Patients with primary antibody deficiency (PAD) remain susceptible to recurrent respiratory tract infections, including infections of the sinuses, throat and upper and lower respiratory tract, despite seemingly appropriate immunoglobulin (Ig) therapy 1–3. Important questions remain as to how best to detect and diagnose these infections and potential subclinical disease, as well as how to modify treatment to improve outcomes.
Common variable immunodeficiency (CVID) is characterized by reduced serum levels of IgG, IgA and/or IgM, with a failure to produce specific antibodies. The standard treatment for CVID patients is intravenous (i.v.) or subcutaneous (s.c.) replacement with IgG. Analyses of large cohorts of patients with CVID show that alongside cancer, chronic lung disease remains the major cause of morbidity and mortality 2.
It has been shown that there is an ongoing decline in lung function above predicted levels in PAD patients and that is greater than the decline which would occur in heavy smokers 4. The results of several studies have shown a decline in lung function over time, which has been associated with lower IgG dose, IgG trough levels <5 g/l, low IgA and low mannose-binding lectin levels 5. Risk of pneumonia in CVID is associated similarly with sinusitis, bronchiectasis, low IgG trough, low IgA and low class-switched memory B cells 5. Patients with levels of IgA closer to normal have a milder phenotype, suggesting that the absence of IgA acts like a second defect or co-factor in addition to the low IgG 6. In addition, IgM antibodies, which are also transported to the mucosal surface, appear to confer a degree of protection against colonization of the airway epithelia with Haemophilus influenzae in patients with hypogammaglobulinaemia 7.
There is a clear relationship between IgG dose and IgG trough levels, with wide interindividual variation and an inverse correlation between trough and infection outcome, supporting the use of higher doses of IgG in reducing infections, particularly severe infections such as pneumonia. Meta-analyses of clinical studies of IgG replacement for primary immunodeficiency (PID) patients have shown that, with each 100 mg/dl increment of IVIg trough level, the occurrence of pneumonia is reduced by 27%, with similar trends observed for SCIg 8,9. However, the IgG trough levels required to prevent breakthrough bacterial infections vary between patients, suggesting that individualized dosing strategies are needed 10. In addition, CVID patients with existing complications, such as bronchiectasis or with particular clinical phenotypes, may require higher IgG replacement doses than those without complications to achieve the same protective trough IgG levels 5. This is also the case for patients with X-linked agammaglobulinaemia (XLA) who require higher IgG doses 5. These findings are corroborated further by the finding of increased risk of infections in the last week of the IVIg dosing cycle, when the IgG levels are at their nadir 11.
Several studies have demonstrated the presence of subclinical infections with bacterial, fungal and viral pathogens 12,13. Patients often describe the coincidence of upper airway sinus and respiratory tract infections, or the development of a secondary bacterial chest infection, following a viral upper respiratory tract infection. Sinusitis and upper respiratory tract infections caused by viral and bacterial pathogens remain the most common reported infections in this group of patients despite IgG replacement therapy.
Bacteria such as non-encapsulated, non-typeable H. influenzae, Streptococcus pneumoniae and Moraxella catarrhalis are the most frequently causative agents of recurrent pneumonia, bronchitis, sinusitis and otitis in PID patients 7 although, without testing, it is unclear how many episodes of bacterial infection are preceded by viral upper airway infections in PAD. Studies in asthma patients, however, show a clear increase in the detection of bacteria during and following a rhinovirus (HRV) upper airway infection with a concomitant increase in asthma exacerbations 14.
Appropriate IgG dosing regimens have significantly reduced the incidence of bacterial pneumonia in PAD; however, the level of possibly less severe respiratory tract infections has continued over time, as shown in Fig. 1.
Figure 1.
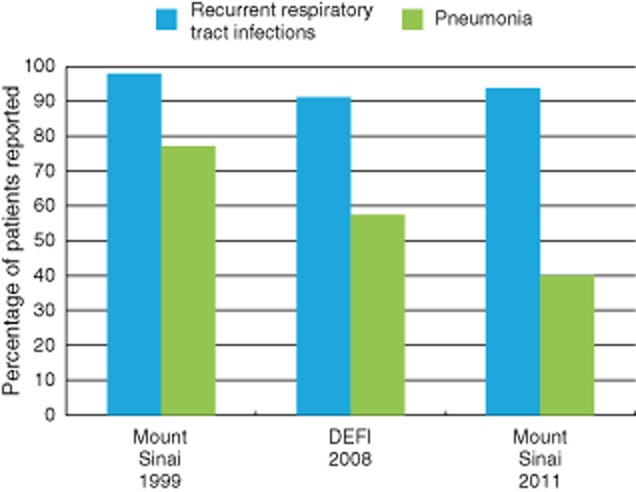
A combined analysis of data on the percentage of patients experiencing pneumonia and recurrent respiratory tract infections is shown in three large series of common variable immunodeficiency (CVID) patients in 1999, 2008 and 2011 [two from the same cohort in the United States 12 years apart (248 and 252 patients) and one from a French cohort (473 patients)]. Accepting the potential difficulties in the interpretation of combined data from several sources, there is a decline in the percentage of patients' pneumonia over time, perhaps representing a change in immunoglobulin (Ig) dosing or greater use of prophylactic antibiotics. In contrast the percentage of patients with recurrent respiratory tract infections has remained high at more than 90% in all of the studies. This term will include infections of the sinuses, throat, upper and lower respiratory tract which do not fulfil criteria for pneumonia.
PAD patients also have a higher frequency of upper respiratory tract infections with HRV, and the period of viral shedding in these patients is much longer than in immunocompetent children or adults (P < 0·001, log-rank test), despite IgG replacement therapy 13,15. The mean (95% confidence interval) duration of HRV shedding was found to be 11·4 (8·2–14·7) days in children, 10·1 (7·4–12·9) days in adults and 40·9 (26·4–55·4) days in patients with hypogammaglobulinaemia (P < 0·001) (Fig. 2). The duration of respiratory tract symptoms correlated with the duration of virus shedding (P = 0·002), and new infections by another HRV type soon after the first episode were common 15. Taken together, these data suggest that upper airway infection, in particular HRV infections, are frequent, prolonged and clinically significant in PAD patients.
Figure 2.
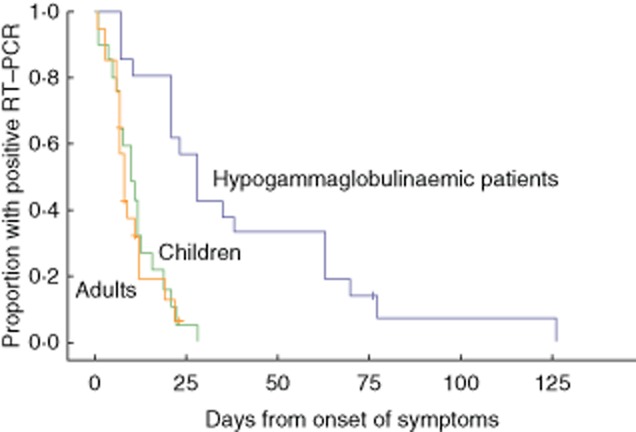
Virus shedding after human rhinovirus (HRV) infection (reproduced from 15, copyright 2013 European Society of Clinical Microbiology and Infectious Diseases, with permission from John Wiley and Sons). The striking difference in the prolongation of duration of shedding of rhinovirus between immunocompetent children and adults alongside primary antibody-deficient patients on immunoglobulin (Ig) replacement therapy is shown.
Additionally, although fungal respiratory tract infections are reportedly rare, in a cohort of 90 patients with confirmed CVID five episodes of fungal infections were identified; one was severe and four were non-invasive moderate infections 5.
Detection of subclinical infections is challenging, but may be achieved with improved clinical, immunological and microbiological vigilance and characterization. The combined use of more aggressive microbiological sampling, molecular detection of pathogens by polymerase chain reaction (PCR), improved delineation of the immunological defect [e.g. pan-hypogammaglobulinaemia (PHG) versus mucosal IgA, combined immunodeficiency (CID)] and improved delineation of the clinical defects (sinusitis, bronchiectasis) using lung and upper airway imaging techniques such as magnetic resonance imaging 16, where repeated assessments are needed, are likely to contribute to a better understanding of the heterogeneity of CVID patients, which will ultimately individualize and improve patient care.
The aetiology of progressive structural lung disease is likely to be multi-factorial, with a role for infection and subclinical infection as well as inflammatory changes which are unrelated to infection. The role of the mucosal immune system (IgA and IgM) in modifying outcomes in PAD has been shown alongside sinusitis and more frequent and prolonged viral infections. The recognition, diagnosis and treatment of pathology of the upper airway must not be forgotten, as this is the gateway for infections of the lower airways and thus ongoing lung damage. This suggests that a more detailed understanding of these mechanisms will help to define how the challenges of subclinical infection could be met by targeted therapies, such as optimized systemic or topical Ig replacement strategies, improved antibiotics, anti-virals and interferon alpha 17, aimed at preventing or treating infection before lasting damage occurs.
Acknowledgments
S. J. is supported by a NISCHR Fellowship. This work summarizes the discussions of a meeting of European Immunologists to address subclinical infection: Hilary Longhurst (UK), Pere Soler-Palacin (Spain), Silvia Sánchez-Ramón (Spain), Esther de Vries (the Netherlands), Isabella Quinti (Italy), Andrea Matucci (Italy), Carlo Agostini (Italy), Stephan Ehl (Germany), Klaus Warnatz (Germany), Benoit Florkin (Belgium), Filomeen Haerynck (Belgium), Louis-Jean Couderc (France), Alison Jones (UK), Nicholas Brodszki (Sweden) and Stephen Jolles (UK).
Disclosures
S. J. has received support for consulting, conferences and/or research from CSL Behring, Baxter, BPL, Biotest, Octapharma, Shire, and SOBI.
References
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1:545–556. doi: 10.1016/j.jaip.2013.09.015. quiz 57. [DOI] [PubMed] [Google Scholar]
- Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stirling RG, Paul E, Hore-Lacy F, Thompson BR, Douglass JA. Longitudinal decline in lung function in patients with primary immunoglobulin deficiencies. J Allergy Clin Immunol. 2011;127:1414–1417. doi: 10.1016/j.jaci.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125:1354–1360. doi: 10.1016/j.jaci.2010.02.040. e4. [DOI] [PubMed] [Google Scholar]
- Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- Micol R, Kayal S, Mahlaoui N, et al. Protective effect of IgM against colonization of the respiratory tract by nontypeable Haemophilus influenzae in patients with hypogammaglobulinemia. J Allergy Clin Immunol. 2012;129:770–777. doi: 10.1016/j.jaci.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Orange JS, Belohradsky BH, Berger M, et al. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin Exp Immunol. 2012;169:172–181. doi: 10.1111/j.1365-2249.2012.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122:210–212. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Bexon MBJ, Rojavin M, Berger M, Zenker O. Increased frequency of infections at the end of the IVIG dosing cycle: effect characterization from three phase III studies. American Academy of Allergy, Asthma and Immunology (AAAAI) Meeting 2012; Abstract 730.
- Kainulainen L, Nikoskelainen J, Vuorinen T, Tevola K, Liippo K, Ruuskanen O. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Respir Crit Care Med. 1999;159:1199–1204. doi: 10.1164/ajrccm.159.4.9807067. [DOI] [PubMed] [Google Scholar]
- Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 2010;126:120–126. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepfer KM, Lee WM, Pappas TE, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307. doi: 10.1016/j.jaci.2014.02.030. 7 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola V, Waris M, Kainulainen L, Kero J, Ruuskanen O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin Microbiol Infect. 2013;19:E322–327. doi: 10.1111/1469-0691.12193. [DOI] [PubMed] [Google Scholar]
- Serra G, Milito C, Mitrevski M, et al. Lung MRI as a possible alternative to CT scan for patients with primary immune deficiencies and increased radiosensitivity. Chest. 2011;140:1581–1589. doi: 10.1378/chest.10-3147. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O, Waris M, Kainulainen L. Treatment of persistent rhinovirus infection with pegylated interferon alpha2a and ribavirin in patients with hypogammaglobulinemia. Clin Infect Dis. 2014;58:1784–1786. doi: 10.1093/cid/ciu169. [DOI] [PMC free article] [PubMed] [Google Scholar]


