Abstract
Acute pancreatitis (AP) can lead to a systemic inflammatory response that often results in acute lung injury and single or multiple organ failure. In a previous study we demonstrated that diabetes aggravates the local pathophysiological process during AP. In this study we explore, if diabetes also increases pancreatitis induced systemic inflammation and causes lung injury. Acute pancreatitis was induced in untreated and streptozotocin-treated diabetic mice by injection of cerulein. Systemic inflammation was studied by IL-6 ELISA in blood plasma and white blood cell count. Lung inflammation and lung injury were quantified by chloroacetate esterase staining, evaluation of the alveolar cellularity index and cleaved caspase-3 immunohistochemistry. In normoglycaemic mice AP increased the IL-6 concentration in plasma and caused lymphocytopenia. Diabetes significantly increased the IL-6 concentration in plasma and further reduced the number of lymphocytes during AP, whereas diabetes had little effect on these parameters in the absence of pancreatitis. However, diabetes only marginally increased lung inflammation and did not lead to cell death of the lung epithelium during AP. We conclude that diabetes increases parameters of systemic inflammation during AP, but that this increase is insufficient to cause lung injury.
Keywords: acute lung injury, chloroacetate esterase staining, cleaved caspase-3, sepsis, systemic inflammatory response syndrome
About 15%–20% of patients with acute pancreatitis (AP) develop severe symptoms, such as pancreatic parenchymal necrosis, a systemic inflammatory response and concomitant single or multiple organ failure (Forsmark & Baillie 2007). The AP-induced inflammatory response often causes acute lung injury (ALI) with a mortality rate of up to 40% (Zhou et al. 2010). This is often associated with lymphopenia and an increase in the concentration of the pro-inflammatory cytokine IL-6 in the blood (Takeyama et al. 2000; Gregoric et al. 2010). IL-6 is not only of prognostic value, but has also been demonstrated to be an essential mediator of pancreatitis-associated lung injury (Zhang et al. 2013). The pro-inflammatory milieu during AP leads to activation and infiltration of neutrophil granulocytes in the lung and to an increase in the alveolar cellularity index (Guice et al. 1989; Frossard et al. 1999; Pastor et al. 2006; Tascilar et al. 2007). Pulmonary inflammation can cause lung injury, which is often characterized by apoptosis of lung epithelial cells (Yuan et al. 2000; Nakamura et al. 2003). Although several proteins, such as the cytokine induced neutrophil chemoattractant, the CD40 ligand or the toll-like receptor 4, have been implicated in the pathophysiological process of AP-induced ALI, underlying mechanisms and contributing parameters of this process are still not well understood (Bhatia et al. 2000; Frossard et al. 2001; Sharif et al. 2009; Zhou et al. 2010).
One parameter that has been described to aggravate AP is diabetes. Several correlative studies in patients have suggested that diabetes leads to a higher risk for pancreatitis (Seicean et al. 2006; Noel et al. 2009; Girman et al. 2010; Xue et al. 2012) and that hyperglycaemia may predispose patients with AP to systemic organ failure (Mentula et al. 2008). In addition, blood glucose level is an accurate predictor of outcome in gallstone pancreatitis and an important criterion for the Ranson score, which is used to assess the prognosis of AP (Ranson et al. 1974; Rajaratnam & Martin 2006).
We have previously demonstrated in experimental settings that diabetes indeed aggravates the local pathophysiological process during acute as well as chronic pancreatitis (Zechner et al. 2012, 2013, 2014). The purpose of this study was to explore if the aggravation of AP by diabetes leads to increased systemic inflammation, and if adequate alterations in the lung can be observed.
Methods
Animals
Eight to 12-week-old C57BL/6J mice were allowed access to water and standard laboratory chow ad libitum. The mice were treated as published previously (Zechner et al. 2012). Diabetes was caused by i.p. injection of 50 mg/kg streptozotocin (STZ; Sigma-Aldrich, Steinheim, Germany) on 5 consecutive days (day 1-5), whereas AP was induced on day 22 and day 23 by administration of eight i.p. injections per day of 50 μg/kg cerulein (Sigma-Aldrich) at a rate of one every hour. All control mice were Sham-treated appropriately (0.9% wt/vol. NaCl solution instead of cerulein, 50 mmol/l sodium citrate pH 4.5 instead of STZ). At 2 h before induction of pancreatitis, and up to the time point of tissue preservation, all mice received drinking water containing 800 mg/l metamizol (Ratiopharm, Ulm, Germany) and 1 g/l BrdU (Sigma-Aldrich Chemie GmbH). For sampling blood and tissue the animals were anaesthetized with 75 mg/kg ketamine (Bela-Pharm, Vechta, Germany) and 5 mg/kg xylazine (Bayer Health Care, Leverkusen, Germany). All experiments were executed in accordance with German legislation, the local animal wellfare committee and the EU-directive 2010/63/EU.
Analysis of plasma and tissue
Blood samples were taken 2 hours after the last cerulein injection on day 23. A differential blood cell count was performed with an automated hematology analyzer Sysmex KX 21 (Sysmex Cooperation, Kobe, Japan) as previously published (Bobrowski et al. 2013). Concentrations of interleukin (IL)-6 were measured in blood plasma with commercially available enzyme-linked immunosorbent assay (ELISA) kits from Thermo Fisher Scientific (Rockford, IL, USA) following the manufacturer's instructions, and data were plotted as fold induction compared to the average of IL-6 in Sham-treated mice. Tissue samples were taken on day 23 (2 hours after the last cerulein injection) or on day 30 (7 days after the last cerulein injection). Naphthol AS-D chloroacetate esterase (CAE) staining, primarily staining neutrophil granulocytes, or hematoxylin/eosin staining was performed on paraffin-embedded tissue to evaluate lung inflammation and histology. To evaluate the alveolar cellularity index (nuclei/septum), the number of nuclei crossing three gridlines of the integrating eyepiece (using a 100× objective) was divided by the number of septa crossing these gridlines (Tascilar et al. 2007; ). We analysed 10 randomly chosen microscopic fields from each lung. Cell death was analysed by immunohistochemistry using a rabbit-anti-mouse cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology Inc., Denver, USA, code 9661, dilution 1:500) and a HRP-conjugated goat-anti-rabbit secondary antibody (code P0448; Dako Deutschland GmbH, Hamburg, Germany). Cell proliferation was evaluated by immunohistochemistry using mouse anti-BrdU (clone: Bu20a, dilution: 1:50) and the Universal LSAB™+ Kit/HRP kit (Dako Deutschland GmbH). Quantification of inflammation, BrdU incorporation and cell death was performed on 10 random fields per mouse using a 40× objective.
Statistics
Data are given as means and standard deviation respectively. The significance of data was assessed by SigmaStat 3.5 software (SigmaStat; Jandel Corporation, San Rafael, CA, USA). In all cases where the assumption of normality or the homogeneity of variance across groups failed, the Mann–Whitney rank sum test was performed, including correction of the α-error according to the Bonferroni probabilities for repeated analysis. In other cases, the unpaired Student's t test including the correction of the α-error according to the Bonferroni was performed. The criterion for significance was P < 0.05 divided by the number of meaningful comparisons.
Results
Diabetes aggravates systemic inflammation during pancreatitis
To evaluate if STZ-induced diabetes has an influence on parameters of systemic inflammation during cerulein-induced AP, the IL-6 concentration in plasma was determined in cerulein-treated diabetic mice (STZ + Cer) and compared to healthy normoglycaemic mice (Sham), cerulein-treated normoglycaemic mice (Cer) and diabetic mice without pancreatitis (STZ). Administration of cerulein in normoglycaemic mice during the acute phase of pancreatitis (on day 23, 2 hours after the last cerulein administration) increased the IL-6 plasma concentrations when compared to healthy mice (Figure1a). STZ plus cerulein treatment lead to an even more pronounced increase in IL-6 concentration when compared to Sham, cerulein or STZ-treated mice (Figure1a). Evaluation of the blood cell count revealed that administration of cerulein only slightly reduced the number of leucocytes in normoglycaemic animals (Figure1b). However, STZ plus cerulein treatment lead to a significant decrease in the number of leucocytes when compared to cerulein-treated mice (Figure1b). A decrease in the number of lymphocytes was observed after administration of cerulein in normoglycaemic mice compared to healthy mice (Figure1c). STZ plus cerulein treatment lead to an even more pronounced decrease in the number of lymphocytes when compared to Sham, cerulein or STZ-treated mice (Figure1c). The number of monocytes plus granulocytes was increased by cerulein administration, but was not significantly influenced by STZ (Figure1d).
Figure 1.
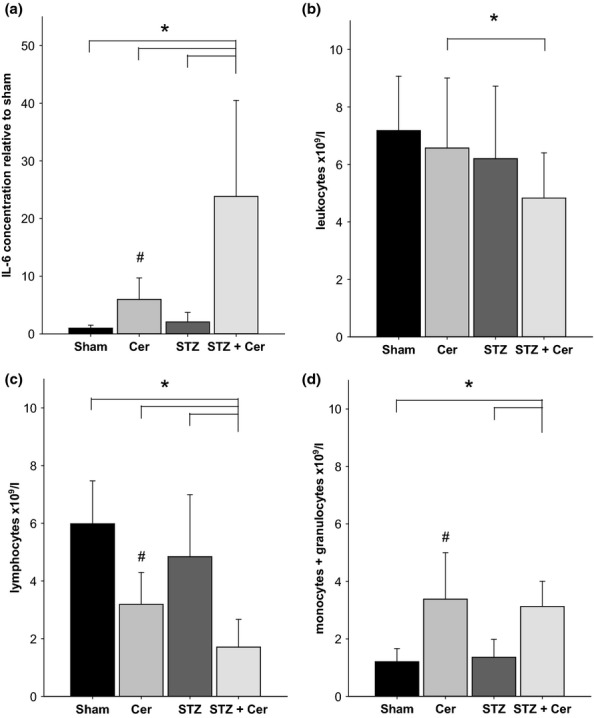
Diabetes aggravates pancreatitis induced systemic inflammation. The concentration of IL-6 in blood plasma (a), and the concentration of leucocytes (b), lymphocytes (c) or monocytes plus granulocytes (d) in the blood was determined on day 23 (2 hours after the last cerulein administration) in control mice (Sham), mice with AP (Cer), diabetic mice (STZ) and diabetic mice with AP (STZ + Cer). Bar charts indicate the average and standard deviation. The number of animals evaluated for each cohort was n = 8 (Sham), n = 19 (Cer), n = 11 (STZ), n = 21 (STZ + Cer) in panel a and n = 6 (Sham), n = 20 (Cer), n = 7 (STZ), n = 19 (STZ + Cer) in panel b to d. Significant differences between the cohorts are indicated, Mann–Whitney rank sum test: *P ≤ 0.001 (a, c, d), *P = 0.008 (b), #P ≤ 0.001 compared to Sham-treated mice (a, c, d).
Diabetes only marginally aggravates lung inflammation during pancreatitis
To evaluate if the observed aggravation of systemic inflammation by diabetes has an influence on lung inflammation, infiltrating inflammatory cells were identified by CAE staining on lung sections on day 23 (Figure2a–d). Administration of cerulein in normoglycaemic or diabetic mice significantly increased the number of CAE+ cells in the lung tissue when compared to Sham or STZ-treated mice (Figure2e). STZ plus cerulein treatment lead to a slight increase (P = 0.023) in the number of CAE+ cells when compared to cerulein-treated mice (Figure2e). The alveolar cellularity index was marginally increased by cerulein treatment (P = 0.143), but barely influenced by STZ treatment (Figure2f).
Figure 2.
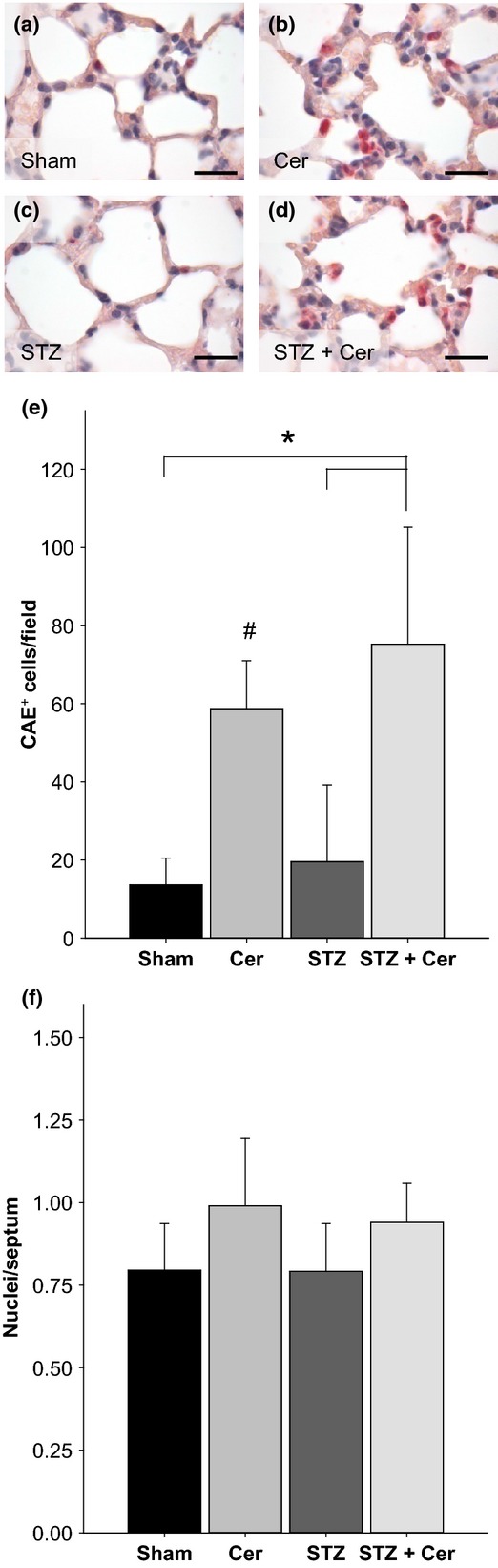
Diabetes and pancreatitis influence lung inflammation and alveolar cellularity on day 23. Representative images of CAE+ inflammatory cells in the lung in control mice (a), mice with AP (b), diabetic mice (c) and diabetic mice with AP (d). The number of CAE+ inflammatory cells per field (e) and the alveolar cellularity index (f) was quantified. Bar charts indicate the average and standard deviation. The number of animals evaluated for each cohort was n = 9 (Sham), n = 17 (Cer), n = 12 (STZ), n = 17 (STZ + Cer) in panel e and n = 4 (Sham), n = 5 (Cer), n = 4 (STZ), n = 6 (STZ + Cer) in panel f. Significant differences between the cohorts are indicated, Mann–Whitney rank sum test: *P < 0.001, #P < 0.001 compared to Sham-treated mice. Bar = 20 μm.
Diabetes does not induce cell death in the lung epithelium
To evaluate if STZ-induced diabetes has an influence on cell death in the lung during the acute phase of pancreatitis (on day 23, 2 hours after the last cerulein administration), cleaved caspase-3+ cells were identified by immunohistochemistry (Figure3a). However, the administration of cerulein, STZ or STZ plus cerulein did not result in an increased number of cleaved caspase-3+ cells in the lung when compared to Sham-treated animals (Figure3b). To evaluate if this lack of cell death in the lung epithelium might be caused by inadequate severity of pancreatitis we evaluated the histology of the pancreas on day 23 and on day 30. Normal histology of the pancreas was observed in Sham and STZ-treated mice (data not shown). In cerulein-treated mice features of AP such as oedema, and tissue-infiltrating inflammatory cells were observed on day 23, but pancreatitis was reversible as judged by histology of the pancreas on day 30 (Figure4a,b). In STZ plus cerulein-treated mice an even stronger induction of oedema and increased infiltration of inflammatory cells was observed in the pancreas on day 23 when compared to cerulein-treated mice (Figure4a,c). In addition, AP continued until day 30 leading to a massive reduction of acinar cells (Figure4d). These data suggest that cerulein induces a mild reversible form of pancreatitis, whereas STZ plus cerulein treatment leads to a more severe form of pancreatitis resulting in an impressive difference in the histology of the pancreas on day 30.
Figure 3.
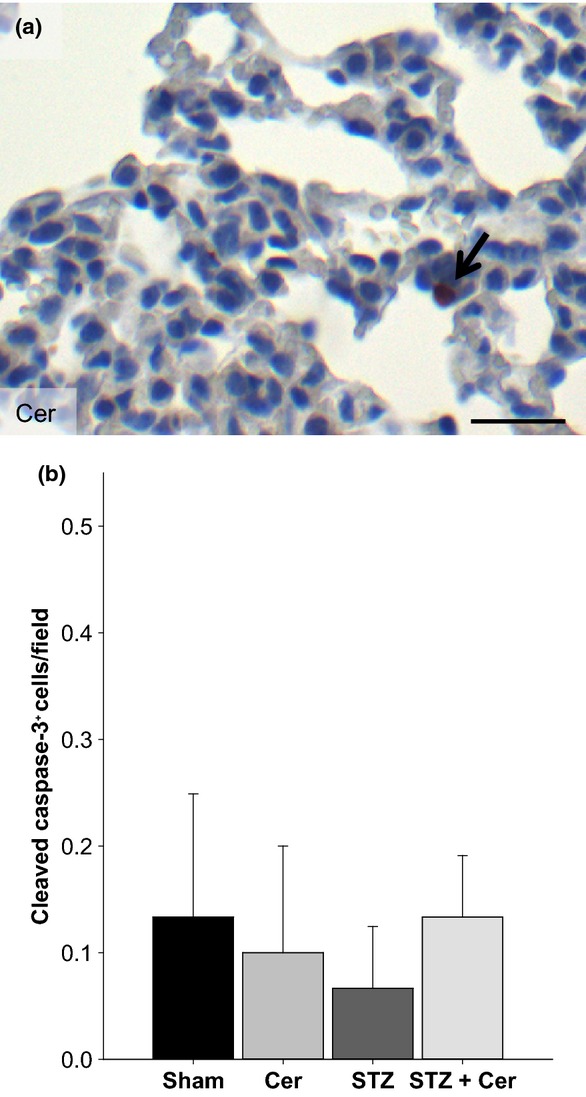
Diabetes does not enhance cell death in the lung epithelium on day 23. Representative image of cleaved caspase-3+ cells in the lung of a cerulein-treated mouse (a). The number of cleaved caspase-3+ cells in the lung epithelium per field (b) was quantified. Bar charts indicate the average and standard deviation. The number of animals evaluated for each cohort was n = 4. No significance was observed by Mann–Whitney rank sum test followed by Bonferroni correction for repeated analysis. Bar = 50 μm.
Figure 4.
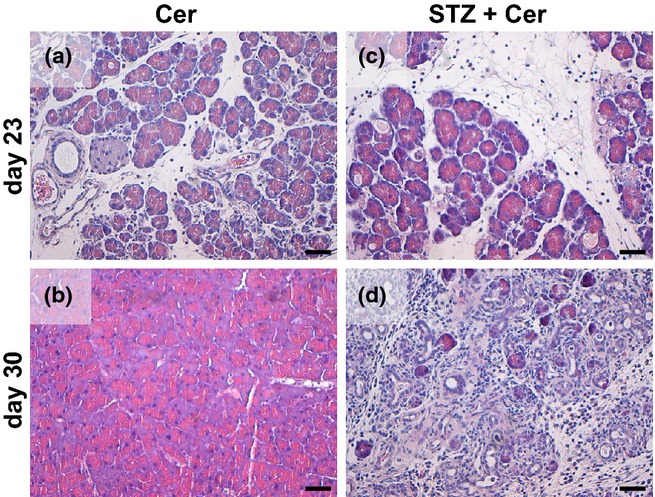
Histology of the pancreas after induction of acute pancreatitis. Representative images of hematoxylin/eosin-stained pancreas sections of cerulein (a, b) or STZ plus cerulein (c, d)-treated mice on day 23 (a, c) and day 30 (b, d). Bar = 50 μm.
Analysis of inflammation and cell death on day 30
On day 30, STZ plus cerulein-treated mice have a marginally increased number of CAE+ cells (P = 0.114) in the lung epithelium when compared to cerulein-treated mice (Figure5a). In addition, the alveolar cellularity index was also slightly increased (P = 0.114) by STZ plus cerulein treatment when compared to cerulein-treated mice (Figure5b). However, we observed no increase in the number of cleaved caspase-3+ cells in STZ plus cerulein-treated mice when compared to cerulein-treated mice (Figure5c). As lung injury can cause proliferation of lung epithelial cells, we also quantified BrdU incorporation into the nuclei of lung epithelial cells during 8 days of AP (day 22–30). We observed no increase in the number of BrdU+ cells in STZ plus cerulein-treated mice when compared to cerulein-treated animals (Figure5d).
Figure 5.
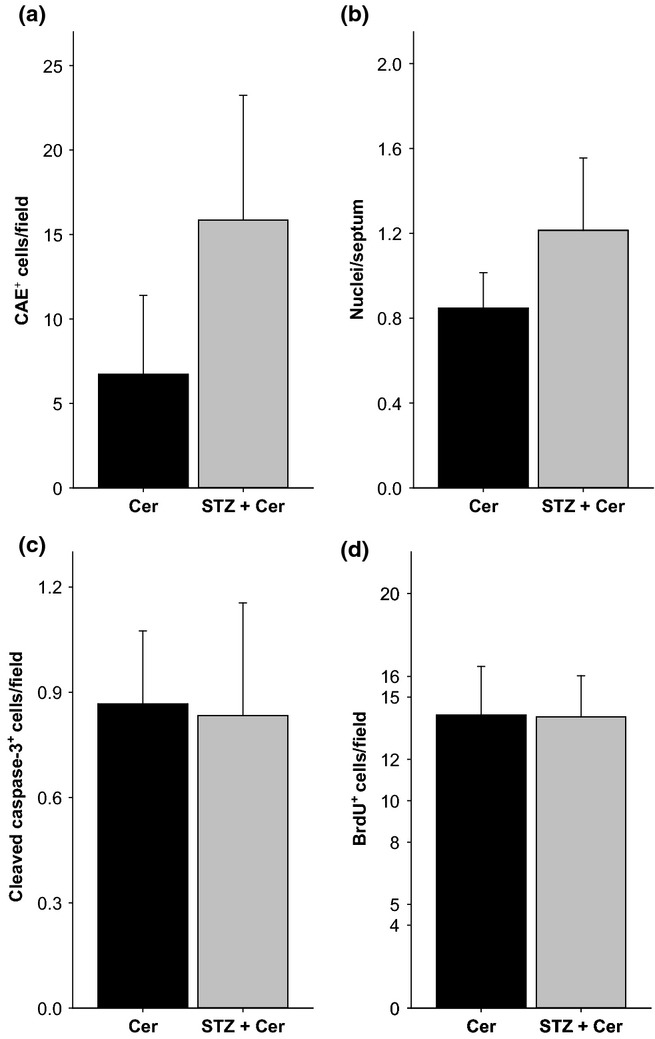
Evaluation of inflammation and cell death in the lung on day 30. The number of CAE+ inflammatory cells per field (a), the alveolar cellularity index (b), the number of cleaved caspase-3+ cells in the lung epithelium per field (c) and the number of BrdU+ cells per field (d) were quantified. Bar charts indicate the average and standard deviation. The number of animals evaluated for each cohort was n = 4. No significance was observed by Mann–Whitney rank sum test followed by Bonferroni correction for repeated analysis.
Discussion
The presented data demonstrate that diabetes during AP (i) enhances IL-6 concentration in blood plasma while decreasing the number of lymphocytes in the blood, (ii) only marginally increases pancreatitis induced lung inflammation, but (iii) does not lead to major cell death or proliferation in the lung epithelium. We conclude that diabetes has a fundamental influence on the progression of pancreatitis at a local level as published previously (Zechner et al. 2012, 2013, 2014) and can also increase systemic inflammatory parameters such as IL-6 concentration in blood plasma. However, these data also suggest that the observed strong aggravation of pancreatitis by diabetes leads neither to strong enhancement of lung inflammation nor to induction of cell death in the lung epithelium.
Redundant administration of cerulein causes an oedematous form of AP, which is associated with lung inflammation and a very mild form of lung injury (Elder et al. 2011). This animal model system should be ideal to test if additional parameters such as diabetes aggravate lung injury, but will not detect a possible inhibition of pancreatitis induced lung injury. The seemingly contradictory result, that diabetes worsens AP leading to a severe form of pancreatitis, but does not cause lung injury, could be explained by the following assumption. Possibly diabetes aggravates pancreatitis, but at the same time reduces the risk for lung injury. This conclusion that lung injury is not aggravated but rather reduced by diabetes is supported by clinical as well as experimental studies. For example, diabetes predicts mortality in critically ill patients, but is not associated with ALI (Koh et al. 2012). A meta-analysis also suggests that pre-existing diabetes leads to reduced rather than increased risk of lung injury in critically ill patients (Gu et al. 2014). Diabetes also does not increase, but reduces the risk for lung dysfunction in patients with sepsis (Esper et al. 2009; Yang et al. 2011). Experiments in rats demonstrate that sepsis-induced ALI is milder in diabetic rats than in normoglycaemic controls (Filgueiras et al. 2012). Although a few studies suggest that in specific model systems, pre-existing diabetes can also increase the risk of lung injury (Hagiwara et al. 2011; Xiong et al. 2012) a consensus seems to develop that diabetes is protective against lung injury (Honiden & Gong 2009). Our presented data and the above cited literature, therefore, suggest that it might be especially valuable to carefully adjust glucose concentration to avoid local complications during AP, but that hyperglycaemia might not increase the risk of lung injury during AP.
Acknowledgments
We thank Berit Blendow, Dorothea Frenz, Eva Lorbeer-Rehfeldt and Maren Nerowski (Institute for Experimental Surgery, University of Rostock) for excellent technical assistance.
Conflict of interests
The authors declare that there is no conflict of interest.
Funding
Supported by the Forschungsförderung der Medizinischen Fakultät der Rostocker Universität (FORUN) (project 889017).
References
- Bhatia M, Brady M, Zagorski J, et al. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838–844. doi: 10.1136/gut.47.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowski A, Spitzner M, Bethge S, Mueller-Graf F, Vollmar B. Zechner D. Risk factors for pancreatic ductal adenocarcinoma specifically stimulate pancreatic duct glands in mice. Am. J. Pathol. 2013;182:965–974. doi: 10.1016/j.ajpath.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Elder AS, Saccone GT, Bersten AD. Dixon DL. Caerulein-induced acute pancreatitis results in mild lung inflammation and altered respiratory mechanics. Exp. Lung Res. 2011;37:69–77. doi: 10.3109/01902148.2010.516307. [DOI] [PubMed] [Google Scholar]
- Esper AM, Moss M. Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit. Care. 2009;13:R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueiras LR, Martins JO, Serezani CH, Capelozzi VL, Montes MB. Jancar S. Sepsis-induced acute lung injury (ALI) is milder in diabetic rats and correlates with impaired NFkB activation. PLoS One. 2012;7:e44987. doi: 10.1371/journal.pone.0044987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsmark CE. Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Frossard JL, Saluja A, Bhagat L, et al. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- Frossard JL, Kwak B, Chanson M, Morel P, Hadengue A. Mach F. Cd40 ligand-deficient mice are protected against cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2001;121:184–194. doi: 10.1053/gast.2001.25483. [DOI] [PubMed] [Google Scholar]
- Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes. Metab. 2010;12:766–771. doi: 10.1111/j.1463-1326.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- Gregoric P, Sijacki A, Stankovic S, et al. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepatogastroenterology. 2010;57:349–353. [PubMed] [Google Scholar]
- Gu WJ, Wan YD, Tie HT, Kan QC. Sun TW. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PLoS One. 2014;9:e90426. doi: 10.1371/journal.pone.0090426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guice KS, Oldham KT, Caty MG, Johnson KJ. Ward PA. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann. Surg. 1989;210:740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Shingu C, Matumoto S, Hasegawa A. Noguchi T. The effect of experimental diabetes on high mobility group box 1 protein expression in endotoxin-induced acute lung injury. J. Surg. Res. 2011;168:111–118. doi: 10.1016/j.jss.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Honiden S. Gong MN. Diabetes, insulin, and development of acute lung injury. Crit. Care Med. 2009;37:2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh GC, Vlaar AP, Hofstra JJ, et al. In the critically ill patient, diabetes predicts mortality independent of statin therapy but is not associated with acute lung injury: a cohort study. Crit. Care Med. 2012;40:1835–1843. doi: 10.1097/CCM.0b013e31824e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentula P, Kylanpaa ML, Kemppainen E. Puolakkainen P. Obesity correlates with early hyperglycemia in patients with acute pancreatitis who developed organ failure. Pancreas. 2008;36:e21–e25. doi: 10.1097/mpa.0b013e31814b22b5. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Honda H, Tashiro M, Taguchi M, Yoshikawa H. Otsuki M. Increased expression of 19-kD interacting protein-3-like protein and the relationship to apoptosis in the lung of rats with severe acute pancreatitis. Crit. Care Med. 2003;31:2527–2534. doi: 10.1097/01.CCM.0000090006.49055.6D. [DOI] [PubMed] [Google Scholar]
- Noel RA, Braun DK, Patterson RE. Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834–838. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor CM, Vonlaufen A, Georgi F, Hadengue A, Morel P. Frossard JL. Neutrophil depletion-but not prevention of Kupffer cell activation-decreases the severity of cerulein-induced acute pancreatitis. World J. Gastroenterol. 2006;12:1219–1224. doi: 10.3748/wjg.v12.i8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam SG. Martin IG. Admission serum glucose level: an accurate predictor of outcome in gallstone pancreatitis. Pancreas. 2006;33:27–30. doi: 10.1097/01.mpa.0000222315.36490.9b. [DOI] [PubMed] [Google Scholar]
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K. Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg. Gynecol. Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- Seicean A, Tantau M, Grigorescu M, Mocan T, Seicean R. Pop T. Mortality risk factors in chronic pancreatitis. J. Gastrointestin. Liver Dis. 2006;15:21–26. [PubMed] [Google Scholar]
- Sharif R, Dawra R, Wasiluk K, et al. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–819. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- Takeyama Y, Takas K, Ueda T, Hori Y, Goshima M. Kuroda Y. Peripheral lymphocyte reduction in severe acute pancreatitis is caused by apoptotic cell death. J. Gastrointest. Surg. 2000;4:379–387. doi: 10.1016/s1091-255x(00)80016-5. [DOI] [PubMed] [Google Scholar]
- Tascilar O, Cakmak GK, Tekin IO, et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J. Gastroenterol. 2007;13:6172–6182. doi: 10.3748/wjg.v13.i46.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XQ, Wang WT, Wang LR, Jin LD. Lin LN. Diabetes increases inflammation and lung injury associated with protective ventilation strategy in mice. Int. Immunopharmacol. 2012;13:280–283. doi: 10.1016/j.intimp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Xue Y, Sheng Y, Dai H, Cao H, Liu Z. Li Z. Risk of development of acute pancreatitis with pre-existing diabetes: a meta-analysis. Eur. J. Gastroenterol. Hepatol. 2012;24:1092–1098. doi: 10.1097/MEG.0b013e328355a487. [DOI] [PubMed] [Google Scholar]
- Yang Y, Salam ZH, Ong BC. Yang KS. Respiratory dysfunction in patients with sepsis: protective effect of diabetes mellitus. Am. J. Crit. Care. 2011;20:e41–e47. doi: 10.4037/ajcc2011391. [DOI] [PubMed] [Google Scholar]
- Yuan YZ, Gong ZH, Lou KX, Tu SP, Zhai ZK. Xu JY. Involvement of apoptosis of alveolar epithelial cells in acute pancreatitis-associated lung injury. World J. Gastroenterol. 2000;6:920–924. doi: 10.3748/wjg.v6.i6.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Spitzner M, Bobrowski A, Knapp N, Kuhla A. Vollmar B. Diabetes aggravates acute pancreatitis and inhibits pancreas regeneration in mice. Diabetologia. 2012;55:1526–1534. doi: 10.1007/s00125-012-2479-3. [DOI] [PubMed] [Google Scholar]
- Zechner D, Sempert K, Genz B, et al. Impact of hyperglycemia and acute pancreatitis on the receptor for advanced glycation endproducts. Int. J. Clin. Exp. Pathol. 2013;6:2021–3029. [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Knapp N, Bobrowski A, Radecke T, Genz B. Vollmar B. Diabetes increases pancreatic fibrosis during chronic inflammation. Exp. Biol. Med. (Maywood) 2014;239:670–676. doi: 10.1177/1535370214527890. [DOI] [PubMed] [Google Scholar]
- Zhang H, Neuhöfer P, Song L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019–1031. doi: 10.1172/JCI64931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MT, Chen CS, Chen BC, Zhang QY. Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J. Gastroenterol. 2010;16:2094–2099. doi: 10.3748/wjg.v16.i17.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]


