Abstract
Background
Some studies have suggested reductions in blood pressure (BP) with statin treatment, particularly in persons with hypertension. Randomized trial evidence is limited.
Methods
We performed a randomized, double-blind, placebo-controlled trial with equal allocation to simvastatin, 20 mg; pravastatin sodium, 40 mg; or placebo for 6 months. Nine hundred seventy-three men and women without known cardiovascular disease or diabetes mellitus, with low-density lipoprotein cholesterol screening levels of 115 to 190 mg/dL, had assessment of systolic and diastolic BP (SBP and DBP, respectively). Blood pressure values were compared for placebo vs statins by intention-to-treat (ITT) analysis. Additional analyses were performed that (1) were confined to subjects with neither high baseline BP (SBP >140 mm Hg or DBP >90 mm Hg) nor receiving BP medications, to exclude groups in whom BP medications or medication changes may have influenced results, and (2) separately evaluated simvastatin and pravastatin (vs placebo). The time course of BP changes after statin initiation and the effect of stopping statins on BP were examined.
Results
Statins modestly but significantly reduced BP relative to placebo, by 2.2 mm Hg for SBP (P = .02) and 2.4 mm Hg for DBP (P<.001) in ITT analysis. Blood pressure reductions ranged from 2.4 to 2.8 mm Hg for both SBP and DBP with both simvastatin and pravastatin, in those subjects with full follow-up, and without potential for influence by BP medications (ie, neither receiving nor meriting BP medications).
Conclusions
Reductions in SBP and DBP occurred with hydrophilic and lipophilic statins and extended to normotensive subjects. These modest effects may contribute to the reduced risk of stroke and cardiovascular events reported on statins.
Trial Registration
clinicaltrials.gov Identifier: NCT00330980
Some studies have suggested reductions in blood pressure (BP) with statin treatment, particularly in patients with hypertension. Many studies reporting BP reductions have been correlational, uncontrolled, tested against other active drugs with uncertain impact on BP, unblinded, nonrandomized, or without assessment of statistical significance.1–9 Some double-blind, randomized studies failed to show an effect but had a small sample size.10,11 Two small, randomized, double-blind, crossover studies12,13 have shown significant (P < .05) benefit, but to our knowledge there are no published articles showing reductions in BP with statins in sizeable randomized trials.
The BP assessments performed in the double-blind, randomized, University of California, San Diego (UCSD) Statin Study were used to assess the impact of randomized assignment to statins vs placebo on systolic and diastolic BP (SBP and DBP, respectively).
METHODS
DESIGN OVERVIEW, SETTING, AND PARTICIPANTS
The UCSD Statin Study was a randomized, double-blind, placebo-controlled trial of 6 months’ duration.
Subjects were 973 men and women from Southern California. A total of 1016 screenees were eligible to participate in the larger trial by virtue of having had a low-density lipoprotein cholesterol (LDL-C) level of 115 to 190 mg/dL (inclusive) at screening; no known cardiovascular disease or diabetes mellitus; and no factors that would preclude 8 months of participation in the study (to convert LDL-C to micromoles per liter, multiply by 0.0259). Blood pressure was not an entry criterion and did not influence eligibility to participate. The 973 subjects for the present BP study are the proper randomized subset of the larger sample in whom BP was assessed at screening. A more complete description of the purpose, eligibility criteria, and study design is presented elsewhere.14,15 The study protocol was approved by the UCSD human subjects committee, and all subjects gave written informed consent to participate. The recruitment period was from April 2000 through July 2003, and all subjects were seen at UCSD.
RANDOMIZATION AND INTERVENTIONS
A computer-generated, blocked, randomization sequence stratified on sex was designed by the statistician (H.L.W.) and provided to the study pharmacist who used the sequence to match assigned treatment to sequentially numbered bottles. Sequential subjects who met eligibility criteria and gave informed consent were assigned sequential study identification numbers and received the bottle with the corresponding number. Subjects received simvastatin, 20 mg; pravastatin sodium, 40 mg; or placebo with equal (33%) probability. A more complete description of procedures is presented elsewhere.14,15 Simvastatin, 20 mg, and pravastatin sodium, 40 mg, were selected as the most lipophilic and hydrophilic statins, given at dosages approximately equivalent in terms of expected LDL-C reduction (the study drug was to be taken at bedtime).16 Neither subjects nor study staff were aware of subject assignment during subjects’ participation.
OUTCOMES AND FOLLOW-UP
Visits occurred at screening, baseline, and at 1 and 6 months during treatment. Subjects received an 8-month (2 months after discontinuation) follow-up visit. Primary outcomes from this study will be reported elsewhere. Brachial BP was assessed but was not a designated primary or secondary end point. Blood pressure was obtained using the auscultatory method by trained psychometrists, in the morning, with subjects seated at rest and arm at chest height using cuff sizes appropriate to the subject. Using calibrated aneroid sphygmomanometers, staff identified SBP and DBP as phase I and phase V of the Karotkoff sounds (unless heard to a BP of 0, wherein phase IV was recorded as the DBP value).17 Although BP was not a primary outcome in the UCSD Statin Study,14,15 it was measured at the screening visit (prior to randomization) and at each on-treatment and posttreatment visit. All subjects whose screening value exceeded 140 mm Hg SBP or 90 mm Hg DBP were given a letter to bring to their physicians stipulating their elevated BP (n=210 subjects). Because of presumptive evidence suggesting a possible benefit of statins to BP and the relative ease of analysis of this end point, we received permission from the Data Safety Monitoring Board to unblind and analyze BP first, and the process of data cleaning for BP commenced prior to the last, 8-month (off-treatment) visit of the last subject. Lack of duplicate BP measurement at each time point must be considered in the context of lack of duplicate measurement of many other variables, including lipids, that show variability: this lack of duplicate measurement was nondifferential across treatment groups, and variance resulting from measurement variability, although potentially eroding power (and producing bias toward the null), can be offset by increased sample size.
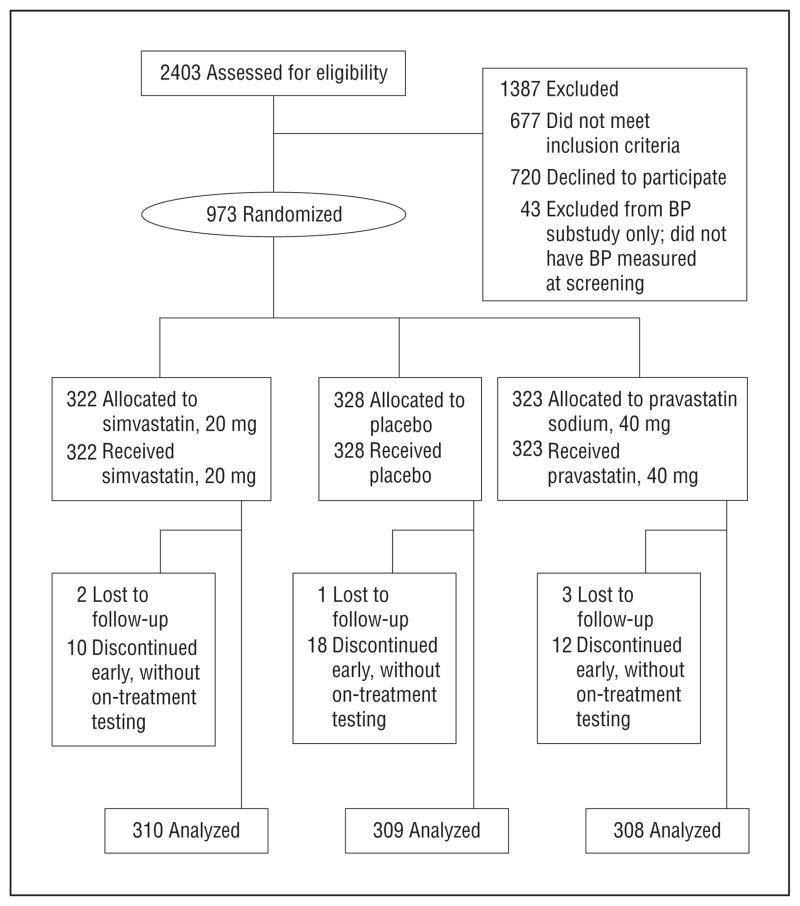
Forty-three of 1016 subjects (4.2%) did not have BP measured at baseline, primarily owing to subjects’ time constraints (Figure 1). The screening visit at which baseline BP was assessed comprised subjects’ first trip to the study site, and some subjects were delayed in reaching the site or abridged their visit owing to other commitments. However, randomization was blinded to and independent of presence or value of screening BP. Thus, the subgroup with BP measurements (n=973 subjects) is equivalent to a proper randomized substudy and forms the sample analyzed herein. (Baseline comparability is shown across treatment arms in this group.)
Figure 1.
Flowchart of participants.
Change scores in BP represented the primary BP end point, subtracting baseline BP from final on-treatment BP. This may enhance power by enabling subjects to serve as their own controls.
STATISTICAL ANALYSES
Analyses were conducted using Stata statistical software (version 8.0; StataCorp, College Station, Texas). The primary BP measure for the present analysis was the change from baseline (screening) BP to 6-month (final on-statin) BP. All analyses were performed for both SBP and DBP.
Baseline comparability across treatment arms was assessed in subjects to address comparability by treatment assignment. We performed t tests to compare the mean change in SBP and DBP across treatment arms, provided that comparability across treatment arms was present at baseline. If baseline disparities across treatment arms were identified, then we performed regression analysis to allow adjustment for baseline disparities. Secondary analyses evaluated the effect on BP separately for pravastatin and for simvastatin vs placebo.
All subjects who received an on-treatment visit (except 3 [2 who received pravastatin and 1 who received simvastatin]) had an on-treatment BP measured and were included in the intention-to-treat analysis. In those with high measured BP at baseline, an “intervention” in the form of a letter to the subjects’ physicians was given—an intervention expected to contribute variance unrelated to treatment assignment, eroding power in samples in which this group is included. In addition, statin effects on BP could differentially affect implementation or modification of BP medications in the statin vs placebo groups in persons with baseline high BP or those receiving BP medications. Therefore, analyses were performed in subjects without high BP at baseline, those with neither high BP nor BP medications at baseline, and those who had never received BP medications to evaluate subjects in whom BP treatment could not have influenced results and for whom results cannot be attributed to statin interactions with BP medications. We examined subjects with higher-normal or lower-normal BP, within the subhypertensive range, by splitting SBP and DBP at the sample median among those with BP lower than 140/90 mm Hg at baseline, selecting the median to maximize power for each analysis. This suite of analyses permitted assessment of the possibility that statin effects occurred primarily at a higher BP as some have proposed12 or solely through drug interactions with hypertensive agents (eg, the “synergistic” effects others have hypothesized4). Finally, because of reports that the endothelial benefits of statins (thought to underlie BP benefits) may fail to extend to subjects with low high-density lipoprotein cholesterol (HDL-C),18 coupled with an absence of observed BP benefits in the CARE study19 (comprising a low HDL-C sample), we analyzed BP effects in those with HDL-C values above and below the median HDL-C levels. The time course of the effect of statins on BP was assessed by examining the change in BP from baseline to 1 month of treatment, to 6 months of treatment, and to 2 months after treatment was discontinued.
RESULTS
Subject participation occurred from April 2000 to March 2004. Analysis supported baseline comparability of analyzed treatment arms on characteristics including age, sex, ethnicity, lipid profiles, glucose, smoking status, SBP, and fraction with elevated BP at baseline (Table 1). However, the baseline difference in DBP comparing pravastatin to placebo was not significant; therefore, t tests were complemented by regression analysis, adjusting for baseline DBP values.
Table 1.
Randomized Substudy Groups Compared at Baseline
| Variable | Placebo | Statin (Pravastatin Sodium or Simvastatin) | P Value, Statin vs Placebo | Pravastatin | P Value, Pravastatin vs Placebo | Simvastatin | P Value, Simvastatin vs Placebo |
|---|---|---|---|---|---|---|---|
| SBP, screening, mm Hg | 126.5 | 126.8 | .73 | 126.3 | .91 | 127.3 | .49 |
| DBP, screening, mm Hg | 74.0 | 75.2 | .05a | 75.4 | .05 | 75.0 | .15 |
| Total cholesterol, mg/dL | 229.3 | 230.0 | .71 | 233.2 | .10 | 226.9 | .31 |
| LDL-C, mg/dL | 150.5 | 151.2 | .68 | 152.8 | .28 | 149.7 | .68 |
| HDL-C, mg/dL | 52.6 | 52.2 | .70 | 53.5 | .48 | 50.9 | .16 |
| Triglycerides, mg/dL | 134.1 | 134.7 | .91 | 134.7 | .91 | 134.6 | .93 |
| Glucose, mg/dL | 89.9 | 90.1 | .75 | 90.0 | .99 | 90.3 | .59 |
| Male, % | 68.0 | 68.1 | .96 | 67.9 | .98 | 68.4 | .91 |
| Age, y | 57.7 | 56.8 | .31 | 57.4 | .72 | 56.3 | .29 |
| White, % | 82.2 | 80.9 | .63 | 80.8 | .66 | 81.0 | .69 |
| Smoker, % | 8.1 | 7.9 | .93 | 8.1 | .99 | 7.7 | .87 |
Abbreviations: DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol, HDL-C, and LDL-C to millimoles per liter, multiply by 0.0259; to convert glucose and triglycerides to millimoles per liter, multiply by 0.0555 and 0.0113, respectively.
The value P = .05 is for the test viewed in isolation; viewing the tests as an ensemble, we obtain a Bonferroni P value of P = .55 (ie, 0.05 × 11 tests) for the multiple hypothesis test of no differences between placebo and statin.
As Table 2 shows, in the intention-to-treat analysis, significant though modest BP reductions were present in the combined statin group relative to placebo, for SBP and for DBP. (The value P =.05 is for the test viewed in isolation. Viewing the tests as an ensemble, we obtain a Bonferroni P value of P =.55; ie, 0.05 × 11 tests, for the multiple hypothesis test of no differences between placebo and statin.) This finding was stronger when subjects with high BP at baseline were excluded (this group received a letter to their physicians which may have led to treatment changes that, although nondifferential by arm, may have amplified variance and attenuated significance). Reductions in BP were seen for subjects without hypertension—those with neither high BP at baseline nor receiving BP medications. This held true, separately, for those with SBP (or DBP) above or below the sample median.
Table 2.
BP Difference, Statin Groups vs Placebo
| Analysis Type |
t Test of Difference in Mean Change (vs Placebo)
|
Regression, Adjusted for Corresponding Baseline (SBP or DBP)
|
||||
|---|---|---|---|---|---|---|
| Statins | Pravastatin Sodiuma | Simvastatina | Statins | Pravastatin | Simvastatin | |
| Intention to treatb | ||||||
| SBP | 2.2 | 1.5 | 2.9 | 2.0 | 1.6 | 2.4 |
| P value | .02 | .20 | .009 | .009 | .095 | .006 |
| DBP | 2.4 | 2.3 | 3.0 | 1.8 | 1.5 | 2.0 |
| P value | <.001 | .002 | <.001 | .001 | .02 | .003 |
| Excluding high BP at baseline (to whom BP “referral” was given)c,d | ||||||
| SBP | 2.9 | 2.5 | 3.4 | 2.7 | 2.3 | 3.0 |
| P value | .001 | .02 | .001 | .002 | .03 | .002 |
| DBP | 2.5 | 2.2 | 2.8 | 2.1 | 1.8 | 2.4 |
| P value | <.001 | .006 | <.001 | .001 | .01 | .001 |
| Excluding high BP or BP medications at baselinec,e | ||||||
| SBP | 2.6 | 2.2 | 3.0 | 2.2 | 1.8 | 2.7 |
| P value | .006 | .048 | .005 | .009 | .07 | .006 |
| DBP | 2.5 | 2.3 | 2.7 | 2.0 | 1.7 | 2.3 |
| P value | <.001 | .006 | .002 | .002 | .02 | .002 |
| Full 6 mo, no BP medicationsf | ||||||
| SBP | 2.7 | 2.6 | 2.8 | 2.2 | 2.3 | 2.3 |
| P value | .009 | .03 | .02 | .02 | .04 | .03 |
| DBP | 2.5 | 2.5 | 2.6 | 2.1 | 1.9 | 2.3 |
| P value | .002 | .008 | .007 | .003 | .02 | .005 |
| BP below median (no HTN, never prescribed BP medications) | ||||||
| SBP if baseline ≤122 | 2.7 | NA | NA | 2.9 | NA | NA |
| P value | .02 | NA | NA | .009 | NA | NA |
| DBP if baseline ≤72 | 3.0 | NA | NA | 2.5 | NA | NA |
| P value | .001 | NA | NA | .002 | NA | NA |
| BP above median (no HTN, never prescribed BP medications) | ||||||
| SBP if baseline ≥122g | 2.8 | NA | NA | 2.2 | NA | NA |
| P value | .03 | NA | NA | .08 | NA | NA |
| DBP if baseline ≥72 | 1.8 | NA | NA | 1.4 | NA | NA |
| P value | .06 | NA | NA | .10 | NA | NA |
| DBP if SBP baseline ≤122 | NA | NA | NA | 2.2 | NA | NA |
| P value | NA | NA | NA | .004 | NA | NA |
| DBP if SBP baseline ≥122 | NA | NA | NA | 1.4 | NA | NA |
| P value | NA | NA | NA | .06 | NA | NA |
| HDL-C above median, 50 mg/dL | ||||||
| SBP | 4.7 | NA | NA | 4.1 | NA | NA |
| P value | <.001 | NA | NA | .001 | NA | NA |
| DBP | 2.8 | NA | NA | 2.2 | NA | NA |
| P value | .01 | NA | NA | .02 | NA | NA |
| HDL-C below median, 50 mg/dL | ||||||
| SBP | 1.5 | NA | NA | 1.2 | NA | NA |
| P value | .30 | NA | NA | .30 | NA | NA |
| DBP | 2.7 | NA | NA | 2.1 | NA | NA |
| P value | .01 | NA | NA | .02 | NA | NA |
Abbreviations: BP, blood pressure; DBP, diastolic BP; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; NA, not assessed; SBP, systolic BP.
SI conversion factor: To convert HDL-C to millimoles per liter, multiply by 0.0259.
Not significant (P>.05): simvastatin vs pravastatin.
Placebo group, n=309; pravastatin group, n=308; simvastatin group, n=310.
Intention to treat within this proper randomized subgroup. “BP referral” refers to letter to physician; see “Methods” section.
For this analysis, the number of subjects in each group are as follows: placebo group, n=244; statin group, n=486; pravastatin group, n=241; simvastatin group, n=245.
For this analysis, the number of subjects in each group are as follows: placebo group, n=207; statin group, n=445; pravastatin group, n=227; simvastatin group, n=218.
For this analysis, the number of subjects in each group are as follows: placebo group, n=171; statin group, n=371; pravastatin group, n=181; simvastatin group, n=190.
Loses significance for BP>median if analysis includes subjects for whom BP medications were prescribed at any time during study participation; remains significant for BP<median.
Table 2 also suggests possible effect modification for SBP based on HDL-C, splitting the sample at the median baseline HDL-C (50 mg/dL). (To convert HDL-C to micromoles per liter, multiply by 0.0259.) The reductions in SBP were indeed focused in the high HDL-C group (mean HDL-C, 65 mg/dL) relative to the low HDL-C group (mean HDL-C, 40 mg/dL). In contrast to SBP, however, DBP reductions were not focused in the high HDL-C subgroup.
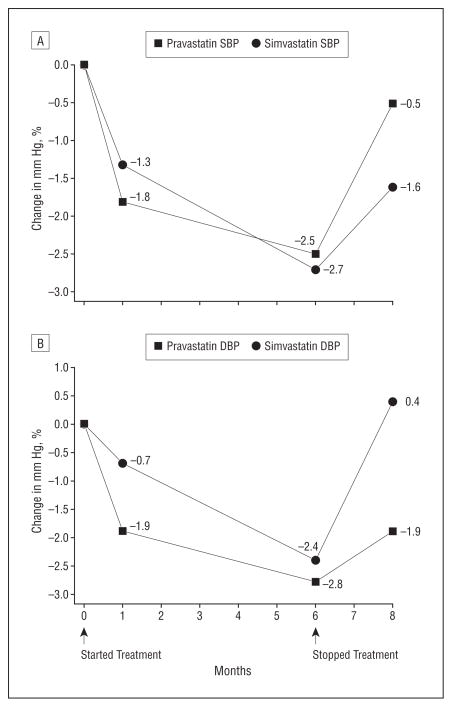
Figure 2A (SBP) and Figure 2B (DBP) show the change in BP from baseline to each time point for the 2 statins relative to placebo, selecting for illustration those subjects without high BP (>140/90 mm Hg) at baseline, who did not receive BP medications, and who had BP measured through the 8-month visit. At 1 month, nonsignificant (P>.05) reductions in SBP and DBP in the statin groups relative to placebo group were seen. By 6 months of treatment, both SBP and DBP differences from baseline showed significant reductions (P<.05) in each of the statin groups relative to placebo. By 2 months after discontinuation of treatment, these changes had dissipated.
Figure 2.
Difference between the statin and placebo groups. A, Systolic blood pressure (SBP), change from baseline. This figure shows values for subjects who were not hypertensive at baseline, were never prescribed BP medications, and had BP measured through the 8-month visit. This mitigates the influence of changes distinct from treatment assignment across time. The change values differed significantly from placebo (P <.05) at month 6 (only) for both pravastatin sodium and simvastatin. Standard errors for pravastatin: month 1, 1.3; month 6, 1.2; month 8, 1.2. Standard errors for simvastatin: month 1, 1.2; month 6, 1.2; month 8, 1.2. P values for pravastatin: month 1, P = .18; month 6, P = .04; month 8, P = .69. P values for simvastatin: month 1, P = .30; month 6, P = .02; month 8, P = .18. B, DBP, change from baseline. This figure shows values for subjects who were not hypertensive at baseline, were never prescribed BP medications, and had BP measured through the 8-month visit. This mitigates the influence of changes distinct from treatment assignment across time. The change values differed significantly from placebo (P ≤.01) at month 6 (only) for both pravastatin and simvastatin. Standard errors for pravastatin: month 1, 0.98; month 6, 0.96; month 8, 1.0. Standard errors for simvastatin: month 1, 0.97; month 6, 0.98; month 8, 1.0. P values for pravastatin: month 1, P = .06; month 6, P = .004; month 8, P = .06. P values for simvastatin: month 1, P = .46; month 6, P = .01; month 8, P = .66.
COMMENT
Both simvastatin and pravastatin reduced SBP and DBP substantially, although the mean absolute magnitude of the change was modest in this largely nonhypertensive sample receiving relatively low statin dosages. To our knowledge, this is the first large, randomized, double-blind, placebo-controlled trial to report that statins lower both SBP and DBP relative to placebo; that the effect extends to persons with “prehypertension,”20 those with lower-normal BP, and persons not receiving BP-lowering medications; and that it occurs and seems to be comparable for similar lipid-lowering potencies of the most hydrophilic and lipophilic statins. Our data enrich information on the time course of the effect: BP reductions with statins were suggestive and not significant in this sample at 1 month of treatment but were manifest and significant at 6 months (Figure 2). At 2 months after statins were discontinued, the difference in BP between the statin and placebo groups had dissipated. These findings extend our understanding of the BP effects of statins, as underscored by a previous statement suggesting that “statins may decrease elevated but not normal blood pressure”12(p1284); the present study modifies that conclusion. Moreover, because those not receiving BP medications showed reductions in SBP and DBP with statin vs placebo that were significant (see Table 2 for P values), the effect of statins on BP could not be attributed purely to a drug interaction with existing antihypertensive medications.4
Mechanisms by which statins may reduce BP include up-regulation and/or activation of endothelial nitric oxide synthase (a potent vasodilator)21,22 and improvements in endothelial function and flow-mediated vasodilation.23–29 Statins may reportedly induce down-regulation of angiotensin II type-I receptor expression.30 Of note, statins’ benefits to endothelial function and vasodilation are thought to be founded on statins’ antioxidant effects27,31 and have been absent or attenuated in some groups—such as persons with low HDL-C or diabetes mellitus.18,32–34 In Cholesterol and Recurrent Events (CARE) study subjects—who showed no BP reduction with statins35—15% had diabetes mellitus and the mean HDL-C level was low (39 mg/dL). In contrast, our sample, evidencing a modest but significant BP reduction (see Table 2 for P values), excluded those with diabetes mellitus and had a higher mean HDL-C level (52 mg/dL). Subgroup analysis supported preferential SBP reduction in those with higher HDL-C. However, the same was not seen for DBP.
Large statin trials have seldom commented on statin effects on BP. For trials of longer duration, more BP reduction in the statin group may result over time in more BP medication use in the placebo group, undermining the ability to detect a statin effect on BP. Successful randomization produces comparability at baseline but cannot protect against differential clinical decisions arising as a result of changes induced by the treatment.
Alternatively, BP effects may genuinely fail to be sustained because physiological responses to statin effects evolve over time (eg, tachyphylaxis). Finally, through effect modification, a true absence of effect may characterize studies that differ from this study in subject selection and/or statin drug or dosage. Future studies can further evaluate the impact of statins on BP with attention to these issues.
One large, randomized, double-blind trial showed no significant effect on BP in nonhyperlipidemic subjects with cardiovascular disease.35 A recent meta-analysis of randomized controlled trials of statins reporting effects on BP, with an aggregate sample size of 828 subjects, reported significant reduction in systolic BP (only).36 The largest of the 20 included studies had a sample size of 100, raising potential concerns about publication bias among small studies. Other recent reviews and meta-analyses have also supported effects but have further emphasized the methodological shortcomings of most of the published literature in this area.37,38 A larger, parallel-design study has reported benefit to BP but has been published only as an abstract.39
We included BP assessment in our randomized controlled trial to address an important finding reported previously in observational and small crossover studies. Blood pressure was not a primary end point. Although duplicate BP measurements were not performed at each study time point, in contrast to the clinical setting (in which duplicate measurements are requisite to improve precision of the estimate for the individual), in the research setting, precision can be enhanced for group level inferences through increased sample size, averaging single values over multiple subjects. Indeed, randomized controlled trials commonly assess the impact of interventions on outcomes that have considerable test-retest variability, and this variability is typically overcome by boosting sample size rather than by duplicate assessment. Any nondifferential measurement error incurred as a result of measurement variability arising from single BP measurements produces expected bias toward the null, making the findings reported herein conservative.
The study addresses persons without diabetes mellitus, known cardiovascular disease, or extreme high or low LDL-C level; findings may not extend to excluded groups. Subjects with hypertension, those most in need of BP reduction, were not strongly represented; however, effects in persons with high-normal BP suggest clinical relevance given reported implications of prehypertension.20,40–42
Some issues were not addressed in this study and will require different study designs. These include the impact of different statin dosages, other statin drugs, and longer duration of treatment.
This study adds to our understanding of the effects of statins, currently the best-selling prescription drugs in the world. It provides the first published confirmation in a large, parallel-design, randomized controlled trial of a finding that has been reported with other study designs: that effects of statins extend to reduction in a second primary cardiovascular risk factor, namely, BP. It suggests that this effect extends to lipophilic and hydrophilic statins. The reduction in BP seen with statins may contribute—among other identified factors43—to some of the “rapid” cardiovascular benefits of statins, arising too swiftly to be explained by effects of statins on plaque accumulation.44 Statin-induced reductions in BP, although modest, could contribute to reductions in transient ischemic attacks and stroke reported with statins,45–50 because stroke incidence, although inconsistently related to LDL-C, is strongly related to BP.
Acknowledgments
Funding/Support: This study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, grant RO1 HL63055-05. The UCSD General Clinical Research Center (NIH MO1 RR00827) also helped to support this effort.
Role of the Sponsors: The funding sources did not influence the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Footnotes
Financial Disclosure: None reported.
Previous Presentations: An abstract of this article was presented at the American Heart Association’s Scientific Sessions; November 7, 2004; New Orleans, Louisiana; and published in Circulation (2004;110[17]:III-402) and Circulation (2005;112[9]:123).
Additional Contributions: We thank Julie Denenberg, MS, for data management and review of data integrity; Marcella Evans, BS, for administrative and editorial assistance; and Hanh Nguyen and Jersey Neilson for graphical assistance. We also thank the UCSD General Clinical Research Center. We are grateful to all of the Statin Study staff members—and subjects—who made this study possible.
Author Contributions: Dr Golomb had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Golomb, Dimsdale, White, and Criqui. Acquisition of data: Golomb, Ritchie, and Criqui. Analysis and interpretation of data: Golomb, Dimsdale, White, and Criqui. Drafting of the manuscript: Golomb, Ritchie, and Dimsdale. Critical revision of the manuscript for important intellectual content: Golomb, Dimsdale, White, and Criqui. Statistical analysis: Golomb and White. Obtained funding: Golomb. Administrative, technical, and material support: Ritchie, Dimsdale, and Criqui. Study supervision: Golomb.
References
- 1.Borghi C, Prandin MG, Costa FV, Bacchelli S, Degli Esposti D, Ambrosioni E. Use of statins and blood pressure control in treated hypertensive patients with hypercholesterolemia. J Cardiovasc Pharmacol. 2000;35(4):549–555. doi: 10.1097/00005344-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Borghi C, Gaddi A, Ambrosioni E, Dormi A. Improved blood pressure control in hypertensive patients treated with statins. J Am Coll Cardiol. 2001;37(suppl A):233A–234A. [Google Scholar]
- 3.Prasad GV, Ahmed A, Nash MM, Zaltzman JS. Blood pressure reduction with HMG-CoA reductase inhibitors in renal transplant recipients. Kidney Int. 2003;63(1):360–364. doi: 10.1046/j.1523-1755.2003.00742.x. [DOI] [PubMed] [Google Scholar]
- 4.Spósito AC, Mansur AP, Coelho OR, Nicolau JC, Ramires JA. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril) Am J Cardiol. 1999;83 (10):1497–1499. A8. doi: 10.1016/s0002-9149(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 5.Kanbay M, Yildirir A, Bozbas H, et al. Statin therapy helps to control blood pressure levels in hypertensive dyslipidemic patients. Ren Fail. 2005;27(3):297–303. [PubMed] [Google Scholar]
- 6.Abetel G, Poget PN, Bonnabry JP. Hypotensive effect of an inhibitor of cholesterol synthesis (fluvastatin): a pilot study [in French] Schweiz Med Wochenschr. 1998;128(7):272–277. [PubMed] [Google Scholar]
- 7.Leibovitz E, Hazanov N, Zimlichman R, Shargorodsky M, Gavish D. Treatment with atorvastatin improves small artery compliance in patients with severe hypercholesterolemia. Am J Hypertens. 2001;14(11 pt 1):1096–1098. doi: 10.1016/s0895-7061(01)02210-5. [DOI] [PubMed] [Google Scholar]
- 8.Terzoli L, Mircoli L, Raco R, Ferrari AU. Lowering of elevated ambulatory blood pressure by HMG-CoA reductase inhibitors. J Cardiovasc Pharmacol. 2005;46(3):310–315. doi: 10.1097/01.fjc.0000175432.56789.e6. [DOI] [PubMed] [Google Scholar]
- 9.Danaog lu Z, Kultursay H, Kayikcioglu M, Can L, Payzin S. Effect of statin therapy added to ACE-inhibitors on blood pressure control and endothelial functions in normolipidemic hypertensive patients. Anadolu Kardiyol Derg. 2003;3(4):331–337. [PubMed] [Google Scholar]
- 10.O’Callaghan CJ, Krum H, Conway EL, et al. Short term effects of pravastatin on blood pressure in hypercholesterolaemic hypertensive patients. Blood Press. 1994;3(6):404–406. doi: 10.3109/08037059409102294. [DOI] [PubMed] [Google Scholar]
- 11.Derosa G, Mugellini A, Ciccarelli L, Fogari R. Randomized, double-blind, placebo-controlled comparison of the action of orlistat, fluvastatin, or both an anthropometric measurements, blood pressure, and lipid profile in obese patients with hypercholesterolemia prescribed a standardized diet. Clin Ther. 2003;25(4):1107–1122. doi: 10.1016/s0149-2918(03)80070-x. [DOI] [PubMed] [Google Scholar]
- 12.Glorioso N, Troffa C, Filigheddu F, et al. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34(6):1281–1286. doi: 10.1161/01.hyp.34.6.1281. [DOI] [PubMed] [Google Scholar]
- 13.Ferrier KE, Muhlmann MH, Baguet JP, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39(6):1020–1025. doi: 10.1016/s0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- 14.Golomb BA, Criqui MH, White H, Dimsdale JE. Conceptual foundations of the UCSD Statin Study: a randomized controlled trial assessing the impact of statins on cognition, behavior, and biochemistry. Arch Intern Med. 2004;164(2):153–162. doi: 10.1001/archinte.164.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golomb BA, Criqui MH, White HL, Dimsdale JE. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178–202. doi: 10.1016/j.cct.2003.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80(1):106–107. [PubMed] [Google Scholar]
- 17.Williams B, Poulter NR, Brown MJ, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18(3):139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 18.Wang TD, Chen WJ, Lin JW, Cheng CC, Chen MF, Lee YT. Efficacy of fenofibrate and simvastatin on endothelial function and inflammatory markers in patients with combined hyperlipidemia: relations with baseline lipid profiles. Atherosclerosis. 2003;170(2):315–323. doi: 10.1016/s0021-9150(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 19.Sacks FM, Pfeffer MA, Moye LA, et al. Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335 (14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and up-regulates endothelial nitric oxide synthase in mice. Stroke. 2001;32(4):980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 22.Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol. 1999;33(1):234–241. doi: 10.1016/s0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- 23.Leu HB, Wu CC, Wu TC, Lin SJ, Chen JW. Fluvastatin reduces oxidative stress, decreases serum monocyte chemotactic protein-1 level and improves endothelial function in patients with hypercholesterolemia. J Formos Med Assoc. 2004;103(12):914–920. [PubMed] [Google Scholar]
- 24.Järvisalo MJ, Toikka JO, Vasankari T, et al. HMG CoA reductase inhibitors are related to improved systemic endothelial function in coronary artery disease. Atherosclerosis. 1999;147(2):237–242. doi: 10.1016/s0021-9150(99)00189-6. [DOI] [PubMed] [Google Scholar]
- 25.Hognestad A, Aukrust P, Wergeland R, et al. Effects of conventional and aggressive statin treatment on markers of endothelial function and inflammation. Clin Cardiol. 2004;27(4):199–203. doi: 10.1002/clc.4960270405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111(18):2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 27.John S, Schneider MP, Delles C, Jacobi J, Schmieder RE. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am Heart J. 2005;149(3):473. doi: 10.1016/j.ahj.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Karatzis E, Lekakis J, Papamichael C, et al. Rapid effect of pravastatin on endothelial function and lipid peroxidation in unstable angina. Int J Cardiol. 2005;101(1):65–70. doi: 10.1016/j.ijcard.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Jorge PA, Almeida EA, Ozaki MR, Jorge M, Carneiro A. Effects of atorvastatin, fluvastatin, pravastatin, and simvastatin on endothelial function, lipid peroxidation, and aortic atherosclerosis in hypercholesterolemic rabbits [in Portuguese] Arq Bras Cardiol. 2005;84(4):314–319. doi: 10.1590/s0066-782x2005000400008. [DOI] [PubMed] [Google Scholar]
- 30.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100(21):2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 31.Mulder HJ, Schalij MJ, van der Laarse A, Hollaar L, Zwinderman AH, Bruschke AV. Improvement of serum oxidation by pravastatin might be one of the mechanisms by which endothelial function in dilated coronary artery segments is ameliorated. Atherosclerosis. 2003;169(2):309–315. doi: 10.1016/s0021-9150(03)00197-7. [DOI] [PubMed] [Google Scholar]
- 32.Beishuizen ED, Tamsma JT, Jukema JW, et al. The effect of statin therapy on endothelial function in type 2 diabetes without manifest cardiovascular disease. Diabetes Care. 2005;28(7):1668–1674. doi: 10.2337/diacare.28.7.1668. [DOI] [PubMed] [Google Scholar]
- 33.Tantikosoom W, Thinkhamrop B, Kiatchusakul S, Jarernsiripornkul N, Srinakarin J, Ojongpian S. Randomized trial of atorvastatin in improving endothelial function in diabetics without prior coronary disease and having average cholesterol level. J Med Assoc Thai. 2005;88(3):399–406. [PubMed] [Google Scholar]
- 34.ter Avest E, Abbink EJ, Holewijn S, de Graaf J, Tack CJ, Stalenhoef AF. Effects of rosuvastatin on endothelial function in patients with familial combined hyperlipidaemia (FCH) Curr Med Res Opin. 2005;21(9):1469–1476. doi: 10.1185/030079905X61910. [DOI] [PubMed] [Google Scholar]
- 35.Tonelli M, Sacks F, Pfeffer M, Lopez-Jimenez F, Jhangri GS, Curhan G. Effect of pravastatin on blood pressure in people with cardiovascular disease. J Hum Hypertens. 2006;20(8):560–565. doi: 10.1038/sj.jhh.1002036. [DOI] [PubMed] [Google Scholar]
- 36.Strazzullo P, Kerry SM, Barbato A, Versiero M, D’Elia L, Cappuccio FP. Do statins reduce blood pressure? a meta-analysis of randomized, controlled trials. Hypertension. 2007;49(4):792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 37.Mangat S, Agarwal S, Rosendorff C. Do statins lower blood pressure? J Cardiovasc Pharmacol Ther. 2007;12(2):112–123. doi: 10.1177/1074248407300380. [DOI] [PubMed] [Google Scholar]
- 38.Sarafidis PA, Kanaki AI, Lasaridis AN. Statins and blood pressure: is there an effect or not? J Clin Hypertens (Greenwich) 2007;9(6):460–467. doi: 10.1111/j.1524-6175.2007.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulter N, Sever PS. Do statins lower blood pressure? evidence from the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA) [abstract 1905] Circulation. 2004;110(suppl III):III-402. [Google Scholar]
- 40.Liszka HA, Mainous AG, III, King DE, Everett CJ, Egan BM. Prehypertension and cardiovascular morbidity. Ann Fam Med. 2005;3(4):294–299. doi: 10.1370/afm.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006;119(2):133–141. doi: 10.1016/j.amjmed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Hsia J, Margolis KL, Eaton CB, et al. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115(7):855–860. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 43.Yildirir A, Muderrisoglu H. Non-lipid effects of statins: emerging new indications. Curr Vasc Pharmacol. 2004;2(4):309–318. doi: 10.2174/1570161043385475. [DOI] [PubMed] [Google Scholar]
- 44.Tsiara S, Elisaf M, Mikhailidis DP. Early vascular benefits of statin therapy. Curr Med Res Opin. 2003;19(6):540–556. doi: 10.1185/030079903125002225. [DOI] [PubMed] [Google Scholar]
- 45.Sirol M, Bouzamondo A, Sanchez P, Lechat P. Does statin therapy reduce the risk of stroke? a meta-analysis [in French] Ann Med Interne (Paris) 2001;152(3):188–193. [PubMed] [Google Scholar]
- 46.Blauw GJ, Lagaay AM, Smelt AH, Westendorp RG. Stroke, statins, and cholesterol: a meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke. 1997;28(5):946–950. doi: 10.1161/01.str.28.5.946. [DOI] [PubMed] [Google Scholar]
- 47.Byington RP, Davis BR, Plehn JF, et al. Reduction of stroke events with pravastatin: the Prospective Pravastatin Pooling (PPP) Project. Circulation. 2001;103(3):387–392. doi: 10.1161/01.cir.103.3.387. [DOI] [PubMed] [Google Scholar]
- 48.Hebert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statin drugs, risk of stroke, and total mortality: an overview of randomized trials. JAMA. 1997;278(4):313–321. [PubMed] [Google Scholar]
- 49.Vaughan CJ, Delanty N, Basson CT. Statin therapy and stroke prevention. Curr Opin Cardiol. 2001;16(4):219–224. doi: 10.1097/00001573-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]