The current European Society for Immunodeficiencies (ESID) registry was established 10 years ago in 2004, when the system was moved from a paper-based to an online system. The purpose of the registry is to collect data on European patients with primary immunodeficiencies (PIDs) and their treatment, with the aim of building an easily accessible database for use by physicians and researchers.
During the 10 years for which the current registry has been operational, the number of patients registered has grown substantially; after 4 years, more than 7000 patients were registered and, as of 25 June, 19 355 patients are registered in Europe as having a PID. Longitudinal data (20 or more data sets, documented every 6 months) is now available for some of the patients in the registry. Children younger than age 15 years represent two-thirds of this cohort. More than half the patients (57%) have an antibody disorder, and this is also the group with the greatest number of adult patients. Of the 19 355 registered, treatment data are available on approximately 14 000, of whom 6476 receive immunoglobulin (Ig) treatment; 4239 patients are receiving intravenous immunoglobulin (IVIg) treatment and approximately 2181 receive subcutaneous immunoglobulin (SCIg).
One of the interesting comparisons arising from the registry data is the difference in minimum prevalence of PIDs between countries. In France, for example, 6·058 cases are documented per 100 000 inhabitants; much higher than in Switzerland (4·157 per 100 000) or Germany (2·105 per 100 000). This is most probably because France has a very efficient documenting system, whereby designated documenters, financed by local grants in France, visit centres to enter patients' data into the registry. Thus, the more efficient the documenting system, the higher the quality of data collected.
Physicians and researchers can apply for access to the registry data by writing to ESID with details of their specific project, or with a proposal to collect new and different data on patients with specific diseases. Despite the large number of patients in the registry and the large amount of data collected, only 23 papers have been published, which is an average of more than two per year over 10 years. However, considering that more than 200 physicians and researchers have access to the database, this still means that the registry is one of the most under-used data sets of its kind in Europe.
In a recent registry publication we analysed the subset of common variable immunodeficiency (CVID) subjects 1. There are more than 4000 CVID subjects in the registry, but because not all have complete data sets, the cohort described in this paper included 2212 subjects.
As CVID has a variable clinical presentation, we investigated the frequencies of different disease phenotypes in the cohort 1. The most common clinical features were pneumonia (32%) and autoimmunity (29%). Other features included splenomegaly (26%), bronchiectasis (23%) and granulomatous disease (10%), and a further 10% had enteropathy. Surprisingly, 5% of patients had solid tumours and 4% had meningitis/encephalitis [conditions associated more usually with X-linked agammaglobulinaemia (XLA)]. Less frequent clinical features were lymphoma, found in 3% of the patients, splenectomy in 2% and lobectomy in 1%.
With regard to how efficiently patients are being diagnosed, CVID has been described as having a bi-modal age of manifestation, with a peak in diagnoses between the ages of 5 and 10 years, a trough around the age of 20, and then another peak between 30 and 40 years. However, when patients are asked about the onset of their symptoms the reported age is lower, with most patients experiencing recurrent infections in childhood, and fewer than half manifesting after age 20 (Fig. 1). In some countries, the delay between onset of symptoms and CVID diagnosis is greater than others, the average being 4·1 years, ranging from 1·8 years in Poland to 7 years in France 1.
Figure 1.
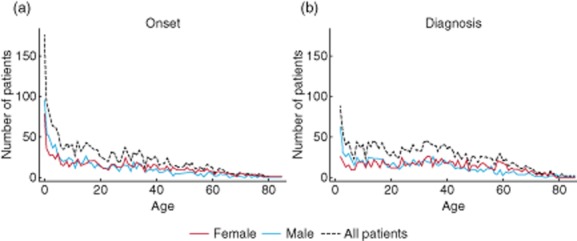
(a) Age at onset of symptoms in the total cohort (n = 1914), among female patients (n = 985) and among male patients (n = 929). (b) Age at diagnosis in the total cohort (n = 2134), among female patients (n = 1094) and among male patients (n = 1040). An age of 0 years means less than 12 months of age (reproduced from 1, copyright 2014, with permission from Elsevier).
We showed that adult CVID cases were diagnosed more promptly than paediatric cases (aged <10 years), suggesting that educational programmes may be required to raise awareness of CVID in general practitioners and paediatricians.
To ascertain whether the diagnostic delay had improved during the last 20 years, we compared age of onset and time of diagnosis, grouped into 3-year intervals, between 1987 and 2010. Throughout that period, we found no improvement in the diagnostic delay (P = 0·14).
Our study also highlighted the variation in the dose of Ig being administered to CVID patients in centres across Europe. This is accounted for by variations in dosing regimens; for example, one of the highest median monthly doses was recorded at Barts and the London Hospital, where the policy is to start patients on very high doses to raise their Ig trough levels quickly, and then subsequently reduce the dose. This centre has a median monthly dosage per patient of 711 mg/kg body weight. In contrast, the centre in Prague had the lowest median monthly dose per patient of 129 mg/kg body weight, due partly to problems with reimbursement of adult patients for Ig treatment. Since the assessment was carried out, the Czech health authorities have altered their policies.
As shown by Orange et al. 2, the CVID subregistry analysis indicated that higher Ig trough levels correlated with fewer serious infections in this cohort. Nevertheless, higher Ig trough levels did not affect the number of days patients spent in hospital. This is due most probably to the fact that, as CVID has such a variety of symptoms, patients are not only hospitalized for infections, but for other comorbid disease including autoimmune conditions, enteropathy and lymphoma.
Finally, we compared outcomes for CVID patients receiving IVIg and those receiving SCIg treatment. We concur with previously published results 2 showing that higher trough Ig levels lead to a reduction of serious infections in patients receiving IVIg, but this could not be shown in the patients receiving SCIg. One possible reason is that physicians are administering SCIg to relatively healthy patients and reserving IVIg for those who are more severely ill, but only a prospective clinical trial will resolve this question.
The registry has recently been redesigned to increase the quality and completeness of data collected in the database. The new system now has three levels of data collection: level 1 encompasses data fields that are mandatory for all who are registered, thus enabling epidemiological studies. Levels 2 and 3 are optional, with level 2 including diagnostic and follow-up data, and level 3 will comprise projects running for limited time-periods to collect data for use in clinical studies.
For the last 10 years, the ESID registry has been funded primarily by the Plasma Protein Therapeutics Association (PPTA) and pharmaceutical company sponsorship. The total cost of the ESID registry has been estimated at approximately €2 million per year, with much of the cost being shouldered by the documenting centres whose doctors, nurses and students record data into the registry. The PPTA has recently withdrawn its funding of the registry, and we envisage the need for core support from the pharmaceutical industry, to support level 3 projects especially, and allow the registry to continue to evolve as a useful tool for research, diagnosis and treatment in the field of PIDs.
Acknowledgments
The author acknowledges the tremendous work of all ESID documenting centres for their participation in the ESID registry project (a list of contributors can be found on http://esid.org/Working-Parties/Registry/Documenting-centers), and specifically Dr Gerhard Kindle and Mr Benjamin Gathmann for collecting the data for this presentation. The author would like to thank Meridian HealthComms Ltd for providing medical writing services.
Disclosure
The author has obtained a speaker's honorarium from CSL Behring for presenting at this meeting.
References
- Gathmann B, Mahlaoui N, Gerard L, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]


