Abstract
Glanders and melioidosis are caused by two distinct Burkholderia species and have generally been considered to have similar disease progression. While both of these pathogens are HHS/CDC Tier 1 agents, natural infection with both these pathogens is primarily through skin inoculation. The common marmoset (Callithrix jacchus) was used to compare disease following experimental subcutaneous challenge. Acute, lethal disease was observed in marmosets following challenge with between 26 and 1.2 × 108 cfu Burkholderia pseudomallei within 22–85 h. The reproducibility and progression of the disease were assessed following a challenge of 1 × 102 cfu of B. pseudomallei. Melioidosis was characterised by high levels of bacteraemia, focal microgranuloma progressing to non-necrotic multifocal solid lesions in the livers and spleens and multi-organ failure. Lethal disease was observed in 93% of animals challenged with Burkholderia mallei, occurring between 5 and 10.6 days. Following challenge with 1 × 102 cfu of B. mallei, glanders was characterised with lymphatic spread of the bacteria and non-necrotic, multifocal solid lesions progressing to a multifocal lesion with severe necrosis and pneumonia. The experimental results confirmed that the disease pathology and presentation is strikingly different between the two pathogens. The marmoset provides a model of the human syndrome for both diseases facilitating the development of medical countermeasures.
Keywords: animal model, Burkholderia, histology
Burkholderia pseudomallei and Burkholderia mallei are the aetiological agents for melioidosis and glanders respectively. Both pathogens are Gram negative, intracellular bacteria and are classified as HHS/CDC Tier 1 agents (7 CFR Part 331, 9 CFR Part 121 and 42 CFR Part 73). Melioidosis is prevalent in SE Asia and northern Australia and presents with diverse clinical manifestations varying from acute sepsis to chronic localised infection to latent infection. Disease presentation is believed to be associated with a number of parameters including bacterial strain, route of entry and host factors (Cheng & Currie, 2005). Naturally occurring infection is primarily through bacterial entry via cuts or skin abrasions or via inhalation of infected soil or water particles (White et al. 1989). However, there is increasing speculation that bacterial ingestion is a potential route of infection, particularly in cases of unexplained origin of the disease (Limmathurotsakul & Peacock 2011). This is supported by reports of B. pseudomallei being isolated from drinking water in both Thailand and Northern Australia (Limmathurotsakul & Peacock 2011). Glanders is generally an equine-associated disease prevalent in parts of the Middle East, Asia, Eastern Europe, Northern Africa and South America. Human infection is primarily by contact with infected animals resulting in acute or chronic forms of either cutaneous (farcy) or nasal-pulmonary (glanders) infection; however, laboratory-acquired infections are reported (Dvorak & Spickler 2008). There are no licensed human vaccines for either of these diseases, and antibiotic treatment is often prolonged, complex and requires intravenous administration. Hence, there is a need to develop reliable, effective medical countermeasures. However, due to the nature of these diseases, it is unlikely that licensure of products will be obtainable solely using traditional Phase 3 human efficacy trials in those at risk of exposure. Therefore, the FDA's Animal Rule may be the most appropriate route for product licensure and will rely on well-characterised animal models of disease to assess the efficacy of medical countermeasures.
To date, mice and hamsters are the most commonly used models to investigate pathogenesis and therapies for both melioidosis and glanders (Fritz et al. 1999, 2000; Jeddeloh et al. 2003; Lever et al. 2003, 2009). Little work has been undertaken in non-human primates (NHP). In the 1940's, six Macaca mulatta were challenged subcutaneously with B. mallei, with all animals surviving the challenge and one animal exhibited a raised fever with an abscess at the site of inoculation (Miller et al. 1948). Studies reported in the 1990's have involved both subcutaneous and intravenous B. mallei challenge in NHP's, specifically baboons, but details of the disease presentation are sparse (Manzeniuk et al. 1996; Khomiakov et al. 1998; Mukhopadhyay et al. 2004). Early studies investigating experimental melioidosis in NHPs were performed in the 1920's and 1940's where macaques were infected by either the oral or subcutaneous route (Stanton & Fletcher 1925; Miller et al. 1948). All animals survived the challenge with minimal, if any, clinical observations. More recently, experimental NHP infection with B. pseudomallei has been described following inhalational challenge in the marmoset (Callithrix jacchus) (Nelson et al. 2011a, Nelson et al. 2013) and in African green monkeys (Chlorocebus aethiops) and rhesus macaques (Macaca mulatta) (Yeager et al. 2012).
The common marmoset, a New World Monkey (NWM) species, is an alternative NHP model to complement the more traditionally used Old World Monkeys (OWM) such as rhesus and cynomolgus macaques. Marmosets have been used to model a number of public health pathogens including Lassa virus (Carrion et al. 2007), Hepatitis C virus (Weatherford et al. 2009), Dengue virus (Omatsu et al. 2009), Herpesvirus (Leibovitch et al. 2013), Junin virus (Weissenbacher et al. 1979), Rift Valley Fever (Smith et al. 2012), SARS (Greenough et al. 2005) and MERS (Falzarano et al. 2014). Marmosets have also been used to model a number of biodefense pathogens including Eastern Equine Encephalitis virus (Adams et al. 2008), Bacillus anthracis (Lever et al. 2008), Francisella tularensis (Nelson et al. 2009, 2010a), B. pseudomallei (Nelson et al. 2011a), Marburg haemorrhagic fever virus (Carrion et al. 2011; Smither et al. 2013), Ebola haemorrhagic fever virus (Carrion et al. 2011) and Variola virus (Kramski et al. 2010).
The aim of these studies was to develop, characterise and compare B. pseudomallei and B. mallei infection by the subcutaneous (s.c.) route of challenge, in a single NHP species, to allow more relevant comparison with the limited human data available.
Materials and methods
Animals
Healthy, sexually mature common marmosets (C. jacchus) were obtained from the Dstl Porton Down breeding colony and housed in vasectomised male and female pairs. For these studies, animals were aged between 1.8 and 4.2 years old and weighed between 319 and 532 g at the time of challenge. All animals were allowed free access to food and water as well as environmental enrichment. All animals were surgically implanted intraperitoneally with a Remo 200 device (Remo Technologies Ltd, Salisbury, UK) under general anaesthesia (Ketamine/Domitor/Isofluorane) to record core body temperature (Tc). Data were transmitted from the devices at 30 s intervals to locally placed antennas and relayed to receivers. Data were analysed using the eDacq software to provide real time and recordable Tc (EMMS; Bordon, Hampshire, UK). The animal studies were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986 and the Codes of Practice for the Housing and Care of Animals used in Scientific Procedures 1989. Following challenge with B. pseudomallei or B. mallei, all animals were handled under animal containment level 3 (CL3) conditions, within a half-suit isolator compliant with British Standard BS5726. Marmosets were challenged subcutaneously in the inner right thigh.
Bacterial strain and culture
Glycerol stocks of B. pseudomallei strain NCTC 13392 and B. mallei NCTC 12938 (also known as ATCC 23344) were provided by the Public Health England (PHE), UK. The B. mallei strain is the type strain of the pathogen and is commonly used in laboratory studies. B. pseudomallei strain NCTC 13392 is closely related to strain K96243 (Sahl et al. 2013). This strain was previously used to develop an inhalational model of melioidosis in the marmoset and for comparability was used for the subcutaneous model. The frozen stock of B. pseudomallei was recovered into Luria-Bertani (LB) broth and incubated at 37 °C with shaking at 180 rpm for 24 h, prior to recovery into phosphate buffered saline (PBS), pH 7.3. The frozen stock of B. mallei was recovered on to LB-agar plates with 5% glycerol (LBG) and incubated at 37 °C for 48 h prior to inoculation into nutrient broth and then further incubated at 37 °C with shaking at 180 rpm for 24 h, prior to recovery into phosphate buffered saline (PBS), pH 7.3. The optical density reading (OD600) of the appropriate bacterial suspension was adjusted to 0.35, equivalent to approximately 1 × 108 cfu/ml. The suspension was serially diluted to the appropriate concentration for challenge. Viable counts were performed on LB-agar plates for B. pseudomallei or LBG for B. mallei, retrospectively, to determine the actual value. All procedures were carried out at CL3, in Class 3 microbiological safety cabinets.
Post-mortem analysis
Post-mortem examinations were performed on all animals in all studies; organs removed were assessed for bacteriology, macroscopic and microscopic features and immunohistochemical analysis. Blood was removed from terminally anaesthetised marmosets by cardiac puncture for assessment of bacteraemia clinical chemistry and haematological parameters.
Bacteriology
Bacterial loads were determined in blood, liver, spleen, kidneys, lungs and brain. Organs were removed aseptically and processed as previously described (Nelson et al. 2009). Appropriate dilutions were subcultured onto LB-agar plates for B. pseudomallei or LBG for B. mallei and incubated at 37 °C for 24 or 48 h respectively. Counts are expressed as cfu/g of tissue or cfu/ml of blood.
Histopathology
Tissues were fixed in 10% neutral buffered formalin and processed for paraffin wax embedding using standard techniques. Thin sections (4 μm) were cut and stained with haematoxylin and eosin (H&E) for histopathological analysis. A scoring system based on the type of lesions and organ distribution was established to allow semi-quantitative comparison (Table1).
Table 1.
The Scoring System used for pathological interpretation
| Numerical value | Symbol | Histopathological description | IHC description |
|---|---|---|---|
| 1 | + | Small focal microgranuloma composed mainly by macrophages, lymphocytes and plasma cells | Very few |
| 2 | ++ | Non-necrotic multifocal solid lesions | Small number |
| 3 | +++ | Focal or multifocal suppurative lesions with the presence of abundant neutrophils and small areas of necrosis | Moderate number |
| 4 | ++++ | Multifocal lesion with severe necrosis | Abundant |
Immunohistochemistry
Immunohistochemical staining (IHC) was performed on selected tissues (liver, spleen, lungs and the inoculation site) for the detection of bacterial antigen, T cells (CD3+), Plasma B cells (CD79+), macrophages and neutrophils (MAC387+) and inducible Nitric oxide synthase (iNOS+) once a lesion had been identified by H&E. A semi-quantitative scoring system was established to allow comparison (Table1).
The avidin biotin complex (ABC, Vector Elite; Vector laboratories, Southgate, Peterborough, UK) method was used for immunolabelling. Four micrometer sections of formalin-fixed wax-embedded tissues were prepared and dried onto polylysine slides (VWR Ltd, East Grimstead, West Sussex, UK). The sections were dewaxed, rehydrated and then treated in hydrogen peroxide 3% (v/v), in methanol for 15 min to eliminate endogenous peroxidase activity. The tissue sections were then pretreated for antigen retrieval by either enzymatic digestion with pronase XIV (0.05% w/v pronase XIV 5.2 U/mg; Sigma 9.c) and trypsin/alpha-chymotrypsin (0.5% trypsin and 0.5% alpha-chymotrypsin; Sigma-Aldrich, Gillingham, Dorset, UK) at 37 °C for 10 min. The sections were then microwaved in citric acid buffer (2.1 g citric acid; Fisher Scientific, Loughborough, Leicestershire, UK, in 1000 ml distilled water pH 6.0) for 18 min at 100 °C, 90% effect (780 W) or microwaved in Dako high pH 9.0 buffer for 10 min. The sections were then mounted in a Sequenza Immunostaining Centre (Shandon Scientific, Runcorn, UK) and rinsed with Tris buffered saline (TBS) pH 7.6, 0.005 M (Sigma–Aldrich, Gillingham, Dorset, UK). Primary antibody cross-reactivity with tissue constituents was prevented using 1.5% normal serum block applied to the sections for 20 min. The serum block matched the host species in which the link antibody was raised. Details of primary antibodies used, specificity, concentration and incubation time are summarised in Table2. All primary antibodies had been previously screened to determine the optimum dilution and incubation temperature. The sections were washed in TBS and then incubated for 30 min with the appropriate biotinylated secondary link antibody (Vector Laboratories) before being washed twice in TBS again. The sections were incubated for 30 min at room temperature with ABC (Vector Elite Kit; Vector Laboratories), and the signal was detected using 3,30-diaminobenzidine tetrahydrochloride (DAB). Finally the sections were lightly counterstained with Mayer's haematoxylin (Surgipath, Peterborough, UK) for 5 min, dehydrated in absolute alcohol and cleared in xylene before being coverslipped.
Table 2.
Immune markers used for immunohistochemistry
| Target | Specificity | Source | Epitope demasking | Primary antibody concentration (incubation time) |
|---|---|---|---|---|
| Human CD3 | Pan T cell marker (Rabbit pc) | DAKO | Trypsin/α-chymotrypsin | 1 μg/ml (O/N at 4 °C) |
| Human CD79 | Pan B cell marker (clone HM57) | DAKO | Microwave-mediated AR | 2.1 μg/ml (1 h at RT) |
| Human MAC387 | Macrophages/monocytes (clone MAC387) | Serotec | Trypsin/α-chymotrypsin | 2 μg/ml (1 h at RT) |
| iNOS | Inducible Nitric Oxyde synthase (Rabbit pc) | Chemicon | Dako high pH microwave buffer | 1 μg/ml (1 h at RT) |
| Bacterial antigen | Burkholderia pseudomallei (3VIE5) | DSTL | Trypsin/α-chymotrypsin | 2 μg/ml (O/N at 4 °C) |
O/N, over night; RT, room temperature; iNOS, inducible Nitric oxide synthase.
IHC techniques for cell markers applied in this study have been successfully used in multiple species tissue samples (CD3+, CD79+, MAC387+ and iNOS+). Bacterial capsular antigen was detected using a B. pseudomallei antibody (3VIE5) that has shown cross-reactivity with B. mallei (Jones et al. 2002). Controls were included in each IHC run. These included sequential sections with an isotype control for each primary antibody and the omission of the primary antibody. All the techniques were performed in a GLP, ISO9001:2008 and ISO 17025 compliant laboratory facility.
Haematology, clinical chemistry and electrolytes
Blood was collected from all animals at post-mortem into blood tubes, anti-coagulated with either EDTA or Lithium heparin. Key haematological parameters were measured from EDTA anti-coagulated blood by use of a laser-flow cytometry-based haematological analyser (ProCyte; IDEXX Laboratories Ltd, Bucks, UK). Heparinised blood was used to assess the clinical chemistry, and electrolyte parameters were analysed using a ‘dry-slide’ technology biochemistry analyser (Catalyst Dx; IDEXX). Postchallenge data were compared to mean prechallenge results.
Statistics
A general linear model with covariate was used for survival comparison. Pearson's correlation analysis was used to determine the relationship of gender, body weight, time to death and inhaled dose. Comparative analysis of bacteriology and blood chemistry data was performed using two-way anova.
Results
Dose ranging studies
Initially, to determine the susceptibility of marmosets to subcutaneous administration of B. pseudomallei or B. mallei, a mixed-sex pair of animals was challenged with either 1.2 × 108 cfu of B. pseudomallei or 5.3 × 107 cfu of B. mallei (Table3). Following challenge with B. pseudomallei, a gradual increase in temperature was observed between 2.5 and 5 h postchallenge (p.c.) remaining high until the animals were humanely euthanised at 22 and 27 h p.c. Following challenge with B. mallei, the animals exhibited a fever within 2 h p.c., which was maintained until the animals were humanely euthanised due to severe clinical signs and a rapid drop in temperature at 120 or 164 h p.c. Further mixed-sex pairs of animals were challenged with between 26 and 1.7 × 103 cfu of B. pseudomallei or 7 and 1.2 × 104 cfu of B. mallei by the subcutaneous route in a stepwise approach (Table3). Following challenge with B. pseudomallei, the febrile response began between 18 and 30 h p.c. Animals remained febrile until the temperature declined prior to the animals reaching the humane endpoint, at between 56 and 85 h p.c. Following challenge with B. mallei, animals became febrile between 2 and 4.5 days p.c. depending on the dose received and were humanely euthanised between 5.9 and 10.6 days p.c. One animal that received 7 cfu of B. mallei survived for the duration of the study and exhibited no overt clinical signs. For both B. mallei and B. pseudomallei, there was a statistically significant relationship between the challenge dose and the survival of the animals using a general linear model that includes a covariate (P < 0.001 for B. pseudomallei and P = 0.01 for B. mallei) with the two pathogens being statistically different from each other (P < 0.0001) (Figure1).
Table 3.
Summary of challenge doses for lethality studies
| Study |
Burkholderia pseudomallei |
Burkholderia mallei |
||||
|---|---|---|---|---|---|---|
| Challenge dose (cfu) |
Time to cull [hours (days)] |
Challenge dose (cfu) |
Time to cull (days) |
|||
| Female | Male | Female | Male | |||
| Susceptibility | 1.2 × 108 | 22 (0.9) | 27 (1.1) | 5.3 × 107 | 6.8 | 5.0 |
| 1 | 1.7 × 103 | 64 (2.7) | 59 (2.5) | 1.2 × 104 | 7.9 | 5.9 |
| 2 | 1.2 × 102 | 85 (3.5) | 76 (3.2) | 1.0 × 102 | 10 | 9.9 |
| 3 | 4.8 × 101 | 78 (3.3) | 74 (3.1) | 7 | N/A | 8.9 |
| 4 | 2.6 × 101 | 75 (3.1) | 66 (2.7) | 82 | 7.1 | 9.8 |
| 5 | 3.8 × 102 | 56 (2.3) | 67 (2.8) | 2.6 × 102 | 10.6 | 7.3 |
| 6 | 3.9 × 102 | 57 (2.4) | 66 (2.7) | 5.7 × 102 | 7.3 | 8.1 |
Figure 1.
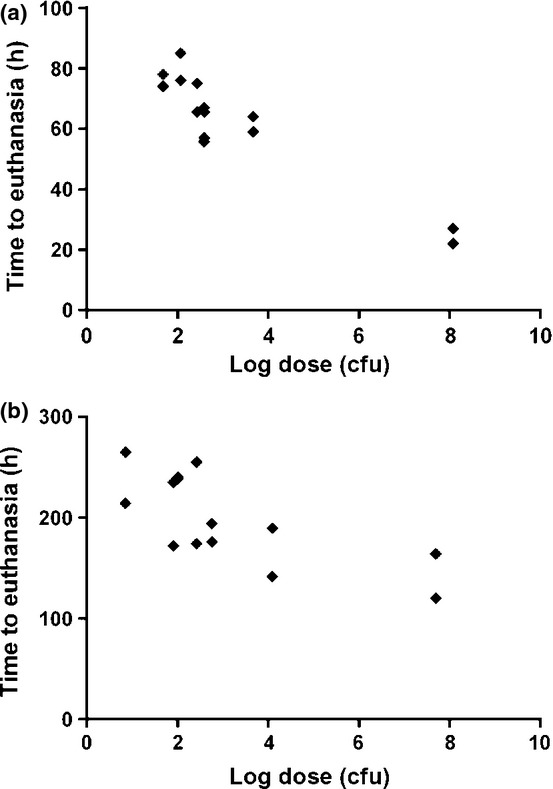
Comparative virulence of different doses of bacteria administered by the subcutaneous route to marmosets. (a) Burkholderia pseudomallei (P < 0.001, general linear model with covariate), (b) Burkholderia mallei (P = 0.01, general linear model with covariate).
To characterise the reproducibility of each disease in NHP's, six animals for each pathogen were challenged with 2.97 × 102 ± 56 cfu of B. pseudomallei or 3.13 × 102 ± 138 cfu of B. mallei. B. pseudomallei-infected animals reached the humane endpoint at 68 ± 5 h. At this challenge dose, the time to onset of fever was 34.4 ± 1.3 h, with a subsequent duration of 35.2 ± 2.6 h, prior to a rapid decline indicative of the animal reaching the humane endpoint (Figure2). In all cases, bacteraemia was observed (range of 2.4 × 104 to 4.9 × 106 cfu/ml, median of 1.5 × 105 cfu/ml), and high concentrations of bacteria were recovered from all organs assessed (6.8 × 105 to 1.4 × 107 cfu/g in the liver, median of 1.7 × 106 cfu/ml; 5.1 × 106 to 7.4 × 107 cfu/g in the spleen, median of 2.6 × 107 cfu/ml; 1.6 × 105 to 1.8 × 106 cfu/g in the kidney, median of 3.5 × 105 cfu/ml; 3.2 × 104 to 6.2 × 107 cfu/g in the lungs, median of 2.3 × 106 cfu/ml and 1.8 × 103 to 1.6 × 106 cfu/g in the brain median, of 8.4 × 103 cfu/ml). Clinical signs were evident from 48 h p.c. for all animals and were typically lethargy and piloerection with a hunched posture.
Figure 2.
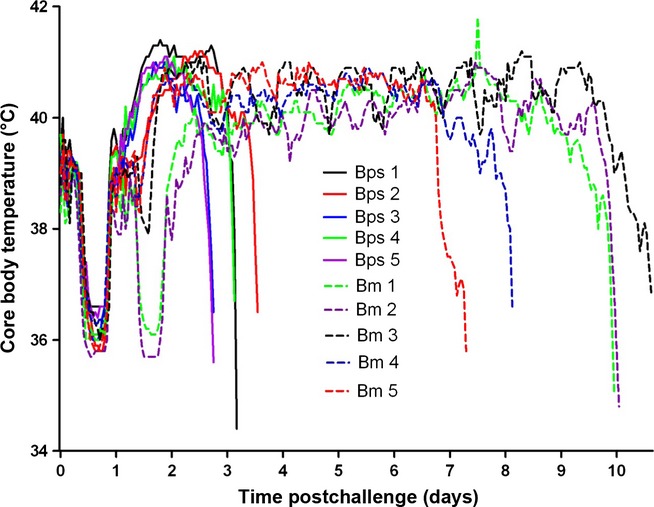
Core body temperature of marmosets postchallenge with either 2.97 × 102 ± 56 cfu of B pseudomallei or 3.65 × 102 ± 105 cfu of Burkholderia mallei by the subcutaneous route.
Burkholderia mallei-infected animals reached the humane endpoint at 8.9 ± 0.6 days. At a challenge dose of 3.13 × 102 ± 138 cfu of B. mallei, the time to onset of fever was 46 ± 4 h, with a subsequent duration of 165 ± 13 h prior to a rapid decline indicative of the animal reaching the humane endpoint (Figure2). In all cases, bacteraemia was observed (range of 3.4 × 103 to 4.9 × 104 cfu/ml, median of 8.7 × 103 cfu/ml), and high concentrations of bacteria were recovered from all organs assessed (5.4 × 104 to 1.4 × 107 cfu/g in the liver, median of 1.7 × 105 cfu/ml; 1.5 × 105 to 2.1 × 107 cfu/g in the spleen, median of 5.7 × 105 cfu/ml; 1.6 × 104 to 6.1 × 106 cfu/g in the kidney, median of 5.3 × 104 cfu/ml; 4.6 × 105 to 8.5 × 107 cfu/g in the lungs, median of 6.2 × 106 cfu/ml and 2.9 × 103 to 6.5 × 104 cfu/g in the brain, median of 9.3 × 103 cfu/ml). Clinical signs were evident from 4 days postchallenge for all animals and typically were lethargy and piloerection with a hunched posture. In the latter stages of infection, most animals presented with a flushed face, with occasional swollen eyes, with or without the presence of nasal or facial lesions and dyspnoea.
A challenge dose of approximately 1 × 102 cfu was therefore selected as a reproducible dose for both pathogens, giving a predicable clinical outcome including lethality, temperature profile, bacteriology and clinical signs. This challenge dose was then used as a target dose for subsequent pathogenesis studies.
Pathogenesis studies
To investigate pathogenesis of Burkholderia pseudomallei induced disease pathogenesis marmosets were challenged by the subcutaneous route in two cohorts for logistically reasons. One cohort received a challenge dose of 1.44 × 102 cfu of B. pseudomallei, and the second cohort received a challenge dose of 2.25 × 102 cfu of B. pseudomallei. Two pairs of animals (one pair from each cohort) were humanely euthanised at 12, 24, 36 and 48 h p.c. These time points were based on the previous temperature data (Figure2) to represent prefever (12 h), early temperature rise (24 h), late temperature rise (36 h) and fever plateau phase (48 h). Data from unchallenged animals (‘naive’) and animals used in the dose ranging studies at a challenge dose of 2.97 × 102 ± 56 cfu (‘terminal’) are included in the discussion and as additional time points, where appropriate.
Bacterial cells spread from the injection site (inner right thigh) to the liver and spleen within the first 12 h p.c. (Figure3). The number of bacterial cells in these organs continued to increase exponentially over 48 h p.c. By 24 h p.c., bacterial cells were recovered from the lung and brain of marmosets. Bacteraemia was evident by 36 h p.c., and bacterial cells were recovered from the kidneys by 48 h p.c. Significantly higher concentrations of bacteria were observed in the liver (P = 0.004), spleen (P = 0.003), kidney (P = 0.002), lung (P = 0.003), brain (P = 0.002) and blood (P = 0.002) at 48 h compared to 12 h p.c.
Figure 3.
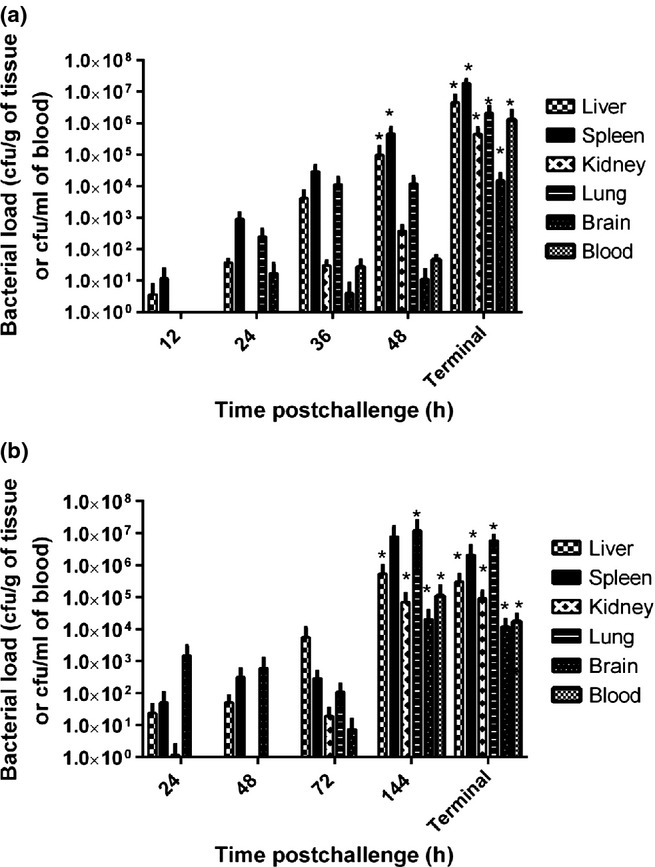
Bacterial load in various marmoset tissues at specific times postchallenge by the subcutaneous route. (a) Burkholderia pseudomallei, mean challenge dose 1.85 × 102 ± 15 cfu. Statistically significant changes in the liver (P = 0.004), spleen (P = 0.003), kidney (P = 0.002), lung (P = 0.003), brain (P = 0.002) and blood (P = 0.001) were observed with time, with most of the significance occurring between t = 12 and t = 48 or for terminal animals (Independent sample Kruskal–Wallis Test). (b) Burkholderia mallei, mean challenge dose 1.79 × 102 ± 14 cfu. Statistically significant changes in the liver (P = 0.004), spleen (P = 0.003), kidney (P = 0.002), lung (P = 0.002), brain (P = 0.002) and blood (P = 0.001) were observed with time, with most of the significance occurring between t = 24 and t = 144 or for terminal animals (Independent sample Kruskal–Wallis Test).
Gross pathological observations were present from 36 h p.c. with 50% animals exhibiting splenomegaly, 100% lymphadenopathy (75% of animals had enlarged inguinal lymph node and the other an enlarged axillary lymph node) and 50% had lung consolidation. By 48 h p.c., 50% of the animals exhibited discolouration of the liver, 75% splenomegaly, 25% lymphadenopathy, 50% mild signs in the lungs including a pale or slightly mottled appearance and 25% exhibited a swelling or lesion at the site of injection. For terminal animals, the following gross features were prevalent: liver discoloration (67%), splenomegaly (50%), small, splenic lesions (100%), a pale discolouration in the lungs (83%), and potential cardiac puncture-associated haemorrhages in the lungs (50%), swollen injection site (17%), injection site-associated abscesses (50%) and lymphadenopathy in the inguinal lymph node (33%). The first evidence of microscopic lesions was observed in the liver and spleens of 75% of animals, and the inoculation site of one of these animals at 36 h p.c. The liver and spleen lesions consisted of small focal microgranulomas to non-necrotic multifocal solid lesions (Table4). The lesions were associated with the presence of bacterial antigen, macrophages, T cells, B cells and iNOS expressing cells. The lesions in these organs increased in frequency and severity as the disease progressed leading to multifocal lesions with severe necrosis observed by 48 h p.c. (Figure4). The multifocal lesion with severe necrosis observed at the inoculation site at 36 h postchallenge (Figure5a) was associated with the presence of bacterial antigen (Figure5b), macrophages and neutrophils (Figure5c), few numbers of T cells (Figure5d) and iNOS producing cells (Figure5f) and the absence of B cells (Figure5e). For terminal animals, variable pathological features were observed in the lungs; 83% of lungs exhibited no pathological features; however, the remaining 17% exhibited multifocal necrotising pneumonia. Small pyogranulomatous lesions were observed in all livers examined, with mid-sized necrotic lesions also present in 33% of these animals. Multifocal necrotic splenitis was observed in all animals. Severe granulomatous and necrotic dermatitis with abundant associated bacteria was observed in all cases where the inoculation site was clearly identified at post-mortem. Focal to severe necrotising lymphadenitis was observed in 33% of inguinal lymph nodes and in the mediastinal lymph node of the animal with multifocal necrotising pneumonia. No disease-related pathological features were observed in the kidneys, brains or mediastinal lymph nodes.
Table 4.
Lesion characterisation including associated bacterial antigen and cell types identified in various organs of marmosets challenged with Burkholderia pseudomallei by the subcutaneous route
| Time (h) |
Lung |
Spleen |
Liver |
Inoculation Site |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | Bp | M | T | B | i | H | Bp | M | T | B | i | H | Bp | M | T | B | i | H | Bp | M | T | B | i | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 36 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 1.5 | 2.25 | 2 | 0.5 | 0.75 | 1.5 | 0.75 | 2.5 | 2.25 | 0.75 | 0.75 | 1 | 1 | 0.5 | 0.25 | 0 | 0.25 |
| 48 | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | 3.75 | 3.5 | 1.75 | 0.5 | 1.25 | 2.25 | 1.75 | 4 | 2.5 | 0.5 | 1 | 1 | 1 | 0.75 | 0.25 | 0 | 0.25 |
| T | 0 | ND | ND | ND | ND | ND | 3.75 | ND | ND | ND | ND | ND | 3.0 | ND | ND | ND | ND | ND | 1.3 | ND | ND | ND | ND | ND |
T, terminal; H, average score of lesion severity; Bp, average score of the presence of bacteria in the lesion detected by IHC; M, average score of the presence of macrophages in the lesion detected by IHC; T, average score of the presence of T cells in the lesion detected by IHC; B, average score of the presence of B cells in the lesion detected by IHC; i, average score of the presence of inducible Nitric oxide synthase (iNOS) in the lesion detected by IHC; ND, not determined.
Figure 4.
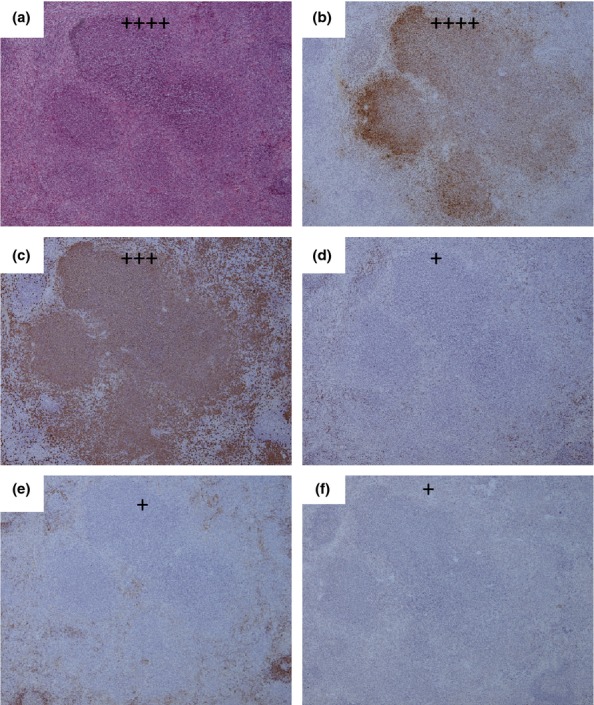
Representative H&E and IHC stained tissue sections from the spleen of a marmoset humanely euthanised at 48 h p.c. challenged with 1.85 × 102 ± 57 cfu of Burkholderia pseudomallei by the subcutaneous route. (a) H & E showing multifocal lesion with severe necrosis (++++), (b) Burkholderia pseudomallei antigen IHC showing abundant bacteria associated with the lesion (++++), (c) IHC staining showing a moderate number of macrophages associated with the lesion (+++), (d) IHC staining showing very few T cells associated with the lesion (+), (e) IHC staining showing very few B cells associated with the lesion (+), (f) IHC staining showing very few inducible Nitric oxide synthase (iNOS) producing cells associated with the lesion (+).
Figure 5.
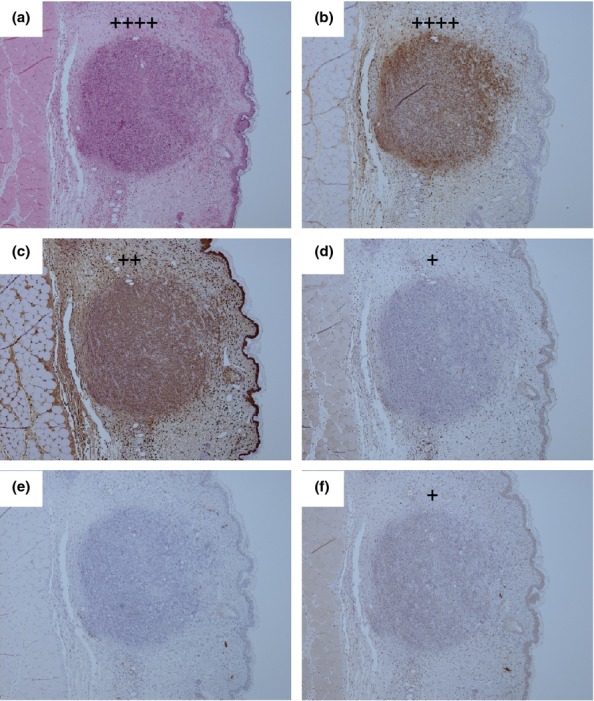
Representative H&E and IHC stained tissue sections from the inoculation site of a marmoset humanely euthanised at 36 h p.c. challenged with 1.85 × 102 ± 57 cfu of Burkholderia pseudomallei by the subcutaneous route. (a) H & E showing multifocal lesion with severe necrosis (++++), (b) B. pseudomallei antigen IHC showing abundant bacteria associated with the lesion (++++), (c) IHC staining showing a small number of macrophages associated with the lesion (++), (d) IHC staining showing very few T cells associated with the lesion (+), (e) IHC staining showing no B cells associated with the lesion, (f) IHC staining showing very few inducible Nitric oxide synthase (iNOS) producing cells associated with the lesion (+).
Key changes in the blood parameters were observed (Figure6). A peak of aspartate aminotransferase (AST) and alanine transaminase (ALT) was observed at 12 h p.c., rising again from 48 h p.c. (Figure6a). A gradual rise in the levels of alkaline phosphatase (ALKP) was also observed from 36 h p.c. Both of which are indicative of liver dysfunction and hepatitis. Other general indicators of an acute, inflammatory response include an increase in levels of glucose as the disease progressed, followed by a decline in the levels by 48 h p.c. and undulating high concentrations of creatine kinase (CK) (data not shown). High levels of lactate dehydrogenase (LDH) were observed from 36 h p.c. (Figure6b). Decreasing levels of amylase (AMYL) (Figure6b) and hypokalemia (data not shown) are suggestive of pancreatitis. An increase in the number of white blood cells (WBC) was observed with time, decreasing in terminal animals (Figure6c). A similar trend was observed with the monocyte levels. Lymphopenia was observed at 24 h p.c. and was associated with neutrophilia, followed by a decrease in the numbers of neutrophils, potentially associated with migration of neutrophils to the sites of infection.
Figure 6.
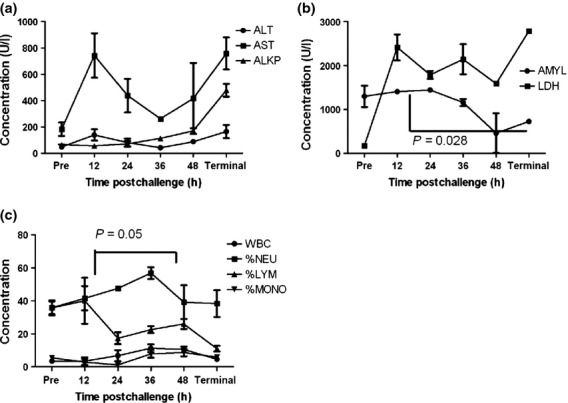
Key blood parameter data from marmosets challenged with 1.85 × 102 ± 57 cfu of Burkholderia pseudomallei by the subcutaneous route at specific times postchallenge. (a) Liver function enzymes, ALT (Alanine transaminase), AST (Aspartate aminotransferase), ALKP (Alkaline phosphatase), (b) AMYL (amylase), LDH (lactate dehydrogenase), (c) White blood cell distribution, WBC (white blood cell count × 109/l), %NEU (per cent neutrophils), %LYM (Per cent lymphocytes), %MONO (per cent monocyte). Each time point represents data from four different animals humanely euthanised at that time, therefore connecting lines are only included to demonstrate the trend of the data sets. Error bars represent the standard error of the mean (SEM).
To investigate Burkholderia mallei induced disease pathogenesis the study was performed as described above, with marmosets challenged by the subcutaneous route in two cohorts, one cohort received a challenge dose of 1.65 × 102 and the second cohort received a challenge dose of 1.93 × 102 cfu of B. mallei. Two pairs of animals (one pair from each cohort) were humanely euthanised at 24, 48, 72 and 144 h p.c. Naive and terminal data were included as described above.
Bacteria spread from the injection site (inside right leg) to the liver, spleen and lung by 24 h p.c. (Figure3). Numbers of bacterial cells were constant within these organs at 48 h p.c. By 72 h p.c., the number of bacterial cells in the liver had increased from 5.9 × 102 cfu to 6 × 103 cfu per gram, with a slight decrease in the numbers recovered from the lungs. At this time, low numbers of bacterial cells were recovered from the kidney and brain. Significantly higher concentration of bacteria were observed in the liver (P = 0.01), spleen (P = 0.001), kidney (P = 0.002), lung (P = 0.01), brain (P = 0.002) and blood (P = 0.001) at 144 h p.c. compared to time 24 h p.c.
Gross pathological features were observed from 72 h p.c. when liver discolouration and lung consolidation were observed in 25% of the animals. By 144 h p.c., liver discolouration was also observed in 50% of animals, as well as splenomegaly (100%), swelling at the injection site (25%) and haemorrhaging and visible lesions in the lung (100%). For terminal animals, the following gross features were observed; liver discoloration (67%), splenomegaly (83%), a pale discolouration in the lungs (50%), raised lesions in the lung (50%), swelling and/or pus-association with injection site (83%) and lymphadenopathy in the inguinal lymph node (67%). The presence of splenomegaly was also indicated by an increase in the organ weight with time (Figure 9a). Additionally, 43% of animals used in the dose ranging studies exhibited swelling and redness of the eyes and one animals exhibited spontaneous pustular eruptions of the face.
Figure 9.
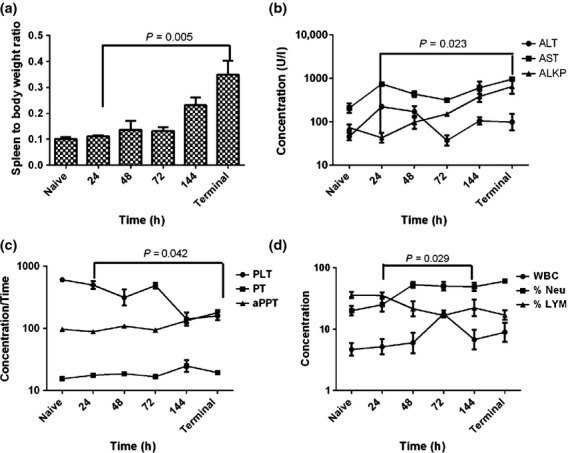
Key disease features from marmosets challenged with 1.79 × 102 ± 20 cfu of Burkholderia mallei by the subcutaneous route at specific times postchallenge. (a) Splenic weight with time, (b) Liver function enzymes, ALT (Alanine transaminase), AST (Aspartate aminotransferase), ALKP (Alkaline phosphatase), (c) Platelet and clotting factors, PLT (Platelet, K/μl), PT (Prothrombin Times, s), aPPT (activated partial thromboplastin time, s), (d) White blood cell distribution, WBC (white blood cell count × 109/l), %NEU (per cent neutrophils), %LYM (per cent lymphocytes). Each time point represents data from four different animals humanely euthanised at that time, therefore connecting lines are only included to demonstrate the trend of the data sets. Error bars represent the standard error of the mean (SEM).
The first evidence of microscopic lesions was observed in the liver and spleens of two animals at 48 h p.c. and consisted of small focal microgranuloma to non-necrotic, multifocal solid lesions (Table5). The lesions in these organs increased in frequency and severity as the disease progressed leading to the multifocal lesion with suppurative lesions observed by 144 h p.c. (Figure7). In all cases, the lesions were associated with the presence of bacterial antigen (Figure7b), macrophages (Figure7c), T cells (Figure7d) and iNOS expressing cells (Figure7f). The presence of B cells was detected in one of four splenic lesions at both 72 and 144 h p.c and in one of four liver lesions at 72 h p.c. Lesions were apparent in two of four lungs and one of four inoculation sites at 144 h p.c. Lesions in the lungs ranged from non-necrotic, multifocal solid lesions in one animal to a multifocal lesion with severe necrosis in the other animal (Figure8). Similarly to the spleen and liver, the lesions were associated with the presence of bacterial antigen (Figure8b), macrophages (Figure8c), T cells (Figure8d) and iNOS expressing cells (Figure8e). However, less B cells were present in the lungs at this time (Figure8e). The small focal microgranulomas observed in the inoculation site of one animal were associated with the presence of bacterial antigen, macrophages, T cells and iNOS expressing cells, without the presence of B cells. No pathological features were observed in any other organs except in the axillary lymph node and adrenal gland of one animal at 144 h p.c. and the inguinal lymph nodes of two animals at 144 h p.c. For all terminal animals, multifocal necrotising pneumonia was observed with small pyogranulomas in the liver and spleen. Severe granulomatous and necrotic dermatitis that infiltrated the underlying muscle was observed in all animals where the inoculation site was visible (83%). The animal that presented with facial lesions had necrotising dermatitis and myositis, with the lesion affecting all the layers of the epidermis and dermis with a high number of bacteria and an associated ulcerative zone. Splenomegaly was also determined in these animals by an increase in the relative organ weight with time (Figure9).
Table 5.
Lesion characterisation including associated bacterial antigen and cell types identified in various organs of marmosets challenged with Burkholderia mallei mallei by the subcutaneous route
| Time (h) |
Lung |
Spleen |
Liver |
Inoculation Site |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | Bm | M | T | B | i | H | Bm | M | T | B | i | H | Bm | M | T | B | i | H | Bm | M | T | B | i | |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 0.75 | 0.75 | 0 | 0.25 | 0.5 | 0.5 | 1 | 1.25 | 0 | 0.25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 72 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | 0.5 | 0.5 | 2 | 1.5 | 0.5 | 0.25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 144 | 1.5 | 1.25 | 1.5 | 0.75 | 0.75 | 1 | 2.25 | 0.75 | 1.5 | 1 | 0.25 | 0.75 | 2.25 | 1.5 | 3 | 1.5 | 0 | 1.25 | 0.25 | 0.25 | 0.5 | 0.25 | 0 | 0.25 |
| T | 4 | ND | ND | ND | ND | ND | 2.75 | ND | ND | ND | ND | ND | 2.75 | ND | ND | ND | ND | ND | 2.5 | ND | ND | ND | ND | ND |
T, terminal; H, average score of lesion severity; Bm, average score of the presence of bacteria in the lesion detected by IHC; M, average score of the presence of macrophages in the lesion detected by IHC; T, average score of the presence of T cells in the lesion detected by IHC; B, average score of the presence of B cells in the lesion detected by IHC; i, average score of the presence of inducible Nitric oxide synthase (iNOS) in the lesion detected by IHC; ND, not determined.
Figure 7.
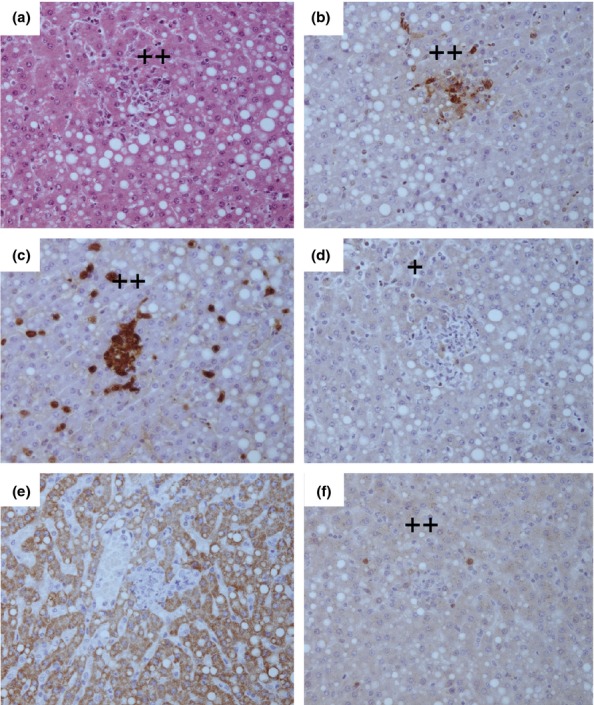
Representative H&E and IHC stained tissue sections from the liver of a marmoset humanely euthanised at 144 h p.c. challenged with 1.79 × 102 ± 20 cfu of Burkholderia mallei by the subcutaneous route. (a) H & E showing non-necrotic multifocal solid lesions (++), (b) Burkholderia mallei antigen IHC showing a small number of bacteria associated with the lesion (++), (c) IHC staining showing a small number macrophages associated with the lesion (++), (d) IHC staining showing very few T cells associated with the lesion (+), (e) IHC staining showing no B cells associated with the lesion, (f) IHC staining showing a small number inducible Nitric oxide synthase (iNOS) producing cells associated with the lesion (++).
Figure 8.
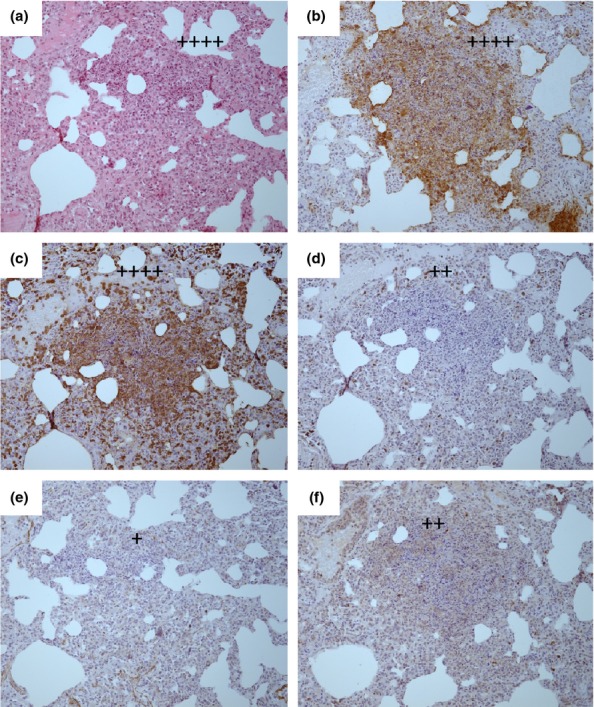
Representative H&E and IHC stained tissue sections from the lungs of a marmoset humanely euthanised at 144 h p.c. challenged with 1.79 × 102 ± 20 cfu of Burkholderia mallei by the subcutaneous route. (a) H & E showing multifocal lesion with severe necrosis (++++), (b) B. mallei antigen IHC showing abundant bacteria associated with the lesion (++++), (c) IHC staining showing abundant macrophages associated with the lesion (++++), (d) IHC staining showing a small number of T cells associated with the lesion (++), (e) IHC staining showing very few B cells associated with the lesion (+), (f) IHC staining showing a small number inducible Nitric oxide synthase (iNOS) producing cells associated with the lesion (++).
Key changes in blood parameters following challenge with B. mallei were observed (Figure9). Levels of ALKP and AST generally increased with time while levels of ALT peaked around 24–48 h p.c. indicating liver dysfunction (Figure9b). Numbers of platelets (PLT) decreased with time, while there was a general increase in the clotting time (prothrombin time, PT and activated partial thromboplastin time, aPPT) towards the end of disease progression indicative of coagulopathy (Figure9c). A general increase in the number of white blood cells was observed until 72 h p.c. which was specifically associated with neutrophilia and lymphopenia (Figure9d). At 144 h p.c., there was a decline in white blood cells, although the levels of neutrophils and lymphocytes remained relatively constant.
Discussion
The strikingly different pathological features observed in these studies reflect the major difference between experimental melioidosis and glanders in the marmoset, apart from the disease duration. The most severe pathological features in marmosets following subcutaneous challenge with B. pseudomallei were present in the spleen and liver. These ranged from multifocal suppurative lesions with small areas of necrosis to multifocal lesions with severe necrosis. Whereas the lesions typically observed in marmosets following subcutaneous challenge with B. mallei were non-necrotic multifocal lesions, conversely, the severity of lesions in the lungs was more pronounced in marmoset glanders than melioidosis, despite the subcutaneous inoculation. Typically, only a third of marmosets had pneumonia following challenge with B. pseudomallei, but multifocal necrotising pneumonia was observed in all animals following challenge with B. mallei. This is highly suggestive of a completely different disease pathogenesis of these two closely related bacteria. The histopathological changes observed with melioidosis in the marmoset are comparable with human reports, where typically pyogranulomatous inflammation was evident in the liver, spleen and lymph nodes (Piggot & Hochholzwe 1970; Wong 1995).
Glanders in the marmoset exhibited many features of human disease (Pilcher 1907; Freeman 1985; Parker 1997; Van Zandt et al. 2013). Human glanders is dependent on the route of infection but typically results in pneumonia, septicaemia and chronic suppurative infection of the skin. Incubation periods in humans are generally between 1 and 14 days prior to onset of symptoms, with signs apparent in marmosets typically at 3 days p.c. with the onset of fever. Bacterial spread in humans, as was observed in the marmosets, occurs from the site of infection, through the lymphatic vessels to the lymph nodes. Swelling is commonly observed at the site of infection, which for the marmosets was associated with purulent material. In the marmosets, lymphadenopathy was observed in the nearest draining lymph node to the inner thigh site of infection, the inguinal lymph node. In the marmoset, although low levels of bacteria were recovered in the liver and spleen at 24 h p.c., a higher concentration of bacteria was recovered from the lungs, suggesting a tropism for this tissue. Presence of bacterial antigen was not assessed in these tissues by IHC until lesions were observed (48 h p.c. for liver and spleen and 144 h p.c. for the lung). In humans, rapid onset of pneumonia is typical and associated with lethal disease, with a mortality of between 90 and 95% if untreated. Chest pain and lung abnormalities were the first symptoms noted in an early case of lethal human glanders following a horse bite (Pilcher 1907). This person, as well as second person also bitten by a horse, went on to present with pneumonia and had lesions in the mucous membranes, specifically around the eyes and nose. Further reports of mucosal tissue involvement, as well as lymphatic tissue involvement, are associated with human glanders (Van Zandt et al. 2013). Facial swelling, swollen and partially closed eyes with occasional lesions, spontaneous pustular eruptions specifically noted on the face and nasal discharge, were commonly observed in the marmoset.
Clinical manifestations of melioidosis vary from acute sepsis with fever, bacteraemia and primary or secondary pneumonia to chronic localised infection associated with abscesses and ulcerations, to latent infection (Dance 1996). The manifestation of disease presentation is dependent on multiple factors; indeed, disease presentation is different in the two main countries of prevalence, Thailand and Australia. In Thailand, there is a higher incidence of lesions in the liver and spleen, 25% of cases compared to 6% (Limmathurotsakul & Peacock 2011) and there is a higher mortality rate, 50% compared to 19% (White 2003). The exact reason for this is unclear but it is likely to be multifactorial and may reflect difference in infecting strains, treatment regimens or route of infection.
The most prevalent natural route of exposure is believed to be inoculation through cuts or skin abrasions (Cheng & Currie 2005). Inoculation of B. pseudomallei can result in three outcomes: subclinical or latent infection, primary cutaneous melioidosis or systemic melioidosis, with only severe cutaneous or systemic melioidosis cases presenting at hospital (Dance 1996). The subcutaneous route of challenge was used in the marmosets to mimic systemic disease. In humans, primary cutaneous melioidosis results in a localised infection causing single lesions ranging from ulcers, with or without purulent exudates, to boils, pustules or even erythematous lesions (Gibney et al. 2008). In the marmosets swollen, purulent, erythematous lesions were observed in many animals at the site of infection. Typically, in the Australian population, this form of disease does not develop any further. However, in Thailand, bacteraemia is a common complication of primary cutaneous melioidosis. Bacteraemia was observed in the marmoset model at 36 h p.c and was associated with onset of fever suggesting the marmoset model may be more representative of disease in Thailand. In humans, if the disease develops into a septicaemic infection, fever is often the first sign, this was observed in the marmosets occurring between 24 and 30 h p.c. Following bacterial dissemination in humans, hepatic and/or splenic abscesses, with or without lung involvement, are typical (Limmathurotsakul & Peacock 2011). In the marmoset, the spleen and liver were the first organs colonised, spreading to the lung by 24 h p.c. However, bacterial levels in the lung were significantly lower than previously reported for marmosets challenged by the inhalational route (Nelson et al. 2011a). Pneumonia is present in approximately 50% of human cases in both Thailand and Australia. However, it is often difficult to determine whether this is primary or secondary pneumonia or identify the route of infection (Gibney et al. 2008; Parameswaran et al. 2012). Indeed, in the Australian study of cutaneous infection, 50% of the cases presented with pneumonia (Gibney et al. 2008).
Marmosets also exhibited other features of human melioidosis including changes in haematological and clinical chemistry parameters. These included reduction in sodium levels as well as increased white blood cell counts, liver function enzymes, creatinine and urea levels (Dance 1996; Mukhopadhyay et al. 2004). An indicator of poor prognosis in humans, also observed in marmosets, was high activation of the coagulation system (Wiersinga et al. 2008), including a prolonged prothrombin time (PT) and activated partial thromboplastin time (aPPT).
The data presented support the potential use of marmoset models of glanders and melioidosis as alternative NHP models used to assess medical countermeasures. For both diseases, the marmoset model is reproducibly lethal at selected challenge doses. Many key aspects of human disease are exhibited, specifically pathological and physiological features. However, one limitation of the marmoset model is the high mortality rate and low infectious dose, specifically for melioidosis, that may not be directly comparable to human disease. The time to the onset of clinical disease in humans is variable, ranging from between 24 h to 62 years (Ngauy et al. 2005). However, it is typically in the region of 1–21 days, with a mean of 9 days (Currie et al. 2000). For both melioidosis and glanders, the marmoset had a uniform, predictable febrile response. This may be a useful biomarker of infection that could be exploited as a trigger to initiate therapy, as well as an additional marker of the success of the intervention. Due to the nature of the diseases in the marmosets, particularly melioidosis, the marmoset models acute, lethal disease that would serve as a stringent model for the medical countermeasure. The marmoset has been previously used to assess medical countermeasures; a vaccine has been tested for Lassa fever (Lukashevicha et al. 2008) and the efficacy of ciprofloxacin, and levofloxacin has been determined as postexposure therapies for anthrax and tularaemia (Nelson et al. 2010b, 2011b). Therefore, the marmoset could be an important, reproducible, lethal model that would complement work with OWMs (e.g. macaques).
In conclusion, these studies are the first reported NHP models of a naturally occurring cutaneous melioidosis and glanders. Despite the causative agents of glanders and melioidosis being genetically similar, the disease presentation is strikingly different when compared directly in the common marmoset. Cutaneous melioidosis presents as an acute, febrile disease resulting in death by multi-organ failure with minimal pulmonary involvement. Whereas, although glanders is also lethal in many cases, the disease progression is slower with the highest severity pathological features were associated with pulmonary tissue. Both diseases in the marmoset, model key features of human disease suggesting marmosets may be an appropriate stringent model to facilitate the development of novel medical countermeasures for these two difficult to treat human diseases.
Acknowledgments
The authors would like to thank Medical Countermeasure Systems (MCS) Joint Project Management Office, Contract Number: HDTRA1-11-C-0039, for funding the work and all their colleagues at Dstl that supported this work in many ways.
References
- Adams AP, Aronson JF, Tardif SD, et al. Common marmosets (Callithrix jacchus) as a nonhuman primate model to assess the virulence of eastern equine encephalitis virus strains. J. Virol. 2008;82:9035–9042. doi: 10.1128/JVI.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R, Jr, Brasky K, Mansfield K, et al. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J. Virol. 2007;81:6482–6490. doi: 10.1128/JVI.02876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R, Jr, Ro Y, Hoosien K, et al. A small nonhuman primate model for filovirus-induced disease. Virology. 2011;420:117–124. doi: 10.1016/j.virol.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AC. Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005;218:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie BJ, Fisher DA, Howard DM, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000;74:121–127. doi: 10.1016/s0001-706x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Dance DAB. Melioidosis and glanders. In: Warrell DA, editor; Weatherall DJ, Ledingham CG, editors. Oxford Textbook of Medicine. Oxford, UK: Oxford Medical Publications; 1996. pp. 590–605. [Google Scholar]
- Dvorak GD. Spickler AR. Glanders. J. Am. Vet. Med. Assoc. 2008;233:570–577. doi: 10.2460/javma.233.4.570. [DOI] [PubMed] [Google Scholar]
- Falzarano D, de Wit E, Feldmann F, et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10:e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BA. Pseudomonas and Legionella. In: Freeman BA, editor. Burrow's Textbook of Microbiology. 22nd edn. Philadelphia, PA: WB Saunders; 1985. pp. 544–557. [Google Scholar]
- Fritz DL, Vogel P, Brown DR. Waag DM. The hamster model of intraperitoneal Burkholderia mallei (glanders) Vet. Pathol. 1999;36:276–291. doi: 10.1354/vp.36-4-276. [DOI] [PubMed] [Google Scholar]
- Fritz DL, Vogel P, Brown DR, Deshazer D. Waag DM. Mouse model of sublethal and lethal intraperitoneal glanders (Burkholderia mallei. Vet. Pathol. 2000;37:626–636. doi: 10.1354/vp.37-6-626. [DOI] [PubMed] [Google Scholar]
- Gibney KB, Cheng AC. Currie BJ. Cutaneous melioidosis in the tropical top end of Australia: a prospective study and review of the literature. Clin. Infect. Dis. 2008;47:603–609. doi: 10.1086/590931. [DOI] [PubMed] [Google Scholar]
- Greenough TC, Carville A, Coderre J, et al. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am. J. Pathol. 2005;167:455–463. doi: 10.1016/S0002-9440(10)62989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddeloh JA, Fritz DL, Waag DM, Hartings JM. Andrews GP. Biodefense-driven murine model of pneumonic melioidosis. Infect. Immun. 2003;71:584–587. doi: 10.1128/IAI.71.1.584-587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Ellis JF, Russell P, Griffin KF. Oyston PC. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J. Med. Microbiol. 2002;51:1055–1062. doi: 10.1099/0022-1317-51-12-1055. [DOI] [PubMed] [Google Scholar]
- Khomiakov I, Manzeniuk IN, Naumov DV. Svetoch EA. The principles of the therapy of glanders in monkeys. Zh. Mikrobiol. Epidemiol. Immunobiol. 1998;1:70–74. [PubMed] [Google Scholar]
- Kramski M, Mätz-Rensing K, Stahl-Hennig C, et al. A novel highly reproducible and lethal nonhuman primate model for orthopox virus infection. PLoS One. 2010;5:e10412. doi: 10.1371/journal.pone.0010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch E, Wohler JE, Cummings Macri SM, et al. Novel marmoset (Callithrix jacchus) model of human Herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization. PLoS Pathog. 2013;9:e1003138. doi: 10.1371/journal.ppat.1003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever MS, Nelson M, Ireland PI, et al. Experimental aerogenic Burkholderia mallei (glanders) infection in the BALB/c mouse. J. Med. Microbiol. 2003;52:1109–1115. doi: 10.1099/jmm.0.05180-0. [DOI] [PubMed] [Google Scholar]
- Lever MS, Stagg AJ, Nelson M, et al. Experimental respiratory anthrax infection in the common marmoset (Callithrix jacchus. Int. J. Exp. Pathol. 2008;89:171–179. doi: 10.1111/j.1365-2613.2008.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever MS, Nelson M, Stagg AJ, Beedham RJ. Simpson AJ. Experimental acute respiratory Burkholderia pseudomallei infection in BALB/c mice. Int. J. Exp. Pathol. 2009;90:16–25. doi: 10.1111/j.1365-2613.2008.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmathurotsakul D. Peacock SJ. Melioidosis: a clinical overview. Br. Med. Bull. 2011;99:125–139. doi: 10.1093/bmb/ldr007. [DOI] [PubMed] [Google Scholar]
- Lukashevicha IS, Carrion R, Salvatoa M. Patterson J. Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine. 2008;26:5246–5254. doi: 10.1016/j.vaccine.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzeniuk IN, Svetoch EA, Diadishev NR, Stepanshin I. Buziun AV. Various indices of the infectious process in treatment of glanders in monkeys. Antibiot. Khimioter. 1996;41:13–18. [PubMed] [Google Scholar]
- Miller WR, Pannell L, Cravitz L, Tanner WA. Rosebury T. Studies on certain biological characteristics of Malleocyes mallei and Malleocyes pseudomallei. II. Virulence and Infectivity for animals. J. Bacteriol. 1948;55:127–135. [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Lee KH. Tambyah PA. Bacteraemic melioidosis pneumonia: impact on outcome, clinical and radiological features. J. Infect. Dis. 2004;48:334–338. doi: 10.1016/j.jinf.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Nelson M, Lever MS, Savage VL, et al. Establishment of lethal inhalational infection with Francisella tularensis (tularaemia) in the common marmoset (Callithrix jacchus. Int. J. Exp. Pathol. 2009;90:109–118. doi: 10.1111/j.1365-2613.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Lever MS, Dean RE, et al. Characterisation of lethal inhalational infection with Francisella tularensis in the common marmoset (Callithrix jacchus. J. Med. Microbiol. 2010a;59:1107–1113. doi: 10.1099/jmm.0.020669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Lever MS, Dean RE, Pearce PC, Stevens DJ. Simpson AJH. Francisella tularensis: the bioavailability and efficacy of levofloxacin in the common marmoset (Callithrix jacchus. Antimicrob. Agents Chemother. 2010b;54:3922–3926. doi: 10.1128/AAC.00390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Dean RE, Salguero FJ, et al. Development of an acute model of inhalational melioidosis in the common marmoset (Callithrix jacchus. Int. J. Exp. Pathol. 2011a;92:428–435. doi: 10.1111/j.1365-2613.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Stagg AJ, Stevens DJ, et al. Post-exposure therapy of inhalational anthrax in the common marmoset. Int. J. Antimicrob. Agents. 2011b;38:60–64. doi: 10.1016/j.ijantimicag.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Nelson M, Dean RE, Salguero FJ, et al. Erratum: development of an acute model of inhalational melioidosis in the common marmoset (Callithrix jacchus. Int. J. Exp. Pathol. 2013;94:74. doi: 10.1111/j.1365-2613.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngauy V, Lemeshev Y, Sadkowski L. Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J. Clin. Microbiol. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu T, Moi ML, Hirayama T, et al. Common marmoset (Callithrix jacchus) as a primate model of dengue virus infection: development of high levels of viraemia and demonstration of protective immunity. J. Gen. Virol. 2009;92:2272–2280. doi: 10.1099/vir.0.031229-0. [DOI] [PubMed] [Google Scholar]
- Parameswaran U, Baird RW, Ward LM. Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009-2010 wet season: comparison with the preceding 20 years. Med. J. Aust. 2012;196:345–348. doi: 10.5694/mja11.11170. [DOI] [PubMed] [Google Scholar]
- Parker MT. Glanders and melioidosis. In: Collier LH, editor; Parker MT, editor. Topley & Wilson's Principles if Bacteriology, Virology and Immunity. 8th edn. Philadelphia, PA: BC Decker; 1997. pp. 392–394. [Google Scholar]
- Piggot JA, Hochholzwe L. A histopathologic study of acute and chronic melioidosis. Arch. Pathol. 1970;90:101–111. [PubMed] [Google Scholar]
- Pilcher JT. Glanders in the human subject. Clinical report of two cases observed in the fourth medical division of Bellevue hospital of New York. Ann. Surg. 1907;45:444–452. doi: 10.1097/00000658-190703000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl JW, Stone JK, Gelhaus HC, et al. Genome Sequence of Burkholderia pseudomallei NCTC 13392. Genome Announc. 2013;1:e00183–13. doi: 10.1128/genomeA.00183-13. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Bird BH, Lewis B, et al. Development of a novel nonhuman primate model for Rift Valley fever. J. Virol. 2012;86:2109–2120. doi: 10.1128/JVI.06190-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither SJ, Nelson M, Eastaugh L, et al. Establishment of lethal Marburg virus haemorrhagic fever by the aerosol route in the common marmoset (Callithrix jacchus. Int. J. Exp. Pathol. 2013;94:156–168. doi: 10.1111/iep.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AT. Fletcher W. Melioidosis and its relation to glanders. J. Hyg. (London) 1925;23:33–35. doi: 10.1017/s0022172400034252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zandt KE, Greer MT. Gelhaus HC. Glanders: an overview of infection in humans. Orphanet J. Rare Dis. 2013;8:131. doi: 10.1186/1750-1172-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherford T, Chavez D, Brasky KM, Lemon SM, Martin A. Lanford RE. Lack of adaptation of chimeric GB virus B/hepatitis C virus in the marmoset model: possible effects of bottleneck. J. Virol. 2009;83:8062–8075. doi: 10.1128/JVI.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher MC, Calello MA, Colillas OJ, Rondinone SN. Frigerio MJ. Argentine hemorrhagic fever: a primate model. Intervirology. 1979;11:363–365. doi: 10.1159/000149059. [DOI] [PubMed] [Google Scholar]
- White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V. Pitakwatchara N. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;2:697–701. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Wiersinga WJ, Meijers JC, Levi M, et al. Activation of coagulation with concurrent impairment of anticoagulant mechanisms correlates with a poor outcome in severe melioidosis. J. Thromb. Haemost. 2008;6:32–39. doi: 10.1111/j.1538-7836.2007.02796.x. [DOI] [PubMed] [Google Scholar]
- Wong KT. The histopathology of human melioidosis. Histopathology. 1995;26:51–55. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Yeager JJ, Facemire P, Dabisch PA, et al. Natural history of inhalation melioidosis in rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus aethiops. Infect. Immun. 2012;80:3332–3340. doi: 10.1128/IAI.00675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


