For more than two decades intravenous immunoglobulin (IVIg) has been the treatment of choice for Guillain-Barré syndrome (GBS), the most acute and severe form of inflammatory neuropathy 1. Despite the frequent usage and proven beneficial effect of IVIg in GBS, the working mechanism remains elusive 2. Patients with GBS show a variable clinical response to IVIg, ranging from a rapid and full recovery to further disease progression, resulting in respiratory failure and considerable long-term disability. The optimal dosage and pharmacokinetics (PK) of IVIg in GBS have not been defined, and may differ between patients 1,3. There is an unmet need for biomarkers to monitoring disease activity or treatment effect of IVIg in individual patients with GBS. Such biomarkers could help to adjust and personalize the treatment with IVIg. GBS is an immune-mediated disorder but has an acute and monophasic clinical course which is different from most classical chronic or relapsing–remitting autoimmune diseases 1. GBS is a typical postinfectious disorder in which the preceding infection results in the production of cross-reactive and neurotoxic antibodies in a subgroup of patients. This pathomechanism is best described for preceding infections with Campylobacter jejuni bacteria, wherein the lipo-oligosaccharides (LOS) mimic carbohydrates expressed on peripheral nerve gangliosides. The subsequent cross-reactive antibody response results in rapidly progressive nerve damage with the typical acute and monophasic weakness in the limbs 1. Sialic acid moieties expressed on both the LOS and the gangliosides seem to be important for this event to occur. The presence of sialic acids in C. jejuni LOS is known to stimulate the immune response and may explain the increased pathogenicity of sialylated strains 4.
In addition, sialic acids as part of immunoglobulin (Ig)G Fc glycosylation may play an important role in the immunomodulatory effects of IVIg. Ravetch and co-workers have shown that in certain animal models the terminal sialic acid, in a α2,6 linkage, confers an anti-inflammatory effect 5,6. While it might not be the predominant mechanism of action in every disease (model) 7, it has led to a surge of interest in IgG glycosylation. At asparagine 297 in the Fc-region, an N-glycan structure is attached to the protein backbone on each CH2 domain. There is a core structure with variation in further glycosylation by the presence or absence of bisecting N-acetylglucosamine, fucose, galactose and sialic acid (Fig. 1) 8. In human disease these glycoforms of serum IgG may reflect the activity of the immune system or disease. In general, the serum IgG Fc glycosylation is stable in a healthy person, but decreases upon inflammation or immunization 8. This feature makes IgG Fc glycosylation a potential biomarker for disease activity, as has been demonstrated for galactosylation in rheumatoid arthritis (RA) and other inflammatory diseases 9.
Figure 1.
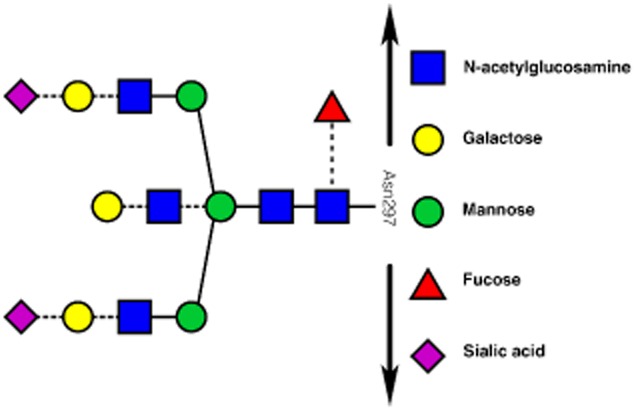
Schematic representation of the immunoglobulin (Ig)G Fc-N-glycan structure (adapted with permission from 8, copyright 2014, The American Chemical Society). Each IgG molecule possesses more than two of these carbohydrate structures attached to asparagine 297 of the protein backbone (black arrows) of the CH2 domain. Possible variation in this structure, leading to distinct glycoforms, is denoted by the dashed lines.
The notion that IgG Fc glycosylation might mediate the anti-inflammatory actions of high-dose IVIg and could serve as a potential biomarker of disease activity and treatment efficacy was assessed recently in a large cohort of patients with GBS 8. All patients had participated previously in two randomized controlled clinical trials (n = 174) and were treated with the same regimen of IVIg (0·4 g/kg of body weight for 5 consecutive days) 10,11. IgG1 and IgG2 glycosylation in pretreatment (n = 150), as well as 2 weeks post-treatment serum samples (n = 150), was assessed by liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS). MS is an extremely reliable method to assess IgG glycosylation and allows for unambiguous determination of the specific glycoforms 8,9. The study showed that, prior to IVIg treatment (n = 91), the IgG Fc galactosylation level in GBS patients was slightly lowered compared to age- and sex-matched healthy controls (n = 91; IgG1: P = 0·013 and IgG2: P = 0·001). The pretreatment IgG Fc glycosylation was not associated with disease severity. Two weeks after the start of the IVIg (n = 150), the total serum IgG Fc glycosylation was increased compared to IgG Fc glycosylation in pretreatment samples (n = 150, P < 0·001). The total serum IgG at that time-point consists presumably of a mixture of both endogenously produced IgG and exogenous IgG derived from the IVIg. The latter reflects the IgG Fc glycosylation profile in blood from the normal healthy population. The increase in galactosylation was more pronounced than the increase in sialylation. However, not all patients showed an increase in serum IgG Fc glycosylation post-IVIg. Indeed, some patients showed a decline in serum IgG glycosylation compared to pretreatment, despite infusion of high-dose IVIg 8.
The PK of total serum IgG in GBS was investigated in a previous study, and it was shown that the serum IgG levels are elevated after IVIg but with a large interpatient variation 3. No correlation was found between the change in total serum IgG levels and Fc glycosylation 8. This finding may imply that the different IgG glycoforms after IVIg have a similar PK, although in that study endogenously produced IgG and IVIg-derived IgG could not be discriminated. It has been shown that where IgG glycoforms bind differentially to the Fcγ-receptor family, the binding to FcRn is not influenced 7. Scavenging by other receptors, mainly of the C-type lectin family, is not ruled out 7. Increased vulnerability to proteolytic degradation might also occur, as the extent of glycosylation is known to influence stability of the protein 12.
Interestingly, the IgG Fc glycosylation in serum 2 weeks after the start of IVIg therapy was associated with disease severity and outcome of the patients with GBS. This was shown for the two predominant IgG subclasses (IgG1 and IgG2) for galactosylation and, to a lesser extent, sialylation. A higher level of glycosylation was associated with a decreased chance of respiratory failure (P < 0·01), less severe muscle weakness after 4 weeks (IgG1: P < 0·01 and IgG2: P = 0·02) and more patients regaining the ability to walk unaided at the end of follow-up (IgG1: P = 0·01 and IgG2: P = 0·02) 8. The finding that Fc galactosylation, more than Fc sialylation, reflects disease activity was also shown recently for RA 13. In the study on patients with GBS, a more anti-inflammatory glycoprofile (increased glycosylation) of serum IgG after IVIg therapy was associated with a more favourable outcome, independent of age and gender (known to influence IgG Fc glycosylation). While total serum IgG Fc glycosylation might portray a role as indicator of IVIg treatment response, it might not reflect the state of inflammation of peripheral nerves and nerve roots seen in this disease. Accordingly, patients with the acute phase of GBS, in general, show no increase in acute phase proteins or cytokines 1.
Further studies are required to determine the value of serum IgG Fc glycosylation as a potential biomarker for disease activity and response to IVIg. A limitation of the current studies is that they were unable to discriminate between endogenously present IgG and those derived from IVIg. It is therefore unknown whether the administration of IVIg influences the endogenous IgG production and glycoforms. A second limitation is that the study measured only the Fc glycosylation of the total pool of serum IgG. Performing Fc glycosylation analysis on pathogenic antibodies in GBS may reflect disease activity and treatment response more accurately. Such studies could have a major impact on IVIg-treated disorders. GBS is an excellent model disease to address these research questions, as GBS is an acute and monophasic disorder, most patients have no co-morbidity and all are treated with the same regimen of IVIg.
Acknowledgments
W.-J. R. F., M. H. C. S., M. W. and B. C. J. would like to thank all other co-authors of the original paper, ‘IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins’, for their efforts in making this study possible.
Disclosures
W.-J. R. F., M. H. C. S., M. W. and B. C. J. have no conflicts of interest to declare.
References
- van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain–Barré syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain–Barré syndrome. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD002063.pub5. :CD002063. [DOI] [PubMed] [Google Scholar]
- Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain–Barré syndrome. Ann Neurol. 2009;66:597–603. doi: 10.1002/ana.21737. [DOI] [PubMed] [Google Scholar]
- Huizinga R, van Rijs W, Bajramovic JJ, et al. Sialylation of Campylobacter jejuni endotoxin promotes dendritic cell-mediated B cell responses through CD14-dependent production of IFN-beta and TNF-alpha. J Immunol. 2013;191:5636–5645. doi: 10.4049/jimmunol.1301536. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- Fokkink WJ, Selman MH, Dortland JR, et al. IgG Fc N-glycosylation in Guillain–Barré syndrome treated with immunoglobulins. J Proteome Res. 2014;13:1722–1730. doi: 10.1021/pr401213z. [DOI] [PubMed] [Google Scholar]
- Albrecht S, Unwin L, Muniyappa M, Rudd PM. Glycosylation as a marker for inflammatory arthritis. Cancer Biomark. 2014;14:17–28. doi: 10.3233/CBM-130373. [DOI] [PubMed] [Google Scholar]
- van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain–Barré syndrome. Dutch Guillain–Barré Study Group. N Engl J Med. 1992;326:1123–1129. doi: 10.1056/NEJM199204233261705. [DOI] [PubMed] [Google Scholar]
- van Koningsveld R, Schmitz PI, Meche FG, Visser LH, Meulstee J, van Doorn PA. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain–Barré syndrome: randomised trial. Lancet. 2004;363:192–196. doi: 10.1016/s0140-6736(03)15324-x. [DOI] [PubMed] [Google Scholar]
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Bondt A, Selman MH, Deelder AM, et al. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res. 2013;12:4522–4531. doi: 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]


