Immunoglobulin (Ig)G therapy is the primary treatment for patients with primary antibody deficiencies (PAD), and can be administered intravenously [intravenous immunoglobulin (IVIg)] or subcutaneously [subcutaneous immunoglobulin (SCIg)]. IVIg has been a well-established treatment for many years; however, SCIg is now gaining popularity among patients and clinicians alike, due in part to the convenience of self-administration, the absence of the wear-off effects experienced with IVIg, lower rates of adverse events (AEs) compared with high bolus IVIg and also the reduced fluctuation of serum IgG levels afforded by more frequent dosing intervals.
When switching patients from IVIg to SCIg, dosage and dosing intervals are based on the current IVIg dose and the patient's response to therapy, and guidance is available to inform best practice for making this transition safely 1,2. However, switching protocols vary between the United States and Europe, adding a further confounding factor. Very little guidance on how to initiate SCIg treatment in newly diagnosed patients naive to Ig therapy currently exists. To address this data gap, we developed a population pharmacokinetic (PK) model to test a range of doses for initial SCIg loading.
Population PKs is useful to predict the behaviour of a compound such as IgG in a larger group of patients while accounting for inter- and intra-individual variability, without the need to evaluate each novel regimen in a clinical trial. The use of such models is supported by the Food and Drug Administration (FDA) and other regulatory bodies, and they are often used to develop quantitative guidelines for drug–dosage individualization. Our model was developed based on data from four trials with SCIg (Hizentra®) and IVIg (Privigen®) in 151 patients, with the aim of determining the serum IgG levels achieved by different dosing regimens. An important component of the model is the endogenous level of IgG, a variable rarely recorded in clinical studies with steady state dosing. The model focuses on how the concentration of IgG in the central compartment differs depending on the route of IgG administration. It also takes into account clearance interactions between the central compartment and the peripheral/secondary compartment, characterized by absorption coefficients and traditional PK variables such as clearance and half-life are presented.
The model has been validated against clinical study data by Borte et al. 3, which investigated IgG serum dynamics after loading 18 treatment-naive patients with Vivaglobin® SCIg, and accepted by several regulatory agencies, including the FDA 4 and European Medicines Agency (EMA). Use of the PK model for loading dose simulations was validated in part by being able to successfully predict the observed IgG levels in the Borte et al. study. Importantly, data from the Borte et al. study subjects were not used in the pharmacometric model data set. We have since used the model to test six different loading regimens (Table 1) in 2500, simulated population-average, 60 kg PAD patients treated de novo with Hizentra®.
Table 1.
Simulated loading regimens. Loading regimens lasted either 1 or 2 weeks, using doses of either 100 or 150 mg/kg, dosed either twice, thrice or five times per week
| Day (week 1) |
Day (week 2) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 1 | 100 | 100 | 100 | 100 | 100 | |||||||||
| 2 | 150 | 150 | 150 | 150 | 150 | |||||||||
| 3 | 100 | 100 | 100 | 100 | ||||||||||
| 4 | 150 | 150 | 150 | 150 | ||||||||||
| 5 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||||
| 6 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||
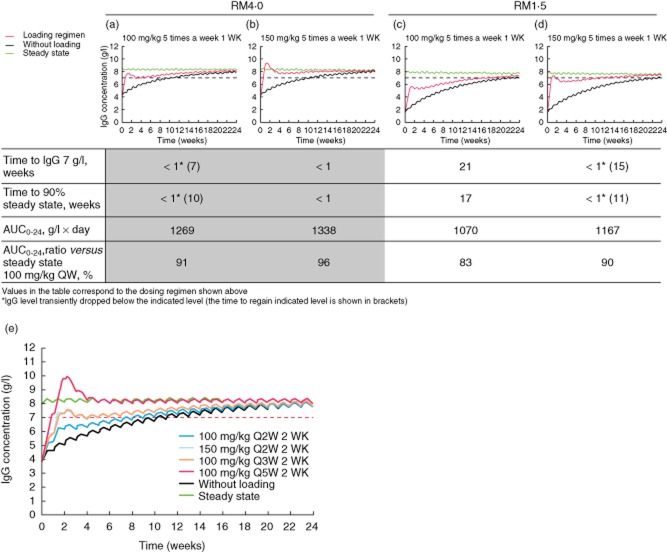
Each of these regimens was to be followed by regular weekly infusions of 100 mg/kg starting on day 8 (for the 1-week regimens) or 15 (for the 2-week regimens). Two populations were simulated; the first with endogenous IgG serum levels of 4 g/l (corresponding to serum levels seen in PAD populations comprising predominantly common variable immunodeficiency patients) and the second with levels of 1·5 g/l (for mostly X-linked agammaglobulinaemia patients). The outcomes included the time for serum IgG levels to reach a target concentration of 7 g/l which, for the purposes of this analysis, was assumed to be protective for the majority of PAD patients; the time to reach 90% of the steady state IgG level (quasi-steady state); and also the area under the curve (AUC), absolute and as a percentage of steady state.
Results are shown in Fig. 1. In the 4 g/l group on regimen 1 (100 mg five times per week for 1 week), quasi-steady state target serum IgG levels were achieved within 1 week of initiating SCIg. After switching to a maintenance dose of 100 mg/kg/week SCIg, plasma IgG levels dropped marginally below 7 g/l for a period of 7 weeks before reaching a steady state above 7 g/l. Those receiving the same loading dose in the 1·5 g/l starting group achieved quasi-steady state after 17 weeks and reached 7 g/l IgG after 21 weeks. Once these levels had been achieved, they were maintained on a continual dose of 100 mg/kg/week.
Figure 1.
Serum immunoglobulin (Ig)G levels of simulated patients during loading and maintenance treatment, compared to steady state and IgG therapy without loading (modified with permission from 5, copyright 2014, open source). (a) Loading regimen of 100 mg/kg five times a week for 1 week (4 g/l starting level). (b) Loading regimen of 150 mg/kg five times a week for 1 week (4 g/l starting level). (c) Loading regimen of 100 mg/kg five times a week for 1 week (1·5 g/l starting level). (d) Loading regimen of 150 mg/kg five times a week for 1 week (1·5 g/l starting level). (e) 2-week loading regimens. RM1·5 is the reference model assuming a baseline (endogenous) IgG concentration of 1·5 g/l; RM4·0 is the reference model assuming an endogenous IgG level of 4 g/l; Q2W: two doses per week; Q3W: three doses per week; Q5W: five doses per week. AUC = area under the curve.
Patients in the 4 g/l starting group on loading regimen 2 (150 mg/kg five times per week for 1 week) achieved quasi-steady state serum IgG in under a week, and were subsequently maintained on 100 mg/kg/week. In the group starting from 1·5 g/l, both end-points were reached in under a week but quickly dropped below the target levels once maintenance therapy was initiated, taking 15 weeks to return above 7 g/l IgG. Data from the 2-week loading regimens are shown in Table 2.
Table 2.
Time taken to reach simulation endpoints in each dosing regimen and each endogenous immunoglobulin (Ig)G level group
| End-point | Endogenous IgG level | Regimen 3 | Regimen 4 | Regimen 5 | Regimen 6 |
|---|---|---|---|---|---|
| Time to IgG 7 g/l, weeks | 4 g/l | 10 | 2* (6) | 2* (6) | <1 |
| 1·5 g/l | 24 | 21 | 21 | 2 | |
| Time to 90% of steady state, weeks | 4 g/l | 13 | 2* (9) | 2* (9) | <1 |
| 1·5 g/l | 21 | 17 | 16 | 2 |
IgG level transiently dropped below the indicated level after the switch to maintenance dose (time to regain indicated level shown in brackets).
Notably, regimen 5 (100 mg/kg/day for 3 consecutive days on each of 2 consecutive weeks, see Table 1 and Fig. 1e) maintained target IgG levels in patients with high endogenous IgG after 2 weeks, once the patients were switched to the maintenance dose of 100 mg/kg/week.
Loading regimens at the beginning of SCIg therapy are critical, to confer a high level of protection against infections from the outset. If no loading dose is used, it usually takes between 4 and 6 months of maintenance therapy to achieve target serum IgG levels, during which time the patients are under-protected and vulnerable to infections.
In most patients with PAD, a loading regimen of 100 mg/kg for 5 consecutive days leads to IgG levels that can be considered protective within 1 week. In patients with more severe disease and/or lower baseline IgG levels, loading with 150 mg/kg on 5 consecutive days may be more appropriate, as this confers lasting near-protective levels of serum IgG within 1 week. In patients who prefer to avoid a schedule of daily infusions, a loading dose of 150 mg/kg twice per week for 2 weeks or 100 mg/kg three times per week for 2 weeks can reach protective levels after 2 weeks. After the loading, SCIg dosing can be adjusted individually depending on the clinical response, which allows for some flexibility in interpreting these results.
This study investigated a population of 2500 simulated patients with a fixed theoretical baseline IgG level, receiving a fixed maintenance dose of IgG. Investigations into a more variable population would be valuable, as in practice baseline IgG and clearance vary from patient to patient. A simulation with a more varied set of parameters would provide a more robust and flexible model which could have more predictive value for a greater number of patients. Similarly, models investigating the effect of 200 mg/kg loading doses may be tested, as clinical experience shows that this dose level is tolerable in a high number of patients. It would also be valuable to test the regimens simulated here in a clinical study.
Potential limitations of this study include the fact that simulations were based on the data obtained in clinical trial subjects, all of whom had received IgG therapies prior to their study participation. As there were no PK data from IgG treatment-naive subjects, methodological assumptions had to be made about their endogenous IgG levels. Based on published reports, we chose to fix endogenous IgG in the model to a value within a range of 1·5–4·0 g/l. Consequently, confirmation of our assessments of various SCIg loading dose possibilities is warranted.
Acknowledgments
M. R., J. S., M. P. and A. H. thank Meridian HealthComms Ltd for providing medical writing services.
Disclosure
M. R., J. S. and A. H. are employees of CSL Behring. M. P. is a consultant of CSL Behring.
References
- Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M Subcutaneous IgG Study Group. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–273. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- Wasserman RL, Melamed I, Nelson RP, Jr, et al. Pharmacokinetics of subcutaneous IgPro20 in patients with primary immunodeficiency. Clin Pharmacokinet. 2011;50:405–414. doi: 10.2165/11587030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Borte M, Quinti I, Soresina A, et al. Efficacy and safety of subcutaneous vivaglobin(R) replacement therapy in previously untreated patients with primary immunodeficiency: a prospective, multicenter study. J Clin Immunol. 2011;31:952–961. doi: 10.1007/s10875-011-9588-5. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. 2014. Clinical pharmacology BLA review: Hizentra . Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM373050.pdf (accessed on 4 August )
- Sidhu J, Rojavin M, Pfister M, Edelman J. Enhancing patient flexibility of subcutaneous immunoglobulin G dosing: pharmacokinetic outcomes of various maintenance and loading regimens in the treatment of primary immunodeficiency. Biol Ther. 2014;4:41–55. doi: 10.1007/s13554-014-0018-0. doi: 10.1007/s13554-014-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]