Immunoglobulin (Ig)G replacement is the cornerstone of treatment for antibody production deficiency. Although monthly administration of intravenous immunoglobulin (IVIg) has been in widespread clinical use for more than 30 years there are several issues with this therapy, including the requirement for intravenous (i.v.) access, monitoring by a health-care professional, mild and severe systemic adverse events (AEs) and impact on patients' quality of life. As a result, subcutaneous immunoglobulin (SCIg) therapy is becoming increasingly popular as i.v. access is unnecessary, self-administration is possible and there is a lower frequency of mild systemic AEs than is seen with IVIg.
Comparative studies of IVIg and SCIg therapy in primary immunodeficiency (PID) patients have shown SCIg to be as efficacious as IVIg. Other studies have shown that SCIg administration is associated with improved patient quality of life 1,2. However, SCIg requires a more frequent administration regimen, typically once every 1 or 2 weeks. In addition, multiple injection sites are required to achieve an infusate volume yielding a dose similar to monthly IVIg. Hyaluronan, an extracellular matrix component that forms a barrier to bulk movement of molecules and fluid in the interstitial space 3, limits the amount of SCIg that can be administered in a single site.
The limited capacity of the subcutaneous (s.c.) space has been addressed by increasing the number of infusion sites, increasing the infusion frequency 4 and increasing the concentration of the Ig product. A completely different strategy has been to facilitate SCIg administration by pre-infusion of recombinant human hyaluronidase (rHuPH20) (Fig. 1). rHuPH20 is a recombinant form of the native sperm-derived hyaluronidase, PH20, and was selected because of its highly specific activity at physiological pH and its extracellular action. This enzyme acts by depolymerizing hyaluronan, thereby increasing the hydraulic conductivity in the interstitium leading to higher infusion rates, dramatically increased infusion volumes and higher IgG bioavailability. The short local half-life of rHuPH20 in the s.c. tissue (<30 min) allows the complete restoration of tissue integrity within 24–48 h 3,5; s.c. administration of 10% Ig with a pre-infusion of rHuPH20 (referred to hereafter as IGHy) allows the infusion of a full month's dose of IgG to most PID patients at a single site with an infusion time comparable to IVIg 6.
Figure 1.
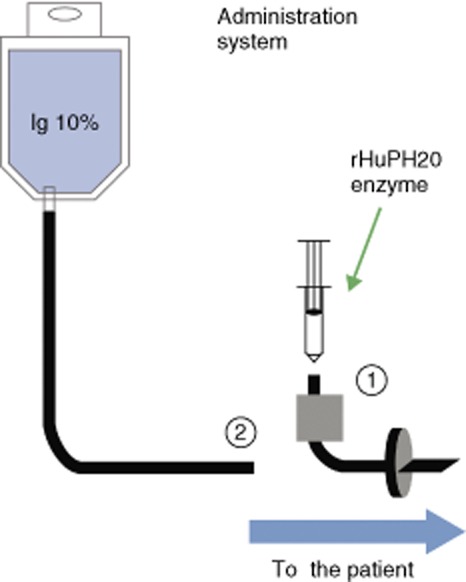
Subcutaneous (s.c.) administration of 10% immunoglobulin (Ig) with a pre-infusion of recombinant hyaluronidase (IGHy). (1) Recombinant hyaluronidase (rHuPH20) is initially infused in the s.c. infusion set; (2) within 10 min, Ig 10% is infused through the same infusion line.
A non-randomized, open-label, prospective, multi-centre Phase III pivotal trial (study 160603; Clinicaltrials.gov identifier: NCT00814320) was conducted to compare the pharmacokinetic characteristics of IGHy with conventional 10% IVIg in PID patients 6. Safety, efficacy and tolerability were compared to historical standards. Of the 89 patients enrolled, 83 received at least one dose of IGHy, five patients withdrew due to related adverse events and 68 completed the study 6.
The rate of acute serious bacterial infections during IGHy treatment in the pivotal trial was 0·025 per patient per year. The overall infection rate was 2·97 per patient per year for IGHy compared with 4·51 for standard IVIg treatment during that trial 6.
The majority of adverse reactions to IGHy were mild (265 of 384) or moderate (114 of 384), with only five severe reactions being reported. Three cases were associated with severe local reactions, which included infusion site pain, infusion site swelling and genital oedema that resulted from the spread of IGHy in the s.c. space. All resolved with no sequelae. The two severe systemic reactions were migraine and oral pain. No drug-related serious AEs occurred in this study 6.
No patients developed anti-rHuPH20 neutralizing antibodies, despite detection of antibodies that bound hyaluronidase in 13 patients, as determined by a highly sensitive immunoreactive bridging assay. In eight of 13 patients, antibody titres exceeded the threshold on only one or two occasions. Anti-rHuPH20 antibodies declined to below threshold in all five patients with titres that were elevated on more than two occasions, despite continued exposure to rHuPH20 in four of five subjects 6. There was no correlation between the presence of these antibodies and local or systemic AEs (including redness, itching, urticaria or laboratory abnormalities).
In order to limit phlebotomy and total blood drawn, the pharmacokinetic study was not conducted in patients aged 2 to <12 years. In this patient group, the trough IgG and specific antibody titres were measured and used to adjust doses to ensure pharmacokinetic equivalence. Serum trough levels were comparable between IGHy and IVIg but, as expected, peak levels were higher in the latter. The median time to reach the peak IgG serum level with IGHy was similar to SCIg (approximately 5 days); however, there was less peak-to-trough variability within the infusion interval with the IGHy preparation (Fig. 2). When administered at 108% of the IVIg dose, the pharmacokinetic equivalence of IGHy relative to IVIg was 93·3% (two-sided 90% confidence interval, 91·4–95·2%), as measured by area under the curve (AUC) 6.
Figure 2.
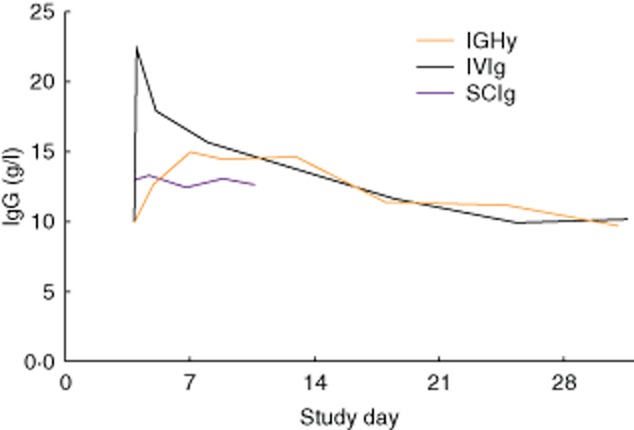
Serum immunoglobulin (Ig)G concentration for 10% Ig with a pre-infusion of recombinant hyaluronidase (IGHy) compared with intravenous immunoglobulin (IVIg) and subcutaneous immunoglobulin (SCIg). Representative pharmacokinetic curves for one subject comparing a 4-week infusion of IVIg, a weekly infusion of SCIg 10% at 143% of the intravenous (i.v.) dose with the same data points extended across the 4-week period to facilitate comparison with the other curves and a 4-week infusion of IGHy at 104% of the i.v. dose (reproduced from 6, copyright 2012, with permission from Elsevier).
Subjects who completed the pivotal trial were eligible to enter the extension study (study 160902; Clinicaltrials.gov identifier: NCT01175213), during which they continued IGHy therapy at the same dose and frequency as that with IVIg, i.e. every 3–4 weeks 7. A total of 66 patients (of 68 who completed the pivotal trial) enrolled and 49 patients completed the extension study. Following 3 months of treatment, some patients switched voluntarily to a 2-week dosing interval to evaluate the effects on IgG trough levels and evaluate tolerability relative to 4-weekly infusions. In these patients, the IgG trough levels increased by 12%. In an interim analysis at 48 weeks' treatment for the last patient enrolled, there were no severe reactions reported and the AEs profile was similar to that in the pivotal trial 3.
Although there were no clinical safety signals regarding the use of IGHy, exposure to the rHuPH20 component of this Ig preparation was discontinued after approximately 3 years, in July 2012, pending review of additional preclinical data by the Food and Drug Administration (FDA) on the potential impact of antibodies that bind rHuPH20. Patients were followed for an additional 24–48 weeks while receiving i.v. or conventional s.c. treatment 3.
In conclusion, infusion of IGHy for the treatment of patients with PID provides the combined benefits of both s.c and i.v. administration: monthly administration in the home setting at an infusion rate comparable to IVIg and a low rate of systemic AEs similar to the rate seen with SCIg.
IGHy was approved by the European Medicines Agency (EMA) for treatment of adults (>18 years of age) with PID in the European Union in March 2013 and by the FDA for treatment of adults (>16 years of age) with PID in the United States in September 2014.
Acknowledgments
R. L. W. would like to thank Meridian HealthComms Ltd for providing medical writing services.
Disclosures
R. L. W. has received investigator support, consultant and speaker honoraria from ADMA, Baxter Healthcare, Bioplasma Laboratories, CSL Behring, Kedrion and Therapure.
References
- Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. doi: 10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;28:779–802. doi: 10.1016/j.iac.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Wasserman RL. Overview of recombinant human hyaluronidase-facilitated subcutaneous infusion of IgG in primary immunodeficiencies. Immunotherapy. 2014;6:553–567. doi: 10.2217/imt.14.34. [DOI] [PubMed] [Google Scholar]
- Shapiro RS. Subcutaneous immunoglobulin therapy given by subcutaneous rapid push vs infusion pump: a retrospective analysis. Ann Allergy Asthma Immunol. 111:51–55. doi: 10.1016/j.anai.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4:427–440. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- Wasserman RL, Melamed I, Stein MR, et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol. 2012;130:951–957. doi: 10.1016/j.jaci.2012.06.021. e11. [DOI] [PubMed] [Google Scholar]
- Melamed I, Wasserman R, Stein MR, et al. 2012. Long-term safety and pharmacokinetics of facilitated-subcutaneous infusion of human immune globulin G, 10%, and recombinant human hyaluronidase: phase 3 extension study in patients with primary immunodeficiencies . Poster P213 presented at the American College of Allergy, Asthma and Immunology Meeting.


