The Fc-gamma receptors (FcγRs) are receptors for the Fc region of immunoglobulin (Ig)G, and are involved in a multitude of innate and adaptive immune responses, including mediating the specific recognition of antigens by leucocytes 1,2. Depending on their affinity for IgG, FcγRs can be divided into the high-affinity FcγRI, which binds monomeric IgG, and the low-affinity receptors FcγRIIa, b and c and FcγRIIIa and b 1,3. FcγRIIb is the only inhibitory receptor and contains an intracellular tyrosine-based inhibition motif (ITIM) (Fig 1). Although the FcγRII and -III receptors display low affinity for monomeric IgG, they are capable of binding to aggregated IgG through multimeric low-affinity, high-avidity interactions 1. FcγRs are widely expressed throughout the haematopoietic system; however, the expression profile of each FcγR varies, with FcγRI being expressed on macrophage, neutrophils and eosinophils (Fig. 1).
Figure 1.
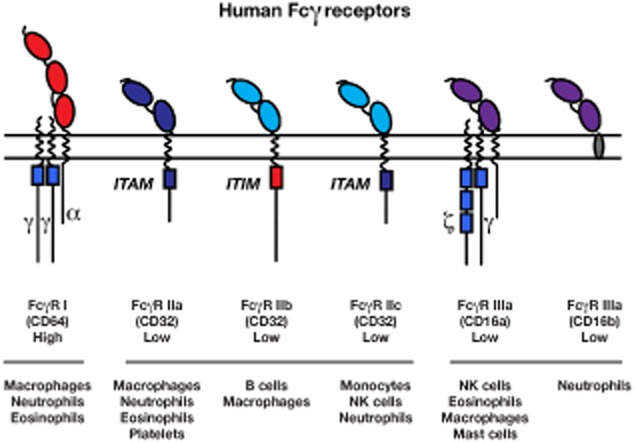
Overview of human Fcγ receptors, including affinity for immunoglobulin (Ig) G and expression profile. ITAM = immunoreceptor tyrosine-based activation motif; NK = natural killer; ITIM = intracellular tyrosine-based inhibition motif.
The genes encoding the FcγRIIa, b c and FcγRIIIb and c are located in a cluster on chromosome 1 3. The FCGR2C gene is located close to FCGR2B, and contains eight exons which are highly homologous to exons 1–6 from FCGR2B and exons 7–8 from FCGR2A, probably resulting from a cross-over event between these two genes 3,4. Multiple genetic variations, including single nucleotide polymorphisms (SNPs) and copy number variation (CNV), have been identified in Fc receptors, in particular the low-affinity FcγRs, which affect receptor function, and have been associated with disease states 1,3,4. For example, the FCGR3A-V158F and FCGR2A-H131R SNPs result in increased affinity for IgG and are associated with idiopathic thrombocytopenic purpura/immune thrombocytopenia (ITP) in paediatric patients 5. Similarly the FCGR2C-ORF genotype predisposes to ITP, potentially by altering the balance of activating and inhibitory FcγRs on immune cells 4. FCGR2C is often considered a pseudogene due to the presence of a stop codon in exon 3; however, a SNP observed in ∼9% of healthy Caucasian individuals results in the stop codon changing to a glutamine that results in an open reading frame (ORF) and the FCGR2C-ORF genotype 4. Using a multiplex ligation-dependent probe amplification (MLPA) assay we have been able to analyse SNPs and CNV genetic variation. Using this assay in more than 1750 subjects we have been able to identify extensive ethnic variation. Our current population studies have identified linkage disequilibrium in these genes; if a subject is positive for the FCGR3A-V158F SNP, there is a high chance that they also carry the FCGR2C-ORF or FCGR2A-H131R SNPs. Furthermore, our ITP cohort has demonstrated that only the FCGR3A-V158F SNP is associated independently with ITP, whereas FCGR2C-ORF seems to be associated due to linkage; clearly, the way we should consider these IgG receptors is through their associated IgG-binding effects, depending on their expression pattern in different cell types. A similar genomewide association study covering 2173 patients of European and Asian descent found that the FCGR2A-H131R SNP conferred elevated risk of Kawasaki disease; however, this study was unable to test for the FCGR2C-ORF 6. The results from these studies in ITP and Kawasaki disease confirm the importance of considering both ethnicity and linkage equilibrium when attempting to carry out comprehensive genotyping of the FCGR2/3 locus 7.
As we have discussed, a multitude of SNPs in the low-affinity Fc receptor have been associated with ITP, a chronic autoimmune disorder characterized by a reduction in platelet count and an increased risk of skin/mucous membrane bleeding. ITP is caused by increased platelet destruction and impaired platelet production as a result of the development of autoantibodies against platelet glycoproteins 8. Following antibody binding, the IgG opsonized platelets are cleared rapidly by spleen and liver macrophages; ITP does not necessarily require treatment, but splenectomy is an effective and durable treatment and, in cases of severe bleeding, treatment with intravenous immunoglobulin (IVIg) is indicated in order to increase platelet levels. Three possible modes of action have been proposed as the mechanism by which IVIg works to suppress autoimmune diseases (Fig. 2): competing with pathogenic IgGs for the activating FcγRs, saturation of activating FcγRs by IVIg or up-regulation of the inhibitory FcγRIIb 9. Using monocyte-derived macrophages from human blood, we observed that the uptake of erythrocytes opsonized by anti-D could be prevented by the blockade of FcγRI, FcγRII and FcγRIII by IVIg 7.
Figure 2.
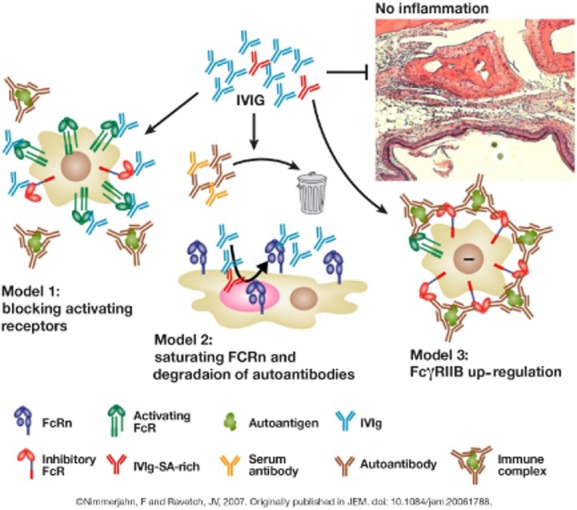
Three models have been proposed to explain the anti-inflammatory activity of intravenous immunoglobulin (IVIg) 9. In the first model, IVIg (consisting of a mixture of sialic acid–rich [red] and sialic acid–low [blue] antibodies) binds to activating FcγRs on immune effector cells, thereby blocking access of immune complexes to these receptors and inhibiting cell activation. The second model proposes that IVIg competes with serum immunoglobulin (Ig) G (including autoantibodies) for recycling mediated by FcRn. Thus, serum and autoantibodies would be cleared more rapidly and not reach the threshold level for initiating tissue destruction; in this model plasmapheresis or exchange could work as an alternative modality for enhanced autoantibody clearance. In the third model, IVIg leads to up-regulation of the inhibitory FcγRIIb on immune effector cells, thus increasing the threshold level for cell activation by immune complexes.
Glycosylation is one factor that may affect erythrocyte uptake by macrophages. All IgG molecules are glycosylated, with a minor population of the Fc regions terminating in sialic acid residues 10. This sialylation of IgG is an impor tant modification, and results in an anti-inflammatory effect mediated by FcγRIIb via several potential mechanisms: FcγRIIb contributes directly by negative signalling upon binding of sialylated IgG, or indirectly through increased macrophage expression of FcγRIIb upon IgG binding to murine-specific intercellular adhesion molecule-3 grabbing non-integrin-related 1 (SIGNR1) or human dendritic cell (DC)-SIGN (same cell) and FcγRIIb up-regulation on effector macrophages as a result of interleukin (IL)-33 release and basophil activation (different cell) 10. We tested the relevance of IgG sialylation by stimulating monocyte-derived macrophages with anti-trinitrophenyl (TNP) IgG, with or without sialylation, and observed no sialylation-dependent effect on erythrocyte uptake. Conversely, IgG dimer-enriched IVIg was shown to improve erythrocyte uptake by macrophages, indicating that most of the direct in vitro effect of IVIg may be derived from the direct competition for FcγR binding by IgG dimers, instead of a result of sialylation 7.
When carrying out these studies, it is also important to ensure that the correct macrophages are being analysed. There are three subtypes of spleen macrophage: the red pulp macrophages (CD163+), perifollicular-zone macrophages (CD169+) and marginal-zone macrophages (no marker known). It is not currently known which macrophage population is responsible for the clearance of IgG opsonized blood cells, and very little is known about the expression of FcγRs on human spleen macrophages. In order to try to answer these questions, we have successfully developed a sequential selection method for isolating red pulp macrophages from the spleen with >90% purity. When examining the phenotype of the isolated red pulp macrophages, we observed a different pattern of FcγR expression. Further tests confirmed that the red pulp macrophages were capable of active phagocytosis via FcγRs. Using this protocol, we will be able to test these macrophages and compare these cells with the standard monocyte-derived macrophages to study the regulation of IgG receptor expression and function in more detail. We are now ready to use splenic macrophages from fully genotyped individuals to examine how FcγR expression and regulation may affect the outcome of IVIg treatment.
In the future we wish to assess FcγR involvement in IgG-bound platelet destruction upon uptake by splenic macrophages, expression regulation of the high-affinity FcγRI and inhibitory FcγRIIb on splenic macrophages and the impact of genetic variation on the process of blood cell destruction.
Acknowledgments
This work was supported by an independent grant (LSBR-0916) for blood transfusion research. T. K. would like to thank Meridian HealthComms Ltd for providing medical writing services.
Disclosure
Part of this research was performed at the Sanquin Research Institute, Amsterdam.
References
- Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin Exp Immunol. 2009;157:244–254. doi: 10.1111/j.1365-2249.2009.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- van der Heijden J, Breunis WB, Geissler J, de Boer M, van den Berg TK, Kuijpers TW. Phenotypic variation in IgG receptors by nonclassical FCGR2C alleles. J Immunol. 2012;188:1318–1324. doi: 10.4049/jimmunol.1003945. [DOI] [PubMed] [Google Scholar]
- Breunis WB, van Mirre E, Bruin M, et al. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. 2008;111:1029–1038. doi: 10.1182/blood-2007-03-079913. [DOI] [PubMed] [Google Scholar]
- Carcao MD, Blanchette VS, Wakefield CD, et al. Fcgamma receptor IIa and IIIa polymorphisms in childhood immune thrombocytopenic purpura. Br J Haematol. 2003;120:135–141. doi: 10.1046/j.1365-2141.2003.04033.x. [DOI] [PubMed] [Google Scholar]
- Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011;43:1241–1246. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- Nagelkerke SDG, Kustiawan I, van de Bovenkamp FS, et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood. 2014 doi: 10.1182/blood-2014-05-576835. ; doi:10.1182/blood-2014-05-576835. [DOI] [PubMed] [Google Scholar]
- Thota S, Kistangari G, Daw H, Spiro T. Immune thrombocytopenia in adults: an update. Cleve Clin J Med. 2012;79:641–650. doi: 10.3949/ccjm.79a.11027. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 2007;204:11–15. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]


