure 3A–C). Evaluation of CERK expression as a function of molecular subtype revealed that CERK expression is higher in the basal and ErbB2 subtypes compared to the luminal A and luminal B subtypes (Supplementary Figure 3D). CERK expression was also associated with high tumor grade (Supplementary Figure 3E), but not with tumor size or lymph node status (data not shown).
Since ER-negative, PR-negative, HER2-positive, basal-like and high grade tumors are each associated with a poor prognosis, we wished to determine whether the association between CERK expression and relapse-free survival in breast cancer patients was independent of the association between CERK expression and these aggressive tumor subsets. To address this, we performed multivariate Cox proportional hazards regression in combination with meta-analyses and adjusted for each of these variables individually. In each case, we found that the association between elevated CERK expression and decreased recurrence-free survival remained significant after adjusting for ER status, PR status, HER2 status, molecular subtype or tumor grade (Supplementary Figure 4A – E). Influence analysis performed on each significant result obtained by meta-analysis confirmed that these results were independent of any single dataset (Supplementary Figure 5).
In aggregate, our results indicate that CERK expression in human breast cancer is associated with an increased risk of recurrence within five years, and is independent of the association between CERK expression and aggressive subtypes of human breast cancer.
Discussion
The importance of ceramide metabolism in cancer has become of increasing interest in recent years (23,26,27). However, relatively little functional data have emerged in describing ceramide, its metabolites and the metabolic enzymes responsible for their generation in tumorigenesis and tumor progression.
In a number of tumor cell lines, Cerk has been reported to exert cytoprotective effects in a variety of in vitro contexts, including serum starvation, TNFα signaling and UV irradiation. To date, however, no evidence has existed for a functional role for Cerk in tumor cell survival in vivo, tumor progression or tumor recurrence. Using genetically engineered mouse models, human breast cancer cell lines and expression data from breast cancer patients, we have now identified a functional role for Cerk as a regulator of tumor cell survival and breast cancer recurrence following HER2/neu down-regulation.
We found that Cerk is spontaneously up-regulated during the process of tumor recurrence in mammary tumors driven by four different oncogenic pathways. Notably, Cerk was up-regulated as early as 96 hr following oncogene down-regulation in mice bearing HER2/neu-driven mammary tumors and, consistent with this, human HER2/neu-amplified breast cancer cell lines also rapidly up-regulated Cerk following HER2/neu pathway inhibition. Indicative of a role for Cerk in the context of HER2/neu inhibition, tumor cells in which Cerk had been knocked down were strongly selected against within 96 hr following HER2/neu down-regulation, with further selection continuing into the dormant phase of tumor regression at 28 days post-deinduction. Whether tumors comprised solely of Cerk knockdown cells would similarly be selected against will require further study. That this is likely to be the case, however, is suggested by additional in vivo and in vitro analyses, which revealed that enforced Cerk expression inhibited apoptosis, whereas Cerk knockdown enhanced apoptosis, following HER2/neu down-regulation in the absence of a competing population of cells.
In accord with the anti-apoptotic effects of Cerk in tumor cells following HER2/neu down-regulation, Cerk expression in orthotopic primary mammary tumor cells promoted tumor recurrence, indicating a functional role for Cerk in this stage of tumor progression. In aggregate, our findings indicate that Cerk is required for tumor cell survival following HER2/neu down-regulation. Ostensibly, this may result in a larger pool of surviving residual tumor cells that can potentially give rise to recurrent tumors. These findings therefore suggest a therapeutic opportunity whereby inhibition of Cerk in concert with treatment with targeted therapies may enhance tumor cell death, reduce the reservoir of residual tumor cells, and thereby prevent tumor recurrence.
In agreement with its functional role in mammary tumor recurrence elucidated in mice, CERK was associated with increased risk of recurrence in human breast cancer patients. CERK expression was associated with aggressive subtypes of breast cancer, including ER-negative, PR-negative, HER2-positive, high grade, and ErbB2 and basal-like tumors. However, the association between CERK and relapse-free survival was independent of its association with these aggressive subsets of tumors. As such, while CERK was previously reported to be associated with decreased recurrence-free survival in ER-negative breast cancer patients (37), our findings extend this analysis by demonstrating that CERK is associated with poor outcome in all breast cancer patients and does so independently of its association with individual aggressive prognostic factors.
Of note, we did not observe a selective effect against Cerk knockdown cells in primary tumors in an orthotopic competition assay, suggesting that Cerk expression in actively proliferating HER2/neu-driven tumor cells may not be rate limiting for growth and/or survival. This, in turn, suggests that rate-limiting pathways regulating tumor cell growth and survival may differ depending upon the stage of tumor progression. As such, pharmacological agents that may be effective in treating primary breast cancers may have little effect in women with minimal residual disease or recurrent breast cancer. As such, the identification of pharmacological targets unique to these stages of tumor progression will be essential for improving long-term survival in this disease. Consequently, the inhibition of pathways that allow cells to survive therapy and eventually recur may be key to preventing the persistence of disseminated tumor cells and subsequence relapse. In aggregate, our findings may identify one such pathway for cell survival in breast cancer cells, as well as define a subset of human breast cancers with a high likelihood of relapse. Additionally, our data underscore the importance of ceramide metabolism and sphingolipids in cancer drug resistance and relapse.
Supplementary Material
Acknowledgements
We thank Jianping Wang for technical support in immunofluorescence studies.
Financial support: NIH R01 CA 164490, NIH 2U01 CA105490, NIH R01 CA98371, NIH R01 CA143296, NIH R01 CA148774, Komen Foundation KG110973, Breast Cancer Research Foundation.
Footnotes
The authors declare that they have no conflicts of interest relevant to this work.
References
- 1.Maxmen A. The hard facts. Nature. 2012;485:S50–S51. doi: 10.1038/485S50a. [DOI] [PubMed] [Google Scholar]
- 2.Bednarz-Knoll N, Alix-Panabières C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res BCR. 2011;13:228. doi: 10.1186/bcr2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boxer RB, Jang JW, Sintasath L, Chodosh LA. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–586. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.D’Cruz CM, Gunther EJ, Boxer RB, Hartman JL, Sintasath L, Moody SE, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med. 2001;7:235–239. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- 5.Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 2003;17:488–501. doi: 10.1101/gad.1051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–461. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 7.Moody SE, Perez D, Pan T, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, et al. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 9.Baran Y, Bielawski J, Gunduz U, Ogretmen B. Targeting glucosylceramide synthase sensitizes imatinib-resistant chronic myeloid leukemia cells via endogenous ceramide accumulation. J Cancer Res Clin Oncol. 2011;137:1535–1544. doi: 10.1007/s00432-011-1016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 11.Bruno AP, Laurent G, Averbeck D, Demur C, Bonnet J, Bettaïeb A, et al. Lack of ceramide generation in TF-1 human myeloid leukemic cells resistant to ionizing radiation. Cell Death Differ. 1998;5:172–182. doi: 10.1038/sj.cdd.4400330. [DOI] [PubMed] [Google Scholar]
- 12.Camgoz A, Gencer EB, Ural AU, Avcu F, Baran Y. Roles of ceramide synthase and ceramide clearence genes in nilotinib-induced cell death in chronic myeloid leukemia cells. Leuk Lymphoma. 2011;52:1574–1584. doi: 10.3109/10428194.2011.568653. [DOI] [PubMed] [Google Scholar]
- 13.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffrézou JP, Levade T, Bettaïeb A, Andrieu N, Bezombes C, Maestre N, et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 1996;15:2417–2424. [PMC free article] [PubMed] [Google Scholar]
- 15.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 16.Senchenkov A, Litvak DA, Cabot MC. Targeting Ceramide Metabolism—a Strategy for Overcoming Drug Resistance. J Natl Cancer Inst. 2001;93:347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 17.Strum JC, Small GW, Pauig SB, Daniel LW. 1-beta-D-Arabinofuranosylcytosine stimulates ceramide and diglyceride formation in HL-60 cells. J Biol Chem. 1994;269:15493–15497. [PubMed] [Google Scholar]
- 18.Zhu Q, Wang Z, Ji C, Cheng L, Yang Y, Ren J, et al. C6-ceramide synergistically potentiates the anti-tumor effects of histone deacetylase inhibitors via AKT dephosphorylation and α-tubulin hyperacetylation both in vitro and in vivo. Cell Death Dis. 2011;2:e117. doi: 10.1038/cddis.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassmé H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, et al. CD95 Signaling via Ceramide-rich Membrane Rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 20.Miyaji M, Jin Z-X, Yamaoka S, Amakawa R, Fukuhara S, Sato SB, et al. Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J Exp Med. 2005;202:249–259. doi: 10.1084/jem.20041685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siskind LJ, Kolesnick RN, Colombini M. Ceramide Channels Increase the Permeability of the Mitochondrial Outer Membrane to Small Proteins. J Biol Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morad SAF, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer. 2013;13:51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Adan-Gokbulut A, Kartal-Yandim M, Iskender G, Baran Y. Novel Agents Targeting Bioactive Sphingolipids for the Treatment of Cancer. Curr Med Chem. 2012;20:108–122. [PubMed] [Google Scholar]
- 26.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 27.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y-Y, Hill RA, Li Y-T. Chapter Three - Ceramide Glycosylation Catalyzed by Glucosylceramide Synthase and Cancer Drug Resistance. In: Norris James S., editor. Adv editor Res Cancer [Internet] Academic Press; 2013. pp. 59–89. [cited 2013 Nov 6] Available from: http://www.sciencedirect.com/science/article/pii/B9780123942746000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeidan YH, Jenkins RW, Korman JB, Liu X, Obeid LM, Norris JS, et al. Molecular targeting of acid ceramidase: implications to cancer therapy. Curr Drug Targets. 2008;9:653–661. doi: 10.2174/138945008785132358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruckhäberle E, Holtrich U, Engels K, Hanker L, Gätje R, Metzler D, et al. Acid ceramidase 1 expression correlates with a better prognosis in ER-positive breast cancer. Climacteric J Int Menopause Soc. 2009;12:502–513. doi: 10.3109/13697130902939913. [DOI] [PubMed] [Google Scholar]
- 31.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Elojeimy S, Turner LS, Mahdy AEM, Zeidan YH, Bielawska A, et al. Acid ceramidase inhibition: a novel target for cancer therapy. Front Biosci J Virtual Libr. 2008;13:2293–2298. doi: 10.2741/2843. [DOI] [PubMed] [Google Scholar]
- 33.Gangoiti P, Granado MH, Alonso A, Goñi FM, Gómez-Muñoz A. Implication of ceramide, ceramide 1-phosphate and sphingosine 1-phosphate in tumorigenesis. Transl Oncogenomics. 2008;3:81–98. [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez-Muñoz A, Kong JY, Parhar K, Wang SW, Gangoiti P, González M, et al. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett. 2005;579:3744–3750. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara S, Rumi MAK, Okuyama T, Kinoshita Y. Effect of prostaglandins on the regulation of tumor growth. Curr Med Chem Anti-Cancer Agents. 2004;4:379–387. doi: 10.2174/1568011043352902. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, DuBois RN. Prostaglandins and Cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruckhäberle E, Karn T, Rody A, Hanker L, Gätje R, Metzler D, et al. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J Cancer Res Clin Oncol. 2009;135:1005–1013. doi: 10.1007/s00432-008-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez JV, Pan T-C, Ruth J, Feng Y, Zhou A, Pant D, et al. Par-4 Downregulation Promotes Breast Cancer Recurrence by Preventing Multinucleation following Targeted Therapy. Cancer Cell. 2013;24:30–44. doi: 10.1016/j.ccr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paddison PJ, Cleary M, Silva JM, Chang K, Sheth N, Sachidanandam R, et al. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat Methods. 2004;1:163–167. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 40.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 41.Chang HY, Nuyten DSA, Sneddon JB, Hastie T, Tibshirani R, Sørlie T, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanrion M, Negre V, Fontaine H, Salvetat N, Bibeau F, Grogan GM, et al. A Gene Expression Signature that Can Predict the Recurrence of Tamoxifen-Treated Primary Breast Cancer. Clin Cancer Res. 2008;14:1744–1752. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin S-F, Wang Y, Thorne NP, Teschendorff AE, Pinder SE, Vias M, et al. Using array-comparative genomic hybridization to define molecular portraits of primary breast cancers. Oncogene. 2007;26:1959–1970. doi: 10.1038/sj.onc.1209985. [DOI] [PubMed] [Google Scholar]
- 44.Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:3207–3214. doi: 10.1158/1078-0432.CCR-06-2765. [DOI] [PubMed] [Google Scholar]
- 45.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic Complete Response Predicts Recurrence-Free Survival More Effectively by Cancer Subset: Results From the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, et al. Pharmacogenomic Predictor of Sensitivity to Preoperative Chemotherapy With Paclitaxel and Fluorouracil, Doxorubicin, and Cyclophosphamide in Breast Cancer. J Clin Oncol. 2006;24:4236–4244. doi: 10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 47.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 48.Ma X-J, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–6745. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24:1656–1664. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- 51.Pawitan Y, Bjöhle J, Amler L, Borg A-L, Egyhazi S, Hall P, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res BCR. 2005;7:R953–R964. doi: 10.1186/bcr1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt M, Victor A, Bratzel D, Boehm D, Cotarelo C, Lebrecht A, et al. Long-term outcome prediction by clinicopathological risk classification algorithms in node-negative breast cancer--comparison between Adjuvant!, St Gallen, and a novel risk algorithm used in the prospective randomized Node-Negative-Breast Cancer-3 (NNBC-3) trial. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2009;20:258–264. doi: 10.1093/annonc/mdn590. [DOI] [PubMed] [Google Scholar]
- 53.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Klijn JGM, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.